Abstract
Background
In patients with severe coronavirus disease 2019 (COVID-19), data are scarce and conflicting regarding whether chronic use of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) influences disease outcomes. In patients with severe COVID-19, we assessed the association between chronic ACEI/ARB use and the occurrence of kidney, lung, heart, and liver dysfunctions and the severity of the inflammatory reaction as evaluated by biomarkers kinetics, and their association with disease outcomes.
Methods
We performed a retrospective longitudinal cohort study on consecutive patients with newly diagnosed severe COVID-19. Independent predictors were assessed through receiver operating characteristic analysis, time-series analysis, logistic regression analysis, and multilevel modeling for repeated measures.
Results
On the 149 patients included in the study 30% (44/149) were treated with ACEI/ARB. ACEI/ARB use was independently associated with the following biochemical variations: phosphorus >40 mg/L (odds ratio [OR], 3.35, 95% confidence interval [CI], 1.83–6.14), creatinine >10.1 mg/L (OR, 3.22, 2.28–4.54), and urea nitrogen (UN) >0.52 g/L (OR, 2.65, 95% CI, 1.89–3.73). ACEI/ARB use was independently associated with acute kidney injury stage ≥1 (OR, 3.28, 95% CI, 2.17–4.94). The daily dose of ACEI/ARB was independently associated with altered kidney markers with an increased risk of +25 to +31% per each 10 mg increment of lisinopril-dose equivalent. In multivariable multilevel modeling, UN >0.52 g/L was independently associated with the risk of acute respiratory failure (OR, 3.54, 95% CI, 1.05–11.96).
Conclusions
Patients chronically treated with ACEI/ARB who have severe COVID-19 are at increased risk of acute kidney injury. In these patients, the increase in UN associated with ACEI/ARB use could predict the development of acute respiratory failure.
Keywords: SARS-CoV-2, severe COVID-19, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, acute kidney injury
The present retrospective longitudinal cohort study on consecutive patients with newly diagnosed severe COVID-19 provides new evidence on the potentially harmful effect of chronic ACEI/ARB use on kidney outcomes and their possible interaction with the occurrence of acute respiratory failure.
A novel human coronavirus, called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing the coronavirus disease 2019 (COVID-19) was identified in China in December 2019 [1]. COVID-19 can induce pulmonary and systemic inflammation and subsequent acute respiratory failure and multi-organ dysfunction [2, 3]. It has been shown that SARS-CoV-2 infects the cells through its binding to the membrane-bound form of receptor-angiotensin converting enzyme 2 (ACE2) and subsequent internalization of the complex by the host cell [1, 3–6]. From a functional point of view, ACE2 represents a key enzymatic component of the renin-angiotensin-aldosterone system (RAAS) [4, 7]. It corresponds to a carboxypeptidase that acts as a negative regulator of the RAAS, serving as a clearance pathway for angiotensin II, a peptide with multiple actions that promote vasoconstriction, fibrosis, and proinflammatory effects [4, 8].
It has been speculated that patients with COVID-19 who receive angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) therapy may be at increased risk for adverse outcomes [9]. In this context, 2 contrasted hypotheses have been proposed regarding the interaction between ACEI/ARB use and the SARS-CoV-2 infection [10]. The “harmful hypothesis” states that ACEI/ARB use increases the expression of ACE2, which in turn promotes the entry of the SARS-CoV-2 into the cells [11]. In this setting, high ACE2 levels might be associated with a higher local viral load [11]. Conversely, the “beneficial hypothesis” states that ACEI/IRB use is associated with an increase in the expression of ACE2 with subsequent reduction of angiotensin II. The reduction of angiotensin II may have anti-inflammatory and antioxidative effects and therefore may be beneficial in the prevention of acute lung injury [12–14].
To date, data are scarce and conflicting regarding whether ACEI/ARB therapy influences disease outcomes among patients with severe COVID-19 [15–17]. Moreover, no data are available on the longitudinal evolution of biomarkers related to kidney, lung, heart, liver, and muscle functions along with the inflammatory status in patients chronically treated with ACEI/ARB and the potential interaction of biochemical alterations with disease outcomes among patients with severe COVID-19.
A retrospective longitudinal cohort study on patients admitted for severe COVID-19 in a tertiary referral university hospital in France used a big-data approach and multilevel modeling adapted for repeated measures to screen the dynamic evolution of 59 biochemical markers during the hospital stay [3]. Using data from this same cohort, derived from the Nancy Biochemical Database (see Supplementary Material) [3, 18], we assessed the association between chronic ACEI/ARB use and the occurrence of kidney, lung, heart, and liver dysfunctions and inflammation as evaluated by biomarker kinetics in patients with severe COVID-19.
METHODS
Study Design, Setting, and Patients’ Inclusion Criteria
We carried out a retrospective, longitudinal cohort study on all newly diagnosed consecutive patients among the first cases of severe COVID-19 that required hospitalization at the University Hospital of Nancy. Inclusion in the cohort began on the day of hospital admission, and each patient was then followed until discharge from hospital or death if it occurred during hospitalization. The inclusion criteria were: (i) a diagnosis of COVID-19 based on the detection of SARS-CoV-2 ribonucleic acids (RNA) from nasopharyngeal swabs (see Supplementary Material); (ii) severe COVID-19 defined by an oxygen saturation of 94% or less while the patient was breathing ambient air or a need for oxygen support [19, 20]; (iii) COVID-19 requiring hospitalization in one of the University Hospital healthcare departments from 1 March 2020 to 25 March 2020. The final date of follow-up was 31 March 2020; and (iv) availability of data regarding ACEI/ARB use at hospital admission. The cohort was observational, that is, all clinical assessments, biochemical explorations, imaging examinations, and clinical diagnoses were carried out at the discretion of the treating physicians. The Ethics committee of the University Hospital of Nancy approved the study.
Data Collected for the Study
The following data were collected in the Nancy Biochemical Database: patient identification number, patient’s age at hospital admission, date and time of blood sampling, and healthcare department. A total of 59 biochemical markers were available, with 46 in the blood and 13 in the urine (see Supplemental Methods in the Supplementary Material). The following clinical data were collected: date of hospital admission; patient’s medical history; chronic treatment with ACEI/ARB (ACEI or ARB use was considered if the patient was receiving these drugs for at least 3 months before hospital admission); ACEI or ARB molecule; ACEI or ARB daily dose calculated and expressed as a lisinopril-dose equivalent as described in the Supplementary Table 1 [21]; patient’s outcomes during the hospitalization for the management of COVID-19: (i) acute respiratory failure diagnosed when the patient presented with acute clinical signs of respiratory distress (respiratory rate ≥21 breaths per minute) and an acute impairment in gas exchange causing hypoxemia (partial pressure of oxygen [PO2] <60 mmHg on room air) with or without hypercapnia, and which required oxygen therapy; (ii) intubation with mechanical ventilation; (iii) pulmonary embolism; and (iv) in-hospital mortality related to COVID-19, defined as the occurrence of death related to a complication of COVID-19 [22]).
Study Aims and Endpoints
The primary aim of the study was to assess the association between chronic ACEI/ARB use and the evolution during the hospital stay of (i) the biochemical markers related to kidney, lung, heart, liver, muscle, and inflammatory status and (ii) the stage of acute kidney injury (AKI). The diagnosis and severity of AKI were classified according to the AKI network criteria, based on the results of serum creatinine [23]. The secondary aims were to assess (i) the association between the biochemical variations significantly associated with ACEI/ARB use (ACEI/ARB-associated biochemical variations) and COVID-19 related acute respiratory failure and in-hospital mortality; (ii) the association between ACEI/ARB use and the viral load of SARS-CoV-2 at diagnosis. The primary endpoint was, for each studied biochemical marker and the AKI stage, the percentage of time below or above a predefined threshold during the hospital stay. The secondary endpoints were (i) the occurrence of COVID-19-related acute respiratory failure and in-hospital mortality and (ii) the viral load of SARS-CoV-2.
Statistical Analyses
All quantitative variables are shown as the median and interquartile range (IQR, 25–75th percentile) and qualitative variables as percentages and 95% confidence interval (95% CI). The design of the statistical analysis is reported in Supplementary Figure 1. Using a multistep approach, we evaluated a set of 20 biochemical parameters with a sufficiently high number of iterations (n > 250, study power analysis not shown) to assess the relationship between their variation over time and the ACEI/ARB use. In step 1, for each biochemical variable and the AKI stage, we assessed the optimal threshold associated with ACEI/ARB use through receiver operating characteristic (ROC) analysis, according to DeLong et al [24]. The classification variable used in the ROC analysis was the ACEI/ARB use. The optimal diagnostic cut-off was defined using the Youden index J. Bias-corrected and accelerated-bootstrap interval after 10 000 iterations for the Youden index and its associated values were performed [25]. In step 2, all the variables that were significantly associated with ACEI/ARB use in ROC analyses were assessed through time-series analysis [26]. The evolution times were calculated from the first day of biochemical assessment and were expressed in days. The time-series analyses aimed to compare the percentage of time below or above the ROC-defined threshold between patients with or without ACEI/ARB use. The calculated summary effects were reported as percentages of the total time of observation with the 95% CI. Normality testing was performed using the D’Agostino-Pearson test. Subgroups comparison regarding the percentage of time below or above the predefined threshold was carried out using the Student’s t-test or the Mann-Whitney U test according to the parametric or nonparametric distribution of the variables, respectively. In step 3, we performed multivariable logistic regression analysis to assess whether ACEI/ARB use was independently associated with the variation of biochemical markers identified in step 2, after adjustment for potential confounders (age, sex, medical history, and time). In each logistic regression model, we used the dichotomized biochemical variable or the dichotomized AKI stage, derived from ROC-analyses, as the dependent variable. All the variables with P < .1 were included in the model, and the variables with P < .05 were retained in the model using the stepwise method. Results were shown as regression coefficient, standard error (SE), odds ratio (OR), and 95% CI for each independent predictor, and the percentage of cases correctly classified by the logistic regression model. We assessed model discrimination using ROC analysis and model calibration using the Hosmer and Lemeshow goodness-of-fit test and Nagelkerke R2 statistics [27]. Then we assessed the association between ACEI/ARB-associated biochemical variations and the occurrence of acute respiratory failure, on the one hand, and in-hospital mortality, on the other hand, by using multivariable multilevel analysis which enabled to take into account the correlation between the studied biochemical parameters and the patient-level characteristics (ie, age, sex, patient’s medical history) (see Supplementary Material). We performed posthoc exploratory sensitivity analyses to assess the stability of the effect sizes for the association between the use of ACEI/ARB and the biochemical alterations that have shown significance in multivariable multilevel analyses. We performed 4 types of sensitivity analyses: (1) forced adjustment for the medical history of chronic kidney disease, (2) ACEI use versus no treatment with ACEI/ARB to assess the specific effect of ACEI, (3) ARB use versus no treatment with ACEI/ARB to assess the specific effect of ARB, and (4) dose-effect analysis to assess the association between each 10 mg increment of the lisinopril-dose equivalent (= 8 mg increment of candesartan) of the daily intake of ACEI/ARB and kidney outcomes (Supplementary Table 1). The comparison of cycle threshold (Ct) values for the IP2 and IP4 targets between subgroups was carried out using the Mann-Whitney U test. Statistical analyses other than multivariable multilevel analyses were performed using MedCalc 19.1 (MedCalc Software, Ostend, Belgium) based on a 2-sided type I error with an alpha level of 0.05. The multivariable multilevel analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Baseline Characteristics
Between 1 March 2020 and 25 March 2020, 162 patients were admitted to one of the University Hospital of Nancy healthcare departments for severe COVID-19. Among them, 149 (92%) had available data for ACEI/ARB use and were analyzed. The median age of the population was 65 years (IQR, 54–77), and the proportion of males was 61% (91/149) (Table 1). Of the 149 patients, 19 (13%) were treated with ACEI and 25 (17%) by ARB, totaling 30% (44/149) of patients receiving ACEI/ARB. The median daily dose of ACEI/ARB, expressed as a lisinopril-dose equivalent, was 20 mg per day (IQR, 10–40) (Supplementary Table 2). During the study period, 1082 biochemical explorations were carried out for up to 59 biochemical parameters (46 in the blood and 13 in the urine), totaling 15 215 biochemical values. The distribution of the 59 biochemical parameters is reported in Supplementary Tables 3 and 4.
Table 1.
Characteristics of the Patients Included in the Study
| Whole Cohort | No ACEI/ARB | ACEI/ARB | P Valuea | ||||
|---|---|---|---|---|---|---|---|
| Age—N, median (IQR) | 149 | 65 (54–77) | 105 | 63 (51–74) | 44 | 70 (63 to 82) | .005b |
| Male sex—n/N, %, (95% CI) | 91/149 | 61 (53–69) | 63/105 | 60 (51–70) | 28/44 | 64 (49–78) | .68 |
| Patients’ medical history—n/N, %, (95% CI) | |||||||
| Hypertensionc | 66/133 | 50 (41–58) | 29/90 | 32 (22–42) | 37/43 | 86 (75–97) | <.0001 |
| Cardiovascular disease | 38/133 | 29 (21–36) | 17/90 | 19 (11–27) | 21/43 | 49 (33–64) | .0004 |
| Type 2 diabetesc | 38/133 | 29 (21–36) | 13/90 | 14 (7–22) | 25/43 | 58 (43–74) | <.0001 |
| Vascular disease | 36/133 | 27 (19–35) | 20/90 | 22 (14–31) | 16/43 | 37 (22–52) | .07 |
| Dyslipidemia | 30/133 | 23 (15–30) | 18/90 | 20 (12–28) | 12/43 | 28 (14–42) | .31 |
| Obstructive sleep apnea syndrome | 17/133 | 13 (7–19) | 9/90 | 10 (4–16) | 8/43 | 19 (6–31) | .17 |
| COPD | 15/133 | 11 (6–18) | 7/90 | 8 (2–13) | 8/43 | 19 (6–31) | .07 |
| Asthma | 8/133 | 6 (2–10) | 5/90 | 6 (1–10) | 3/43 | 7 (0–15) | .75 |
| Cancer | 8/133 | 6 (2–10) | 6/90 | 7 (1–12) | 2/43 | 5 (0–11) | .65 |
| Chronic kidney disease | 8/133 | 6 (2–10) | 4/90 | 4 (0–9) | 4/43 | 9 (0–18) | .27 |
| Outcomes—n/N, %, (95% CI) | |||||||
| Acute respiratory failure | 76/146 | 52 (44–60) | 50/103 | 49 (39–58) | 26/43 | 61 (45–76) | .19 |
| Intubation and mechanical ventilation | 54/146 | 37 (29–45) | 34/103 | 33 (24–42) | 20/43 | 47 (31–62) | .12 |
| COVID-19 related death | 19/147 | 13 (7–18) | 9/104 | 9 (3–14) | 10/43 | 23 (10–36) | .02 |
| Pulmonary embolism | 2/146 | 1 (0–3) | 1/103 | 1 (0–3) | 1/43 | 2 (0–7) | .52 |
Abbreviations: ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blockers; CI, confidence interval; COPD, chronic obstructive pulmonary disease; IQR, interquartile range, 25th–75th percentile; n, number of observations; N, number of patients.
aχ 2 test or Fisher exact test, as appropriate.
bMann-Whitney U test.
cHypertension and type 2 diabetes were significantly correlated (Spearman rank correlation coefficient = 0.378; P < .0001). To avoid the multicollinearity issue in the multivariable models, hypertension and type 2 diabetes were assessed separately in the logistic regression analysis (model 1: hypertension, cardiovascular disease; model 2: type 2 diabetes, cardiovascular disease) and the multivariable multilevel analysis (model 1: type 2 diabetes; model 2: hypertension).
Biochemical Alterations Associated with ACEI/ARB Use
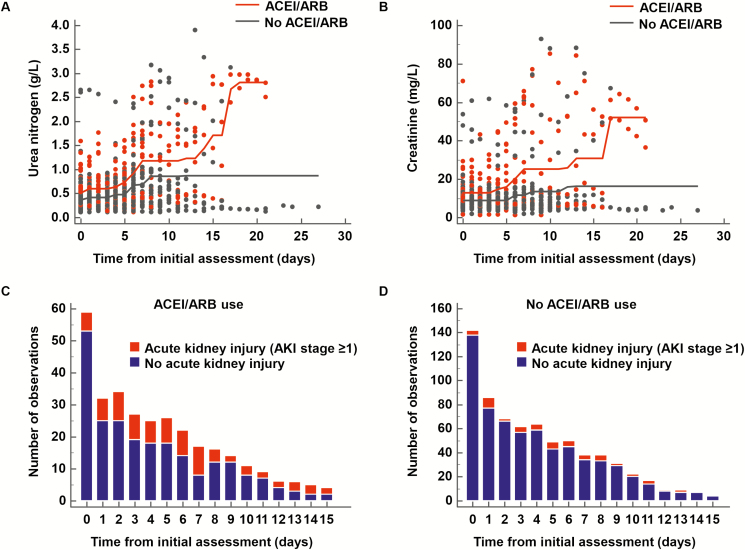
In ROC analysis, 12 of the 20 studied biochemical variables, and the AKI stage had a significant threshold in association with ACEI/ARB use (Table 2). In time-series analyses, 6 of the 12 dichotomized biochemical variables and the AKI stage differed significantly between patients with or without ACEI/ARB use (urea nitrogen >0.52 g/L, AKI stage ≥1, total bilirubin ≤5.8 mg/L, phosphorus >40 mg/L, creatinine >10.1 mg/L, partial pressure of carbon dioxide [PCO2] >39 mmHg, and potassium >4.43 mmol/L) (Table 2 and Supplementary Figure 2). Regarding patients’ medical history, hypertension, cardiovascular disease, and type 2 diabetes were significantly associated with ACEI/ARB use in univariate analysis (Table 1). In the multivariable analysis using logistic regression, after accounting for age, sex, medical history, and time, the ACEI/ARB use was independently associated with the following biochemical variations (decreasing order of the highest OR): phosphorus >40 mg/L (OR, 3.35 [95% CI, 1.83–6.14]; P = .0001), creatinine >10.1 mg/L (OR, 3.22 [95% CI, 2.28–4.54]; P < .0001), urea nitrogen >0.52 g/L (OR, 2.65 [95% CI, 1.89–3.73]; P < .0001), total bilirubin ≤5.8 mg/L (OR, 2.08 [95% CI, 1.24–3.49]; P = .006), and PCO2 >39 mmHg (OR, 1.70 [95% CI, 1.24–2.34]; P = .001) (Table 3). Consistently, ACEI/ARB use was independently associated with AKI stage ≥1 (OR, 3.28 [95% CI, 2.17–4.94]; P < .0001) (Table 3, Figure 1, andSupplementary Figure 3).
Table 2.
Biochemical Variations and Acute Kidney Injury Stage Associated With the Use of ACEI/ARB Among Patients With Severe COVID-19
| Biological Variable | n | ROC, P Valuea | ROC-defined Cutoff | Time-series Analysis P Valueb | Percentage of Time According to the Threshold (95% CI), No ACEI/ARB | Percentage of Time According to the Threshold (95% CI), ACEI/ARB |
|---|---|---|---|---|---|---|
| Electrolytes, kidney markers | ||||||
| Sodium (mmol/L) | 805 | .08 | — | — | — | |
| Potassium (mmol/L) | 811 | .01 | >4.43 | .007 | 11.2 (7.1–15.4) | 21.0 (12.8–29.2) |
| Chloride (mmol/L) | 757 | .0002 | ≤103 | .15 | 27.2 (20.1–34.3) | 39.2 (26.1–52.2) |
| Urea nitrogen (g/L) | 802 | <.0001 | >0.52 | .01 | 20.6 (14.1–27.0) | 41.3 (27.4–55.2) |
| Creatinine (mg/L) | 800 | <.0001 | >10.1 | .002 | 18.8 (11.8–25.7) | 37.3 (24.5–50.1) |
| AKI stagec | 800 | <.0001 | ≥1 | .002 | 8.6 (3.8–13.3) | 28.7 (15.7–41.7) |
| Calcium (mg/L) | 257 | .52 | — | — | — | |
| Phosphorus (mg/L) | 292 | <.0001 | >40 | .004 | 6.4 (2.0–10.8) | 25.3 (11.0–39.6) |
| Blood gas | ||||||
| Hemoglobin (g/dL) | 744 | <.0001 | ≤11 | .10 | 16.9 (11.1–22.9) | 30.3 (17.2–43.4) |
| pH | 742 | <.0001 | ≤7.42 | .05 | 22.9 (16.0–29.8) | 38.4 (25.1–51.7) |
| PO2 (mm/Hg) | 741 | .01 | >75.7 | .20 | 39.1 (31.3–46.9) | 49.1 (35.8–62.4) |
| PCO2 (mmHg) | 742 | .0003 | >39 | .02 | 26.9 (18.6–35.2) | 41.2 (27.1–55.3) |
| Bicarbonate (HCO−) (mmol/L) | 719 | .76 | — | — | — | |
| Lactates (mmol/L) | 675 | .28 | — | — | — | |
| Liver, nutrition, inflammation | ||||||
| ASAT (U/L) | 473 | .50 | — | — | — | |
| ALAT (U/L) | 472 | .01 | >41 | .07 | 38.2 (29.0–47.3) | 54.0 (38.2–69.7) |
| Bilirubin, total (mg/L) | 448 | <.0001 | ≤5.8 | .02 | 23.2 (15.7–30.7) | 42.7 (28.4–57.0) |
| Total proteins (g/L) | 720 | .49 | — | — | — | |
| C-reactive protein (mg/L) | 358 | .40 | — | — | — | |
| Cardiac and muscle markers | ||||||
| hs-c Troponin I (pg/mL) | 245 | <.0001 | >37.7 | .17 | 17.9 (9.2–26.7) | 23.6 (8.3–38.9) |
| 262 | .08 | — | — | — |
Abbreviations: AKI, acute kidney injury; ALAT, alanine aminotransferases; ASAT, aspartate aminotransferases; CK, creatine kinase; COVID-19, coronavirus disease 2019; hs-c Troponin I, high-sensitivity cardiac troponin I; n, number of observations; PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen; ROC, receiver operating characteristics.
aROC analysis, according to DeLong et al with Bias-corrected and accelerated (BCa)-bootstrap interval after 10 000 iterations for the Youden index.
bTime-series analysis was performed using a nonparametric test.
cThe diagnosis and severity of AKI were classified according to the AKI network criteria [23].
Table 3.
Association Between ACEI/ARB Use and Biochemical Alterations in Multivariable Logistic Regression Analysis
| Logistic Regression Models and Covariates | Coef. | SE | aOR (95% CI) | P Valuea | Percent correctb | AUROCb (95% CI) |
|---|---|---|---|---|---|---|
| Potassium >4.43 mmol/Lc (model 1): ACEI/ARB, age, male sex, cardiovascular disease, hypertensiond | 79% | .629 (.593–.664) | ||||
| Male sex | 0.48 | 0.22 | 1.62 (1.05–2.48) | .03 | … | … |
| Cardiovascular disease | 0.72 | 0.20 | 2.06 (1.40–3.03) | .0003 | … | … |
| Potassium >4.43 mmol/Lc (model 2): ACEI/ARB, age, male sex, cardiovascular disease, type 2 diabetesd | 79% | .630 (.593–.665) | ||||
| Male sex | 0.52 | 0.22 | 1.68 (1.08–2.60) | .02 | … | … |
| Cardiovascular disease | 0.70 | 0.20 | 2.01 (1.36–2.97) | .0005 | … | … |
| Urea nitrogen >0.52 g/Lc (model 1): ACEI/ARB, age, male sex, cardiovascular disease, hypertensiond | 72% | .771 (.739–.801) | ||||
| ACEI/ARB (Yes) | 0.52 | 0.20 | 1.68 (1.13–2.49) | .01 | … | … |
| Age | 0.02 | 0.01 | 1.03 (1.01–1.04) | .0002 | … | … |
| Male sex | 1.16 | 0.20 | 3.18 (2.13–4.74) | <.0001 | … | … |
| Hypertension | 0.91 | 0.21 | 2.48 (1.63–3.77) | <.0001 | … | … |
| Urea nitrogen >0.52 g/Lc (model 2): ACEI/ARB, age, male sex, cardiovascular disease, type 2 diabetesd | 73% | .766 (.733–.796) | ||||
| ACEI/ARB (Yes) | 0.98 | 0.17 | 2.65 (1.89–3.73) | <.0001 | … | … |
| Age | 0.04 | 0.01 | 1.04 (1.02–1.05) | <.0001 | … | … |
| Male sex | 1.21 | 0.21 | 3.36 (2.24–5.02) | <.0001 | … | … |
| Creatinine >10.1 mg/Lc (model 1): ACEI/ARB, age, male sex, cardiovascular disease, hypertensiond | 75% | .783 (.752–.813) | ||||
| Male sex | 1.45 | 0.23 | 4.27 (2.73–6.69) | <.0001 | … | … |
| Hypertension | 1.70 | 0.19 | 5.45 (3.78–7.86) | <.0001 | … | … |
| Cardiovascular disease | 0.67 | 0.20 | 1.96 (1.33–2.88) | .0006 | … | … |
| Creatinine >10.1 mg/Lc (model 2): ACEI/ARB, age, male sex, cardiovascular disease, type 2 diabetesd | 73% | .769 (.737–.800) | ||||
| ACEI/ARB (Yes) | 1.17 | 0.18 | 3.22 (2.28–4.54) | <.0001 | … | … |
| Age | 0.03 | 0.01 | 1.04 (1.02–1.05) | <.0001 | … | … |
| Male sex | 1.47 | 0.23 | 4.36 (2.76–6.89) | <.0001 | … | … |
| PCO2 >39 mmHgc (model 1): ACEI/ARB, age, male sexe | 56% | .606 (.569–.641) | ||||
| ACEI/ARB (Yes) | 0.53 | 0.16 | 1.70 (1.24–2.34) | .001 | … | … |
| Age | 0.01 | 0.01 | 1.01 (1.00–1.03) | .02 | … | … |
| Male sex | 0.69 | 0.20 | 2.00 (1.34–2.98) | .0007 | … | … |
| Bilirubin, total ≤5.8 mg/Lc (model 1): ACEI/ARB, age, male sexe | 64% | .706 (.658–.750) | ||||
| ACEI/ARB (Yes) | 0.73 | 0.27 | 2.08 (1.24–3.49) | .006 | … | … |
| Male sex | −1.08 | 0.25 | .34 (.21–.55) | <.0001 | … | … |
| Hypertension | 0.80 | 0.25 | 2.24 (1.38–3.63) | .001 | … | … |
| Phosphorus >40 mg/Lc (model 1): ACEI/ARB, age, male sexe | 80% | .740 (.686–.790) | ||||
| ACEI/ARB (Yes) | 1.21 | 0.31 | 3.35 (1.83–6.14) | .0001 | … | … |
| AKIf stage 1 or more (model 1): ACEI/ARB, age, male sex, cardiovascular disease, hypertensiond | 81% | .787 (.756–.817) | ||||
| ACEI/ARB (Yes) | 0.63 | 0.24 | 1.87 (1.18–2.97) | .008 | … | … |
| Male sex | 0.76 | 0.27 | 2.13 (1.25–3.63) | .005 | … | … |
| Hypertension | 1.20 | 0.26 | 3.33 (2.00–5.55) | <.0001 | … | … |
| Cardiovascular disease | 0.76 | 0.22 | 2.13 (1.38–3.30) | .0007 | … | … |
| AKIf stage 1 or more (model 2): ACEI/ARB, age, male sex, cardiovascular disease, type 2 diabetesd | 80% | .766 (.734–.797) | ||||
| ACEI/ARB (Yes) | 1.19 | 0.21 | 3.28 (2.17–4.94) | <.0001 | … | … |
| Male sex | 0.80 | 0.27 | 2.22 (1.31–3.76) | .003 | … | … |
| Cardiovascular disease | 0.63 | 0.22 | 1.89 (1.22–2.91) | .004 | … | … |
Abbreviations: ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blockers; AKI, acute kidney injury; aOR, adjusted odds ratio; AUROC, area under the receiver operating characteristic curve; CI, confidence interval; Coef., coefficient; SE, standard error.
aMultivariable logistic regression model.
bPercentage of cases correctly classified by the logistic regression model and AUROC for model discrimination.
cThreshold calculated using receiver operating characteristics, according to DeLong et al [24].
dHypertension and type 2 diabetes were significantly correlated (Spearman rank correlation coefficient = 0.378; P < .0001). To avoid the multicollinearity issue in the multivariable regression analysis, these variables were assessed separately: model 1 with hypertension and model 2 with type 2 diabetes. All the logistic regression models were adjusted for the time interval from the initial assessment, using the “Stepwise” method.
eTime delay from the first assessment and patient’s medical histories of cardiovascular disease, hypertension, and diabetes were not retained in the logistic regression model using the “Stepwise” method. Thus, only one multivariable model was reported and included the significant covariates in the stepwise model with an adjustment for the time interval from the initial assessment using the “Enter” method.
fThe diagnosis and severity of AKI were classified according to the AKI network criteria [23].
Figure 1.
Evolution over time of (A) urea nitrogen and (B) creatinine among patients with severe COVID-19 according to ACEI/ARB use. Evolution over time of the number of cases with AKI (stage 1 or more) in patients with (C) and without (D) ACEI/ARB use. The diagnosis and severity of acute kidney injury (AKI) were classified according to the AKI network criteria [23]. ACEI/ARB: angiotensin-converting enzyme inhibitor/angiotensin receptor blockers. Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; COVID-19, coronavirus disease 2019.
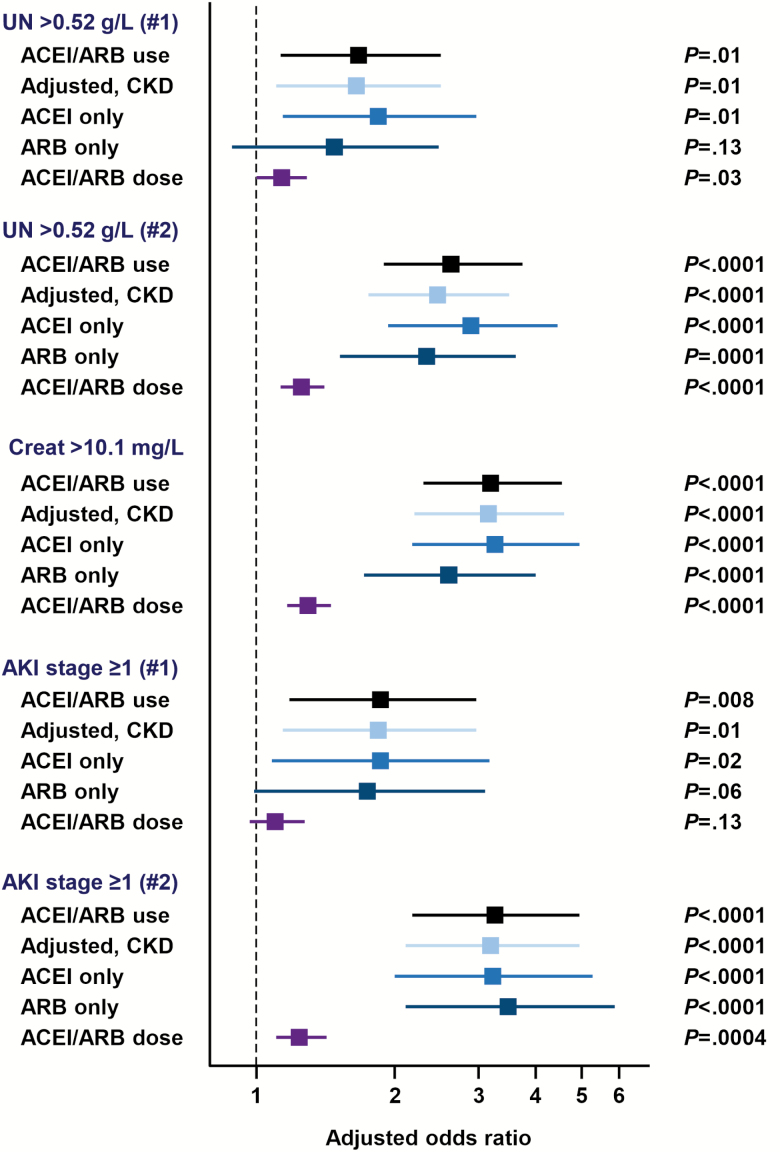
In post hoc exploratory analyses, effect sizes were similar for the association between ACEI/ARB use and the risk of kidney markers alterations (urea nitrogen >0.52 g/L, creatinine >10.1 mg/L, and AKI stage ≥1) after forced adjustment for the medical history of chronic kidney disease (Figure 2 and Supplementary Table 5). Effect sizes were also similar when the treatment by ACEI or ARB was considered separately in comparison to no ACEI/ARB therapy (Figure 2 and Supplementary Tables 6 and 7). In the dose-effect analysis, the daily dose of ACEI/ARB was independently associated with altered kidney markers with an increased risk of +25 to +31% per each 10 mg increment of the lisinopril-dose equivalent, with the following ORs (decreasing order of the highest OR): creatinine >10.1 mg/L (OR, 1.31 [95% CI, 1.17–1.46]; P < .0001), urea nitrogen >0.52 g/L (OR, 1.26 [95% CI, 1.13–1.41]; P < .0001), and AKI stage ≥1 (OR, 1.25 [95% CI, 1.11–1.42]; P = .0004) (Figure 2 and Supplementary Table 8).
Figure 2.
Forest plot reporting the results of sensitivity analyses to assess the stability of the effect sizes for the association between the use of ACEI/ARB and the biochemical alterations that have shown significance in multivariable multilevel analyses. Four types of sensitivity analyses were performed: (1) forced adjustment for the medical history of chronic kidney disease, (2) ACEI use versus no treatment with ACE/ARB to assess the specific effect of ACEI, (3) ARB use versus no treatment with ACE/ARB to assess the specific effect of ARB, and (4) dose-effect analysis to assess the association between each 10 mg increment of the lisinopril-dose equivalent of the daily intake of ACE/ARB and kidney outcomes. Abbreviations: ACE, angiotensin converting enzyme; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Association Between ACEI/ARB-associated Biochemical Variations and the Risk of COVID-19 Related Acute Respiratory Failure and Death
An acute respiratory failure occurred in 61% (26/43) and 49% (50/103) of patients with or without ACEI/ARB use. The corresponding figures regarding COVD-19 related death were 23% (10/43) and 9% (9/104), respectively. We used multivariable multilevel modeling to assess whether ACEI/ARB-associated biochemical variations were independently associated with acute respiratory failure and death after adjusting for potential confounders. Regarding the “acute respiratory failure” secondary endpoint, we constructed 6 models to avoid multicollinearity concerning the biochemical variables that were maintained in the first step of the HLM model (urea nitrogen, creatinine, and AKI stage; Spearman rank correlation coefficient ranging from 0.60 to 0.76 with P < .0001 for the 3 pairwise correlations) and patients’ medical history (Supplementary Table 9). Among the studied ACEI/ARB-associated biochemical variations, urea nitrogen >0.52 g/L was the only variable to be independently associated with the risk of acute respiratory failure (OR, 3.54 [95% CI, 1.05–11.96]; P = .04), along with male sex (highest OR, 8.18 [95% CI, 1.96–34.16]; P = .004) and medical history of hypertension (highest OR, 6.35 [95% CI, 1.28–31.47]; P = .02) (Table 4). Regarding the “in-hospital mortality” secondary endpoint, we constructed two models to avoid multicollinearity (Supplementary Table 10). No ACEI/ARB-associated biochemical marker was retained in the models. Age was significantly associated with the risk of death (highest OR, 1.13 [95% CI, 1.04–1.23]; P = .005) and medical history of chronic obstructive pulmonary disease had borderline significance (highest OR, 10.52 [95% CI, .83–133.32]; P = .07 (Table 4).
Table 4.
Association Between the Biochemical Markers Associated with ACEI/ARB Use and the Risk of COVID-19 Related Acute Respiratory Failure and Death in Multivariable Multilevel Analyses
| Multivariable multilevel models and covariates | Estimation | SE | aOR (95% CI) | P Valuea |
|---|---|---|---|---|
| Acute respiratory failure (model 1)b: Urea nitrogen >0.52 g/L, age, sex, type 2 diabetesc | ||||
| Male sex | 1.85 | 0.73 | 6.37 (1.51–26.76) | .01 |
| Urea nitrogen >0.52 g/L | 1.26 | 0.62 | 3.54 (1.05–11.96) | .04 |
| Type 2 diabetes | 1.13 | 0.80 | 3.10 (.65–14.91) | .16 |
| Age | −0.01 | 0.02 | .99 (.95–1.03) | .70 |
| Acute respiratory failure (model 2)b: Urea nitrogen >0.52 g/L, age, sex, hypertensionc | ||||
| Male sex | 1.97 | 0.72 | 7.16 (1.73–29.61) | .007 |
| Hypertension | 1.63 | 0.80 | 5.12 (1.06–24.65) | .04 |
| Urea nitrogen >0.52 g/L | 1.06 | 0.62 | 2.88 (.86–9.68) | .09 |
| Age | −0.02 | 0.02 | .98 (.93–1.02) | .31 |
| Acute respiratory failure (model 3)b: Creatinine >10.1 mg/L, age, sex, type 2 diabetesc | ||||
| Male sex | 1.99 | 0.76 | 7.30 (1.65–32.22) | .009 |
| Type 2 diabetes | 1.22 | 0.82 | 3.40 (.68–16.84) | .14 |
| Creatinine >10.1 mg/L | 0.27 | 0.66 | 1.31 (.36–4.76) | .68 |
| Age | 0.00 | 0.02 | 1.00 (.96–1.04) | .91 |
| Acute respiratory failure (model 4)b: Creatinine >10.1 mg/L, age, sex, hypertensionc | ||||
| Male sex | 2.10 | 0.75 | 8.14 (1.88–35.30) | .005 |
| Hypertension | 1.85 | 0.82 | 6.35 (1.28–31.47) | .02 |
| Creatinine >10.1 mg/L | 0.12 | 0.67 | 1.12 (.30–4.21) | .86 |
| Age | −0.02 | 0.02 | .98 (.94–1.03) | .40 |
| Acute respiratory failure (model 5)b: AKI stage ≥1, age, sex, type 2 diabetesc | ||||
| Male sex | 2.01 | 0.74 | 7.43 (1.74–31.65) | .007 |
| Type 2 diabetes | 1.22 | 0.81 | 3.39 (.69–16.65) | .13 |
| AKI stage ≥1 | 0.72 | 0.85 | 2.06 (.39–11.01) | .40 |
| Age | 0.00 | 0.02 | 1.00 (.96–1.04) | .91 |
| Acute respiratory failure (model 6)b: AKI stage ≥1, age, sex, hypertensionc | ||||
| Male sex | 2.10 | 0.73 | 8.18 (1.96–34.16) | .004 |
| Hypertension | 1.81 | 0.82 | 6.10 (1.23–30.18) | .03 |
| AKI stage ≥1 | 0.46 | 0.86 | 1.58 (.29–8.5) | .59 |
| Age | −0.02 | 0.02 | .98 (.94–1.03) | .39 |
| Death (model 1)d: Creatinine >10.1 mg/L, age, sex, hypertension, cardiovascular disease, COPD | ||||
| Age | 0.11 | 0.04 | 1.12 (1.03–1.21) | .008 |
| COPD | 2.38 | 1.27 | 10.84 (.89–131.22) | .06 |
| Male sex | 1.41 | 1.01 | 4.11 (.56–29.99) | .16 |
| Creatinine >10.1 mg/L | 0.97 | 0.82 | 2.65 (.53–13.24) | .24 |
| Cardiovascular disease | 0.85 | 0.97 | 2.35 (.35–15.68) | .38 |
| Hypertension | 0.33 | 1.06 | 1.39 (.17–10.99) | .76 |
| Death (model 2)d: AKI stage ≥1, age, sex, hypertension, cardiovascular disease, COPD | ||||
| Age | 0.12 | 0.04 | 1.13 (1.04–1.23) | .005 |
| Medical history of COPD | 2.35 | 1.30 | 10.52 (.83–133.32) | .07 |
| Male sex | 1.11 | 0.98 | 3.02 (.44–20.77) | .26 |
| Medical history of cardiovascular disease | 1.07 | 0.95 | 2.90 (.45–18.66) | .26 |
| AKI stage ≥1 | 0.50 | 0.90 | 1.65 (.28–9.68) | .58 |
| Medical history of hypertension | 0.41 | 1.06 | 1.50 (.19–11.94) | .70 |
Abbreviations: AKI, acute kidney injury; aOR, adjusted odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; SE, standard error.
aTwo-level hierarchical logistic model (HLM), using the predictive quasi-likelihood method.
bThe multilevel model included 129 patients.
cHypertension and type 2 diabetes were significantly correlated (Spearman rank correlation coefficient = 0.378; P < .0001). To avoid the multicollinearity issue in the multivariable multilevel analysis, these variables were assessed separately: model 1 with type 2 diabetes and model 2 with hypertension.
dMultilevel model included 130 patients.
Association Between ACEI/ARB Use and SARS-CoV-2 Viral Load at Diagnosis
Data regarding Ct values were available for 106 on the 149 studied patients (71%). The median Ct value for the IP2 target at baseline did not differ between patients with (n = 36) or without (n = 70) ACEI/ARB use (27 [IQR, 21–34] vs 27 [IQR, 22–32], respectively; P = .53). Consistently the median Ct value for the IP4 target at baseline did not differ between patients with or without ACEI/ARB use (28 [IQR, 22–33] vs 26 [IQR, 22–32]; P = .62). Of the 106 patients, 30 had their SARS-CoV-2 viral load monitored within 2 weeks of the first assessment. Consistently with the initial results, the median Ct values for both IP2 and IP4 targets did not differ between patients with (n = 11) or without (n = 19) ACEI/ARB use (IP2: 29 [IQR, 24–35) vs 31 (24–35), respectively; P = .96; and IP4: 31 [IQR, 25–37] vs 30 [IQR, 25–35], respectively; P = .40).
DISCUSSION
The results of the present study support the hypothesis of a deleterious effect of long-term therapy with ACEI/ARB among patients with severe COVID-19 with regards to their risk of developing acute kidney injury and acute respiratory failure. Current data in the literature do not allow drawing formal conclusions on the causal link between long-term exposure to ACEI/ARB and disease outcomes in patients with severe COVID-19. A retrospective study from China reported a higher prevalence of ACEI/ARB therapy in patients with moderate COVID-19 in comparison to patients with severe disease [28]. Another retrospective study from the Hubei Province in China assessed the association between in-hospital use of ACEI/ARB and all-cause mortality in COVID-19 patients with hypertension [15]. In-hospital use of ACEI/ARB was associated with a decreased mortality [15]. It is worthy to note that the proportion of patients treated with antiviral therapy was significantly higher in the ACEI/ARB group, suggesting that these patients may have had a more severe form [15]. In our study, the patients did not receive antiviral therapy, which had the effect of reducing the risk of bias. A retrospective case series on 187 patients from Wuhan (China) evaluated the association of underlying cardiovascular disease and myocardial injury with fatal outcomes in patients with COVID-19 [16]. In this study, the global mortality rate (23%) was higher among the patients that were chronically treated with ACEI/ARB when compared to those without ACEI/ARB therapy (36.8% vs 25.6%, respectively) [16]. Although the overall mortality rates between the Chinese study by Guo et al [16] and ours are not similar (23% vs 13%, respectively), it is interesting to note that the difference in mortality rates between patients with or without ACEI/ARB use was comparable between the 2 studies (11% vs 14%, respectively). To date, the few studies that have evaluated the relationship between ACEI/ARB use and the severity of COVID-19 differ in their study design, selection criteria, and study outcomes, and thus do not allow a comprehensive assessment of the data.
Several lines of evidence have suggested mechanistic clues for the interaction between SARS-CoV-2 and ACE2 [29]. SARS-CoV-2 binds to the sensitive cells that express ACE2 after contacting the airway surface, with a potentially toxic effect on type II alveolar epithelial cells, thereby causing lung damage and acute respiratory failure [29, 30]. Data from single-cell RNA sequencing have demonstrated that 80% of total ACE2 expression in the human lung cells was found in type II alveolar epithelial cells [30]. ACE and ACE2 have differential expression patterns in the human body. ACE is mainly expressed in the lungs, kidneys, heart, and blood vessels, whereas ACE2 is mainly expressed in the lungs, liver, spleen, brain, intestine, heart with the highest expression in kidney, and cardiovascular and gastrointestinal systems [3, 29, 31–33]. ACE- and ACE2-related signaling pathways have balanced effects on maintaining RAAS homeostasis [29]. ACEI/ARB therapies induce an increase in ACE2 gene expression and activity; however, data regarding the expression of ACE2 in the lungs in the setting of chronic ACEI/ARB therapy remains unclear [12]. Furthermore, there is no evidence regarding increased lung susceptibility to SARS-CoV-2 among patients treated with ACEI/ARB therapy. In this setting, we did not find a significant difference in the SARS-CoV-2 load between patients with or without ACEI/ARB use.
In patients with a severe COVD-19, our results highlight the association between ACEI/ARB use and a significant increase in the risk of AKI. Among these abnormalities, a high level of urea nitrogen was identified as independently associated with the risk of acute respiratory failure. Our results allow us to consider the hypothesis that ACEI/ARB therapy does not have a direct effect on host-pathogen interaction in the lung but rather on the kidney with subsequent alterations in renal homeostasis, which could precede the alterations in pulmonary function in the context of a lung-kidney crosstalk [34, 35]. In this context, the consensus conference on the spectrum of lung-kidney interactions stated that AKI is associated with increased susceptibility to respiratory failure, related pulmonary complications, and delay in weaning and liberation from invasive mechanical ventilation [36]. Nevertheless, the exact mechanisms underlying these observations are poorly understood [36].
We acknowledge several potential limitations of the study that should be considered in the interpretation of our findings. Our study is retrospective and relied on a relatively limited number of patients and needs to be confirmed in independent studies with a longer follow-up. Given the observational design of our study, a causal relationship between the use of ACEI/ARB and kidney outcomes cannot be formally demonstrated. However, the reported associations meet the Bradford Hill criteria for causality [37], including strength, consistency, temporality, plausibility, coherence, and especially a dose-effect relationship reflecting a trend toward a more severe renal impairment with increasing doses of ACEI/ARB. The results of the present study reflect observations on patients that were chronically treated with ACEI/ARB with specific adaptive mechanisms that may differ from those observed in patients receiving acute ACEI/ARB therapy in the context of an anti-SARS-CoV-2 therapeutic strategy [14]. Moreover, the design of our study did not allow us to assess the effect of ACEI/ARB discontinuation during the hospital stay on disease outcomes, which deserves to be addressed in future studies designed for this purpose. Patients with severe COVID-19 can exhibit a cytokine storm, which could impact the risk of lung injury and fatal outcomes [38]. In our study, no association was found between the use of ACEI/ARB and C-reactive protein level. Interleukin 6 and procalcitonin—which serves as an indirect marker of innate immunity—have not been sufficiently assessed in patients to be tested in statistical analyses for their potential association with the use of ACEI/ARB. Our study has several strengths. First, we report an exhaustive description of the biochemical abnormalities and their kinetics of evolution over time, according to ACEI/ARB use in patients with severe COVID-19. Second, we assessed through a multilevel modeling approach adapted for repeated measures the relationship between ACEI/ARB-associated biochemical variations and disease-related complications. Third, our study highlighted the possibility of a lung-kidney crosstalk to better understand the severity of COVID-19 presentation and disease outcomes in association with ACEI/ARB use.
In conclusion, our study provides new data on the potentially harmful effect of chronic ACEI/ARB use on the renal function of patients with severe COVID-19 and its possible interaction with the occurrence of acute respiratory failure. Several guidelines have been updated regarding the use of ACEI/ARB in patients with COVID-19 given the current state of the evidence [14, 39]. Further studies and prospective trials are urgently needed to address the safety profile of ACEI/ARB use before recommending the withdrawal of these drugs in patients at risk of adverse outcomes from COVID-19 or those with a suspected or proven diagnosis of COVID-19.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors warmly thank the technical staff of the Laboratory of Biology and Biopathology of the University Hospital of Nancy for their valuable contribution to the present work.
List of collaborators: Matthieu Garcia, MSc1; Isabelle Chouviac, PharmD1; Sibel Berger, PhD4; Audrey Jacquot, MD8; Matthieu Koszutski, MD8; Philippe Guerci, MD, PhD7; Ombeline Empis de Vendin, MD7; Matthieu Delannoy, MD7; Laura Chenard, MD7; Jean-Marc Lalot, MD7; Emmanuel Novy, MD7; Jean-Pierre Pertek, MD7; Noël Boussard, MD11; Asma Alla, MD4; Alice Corbel, MD5; Benjamin Lefevre, MD6; Hélène Jeulin, PharmD4; Cédric Hartard, PharmD, PhD4; Zakia Aitdjafer, MD1; Véronique Venard, PharmD, PhD4; Alain Lozniewski, MD, PhD12; Gérard Audibert, MD, PhD13; Pierre-Edouart Bollaert, MD, PhD910Pediatric Intensive Care Unit, University Hospital of Nancy, Brabois Children’s Hospital, Nancy, France, 11Department of Bacteriology, University Hospital of Nancy, Central Hospital, Nancy, France, and 12Department of Anesthesiology and Intensive Care Medicine, University Hospital of Nancy, Central Hospital, Nancy, France
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Collaborators are listed in the acknowledgement section.
References
- 1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oussalah A, Gleye S, Clerc Urmes I, et al. Follow-up of multi-organ dysfunction and inflammation using biomarker kinetics in patients with severe COVID-19 disease and association with disease putcomes: results from a referral center cohort in the North East of France. 2020. doi:10.2139/ssrn.3590489. Available at: https://ssrn.com/abstract=3590489 or 10.2139/ssrn.3590489 [DOI] [Google Scholar]
- 4. South AM, Diz D, Chappell MC. COVID-19, ACE2 and the cardiovascular consequences. Am J Physiol-Heart C 2020. doi:10.1152/ajpheart.00217.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020; 367:1444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res 2006; 98:463–71. [DOI] [PubMed] [Google Scholar]
- 8. Benter IF, Ferrario CM, Morris M, Diz DI. Antihypertensive actions of angiotensin-(1-7) in spontaneously hypertensive rats. Am J Physiol 1995; 269:H313–9. [DOI] [PubMed] [Google Scholar]
- 9. Talreja H, Tan J, Dawes M, et al. A consensus statement on the use of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in relation to COVID-19 (corona virus disease 2019). N Z Med J 2020; 133:85–7. [PubMed] [Google Scholar]
- 10. South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol 2020; 16:305–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo J, Huang Z, Lin L, Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome Coronavirus 2 infection. J Am Heart Assoc 2020; 9:e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005; 111:2605–10. [DOI] [PubMed] [Google Scholar]
- 13. Soro-Paavonen A, Gordin D, Forsblom C, et al. ; FinnDiane Study Group Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens 2012; 30:375–83. [DOI] [PubMed] [Google Scholar]
- 14. Bavishi C, Maddox TM, Messerli FH. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol 2020. doi:10.1001/jamacardio.2020.1282 [DOI] [PubMed] [Google Scholar]
- 15. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020; 126:1671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;e201017. doi:10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sommerstein R, Kochen MM, Messerli FH, Grani C. Coronavirus disease 2019 (COVID-19): do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J Am Heart Assoc 2020; 9:e016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oussalah A, Ferrand J, Filhine-Tresarrieu P, et al. diagnostic accuracy of procalcitonin for predicting blood culture results in patients with suspected bloodstream infection: an observational study of 35,343 consecutive patients (A STROBE-Compliant Article). Medicine 2015; 94:e1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 2020; 382:2327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Organization WH. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization, 2020. Available at: https://apps.who.int/iris/handle/10665/331446 [Google Scholar]
- 21. Houston BA, Schneider AL, Vaishnav J, et al. Angiotensin II antagonism is associated with reduced risk for gastrointestinal bleeding caused by arteriovenous malformations in patients with left ventricular assist devices. J Heart Lung Transplant 2017; 36:380–5. [DOI] [PubMed] [Google Scholar]
- 22. Vincent JL, Taccone FS. Understanding pathways to death in patients with COVID-19. Lancet Respir Med 2020; 8:430–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mehta RL, Kellum JA, Shah SV, et al. ; Acute Kidney Injury Network Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44:837–45. [PubMed] [Google Scholar]
- 25. Efron B, Tibshirani RJ.. An Introduction to the Bootstrap. Boca Raton, FL: Taylor & Francis, 1994. [Google Scholar]
- 26. Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990; 300:230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagelkerke NJ. A note on a general definition of the coefficient of determination. Biometrika 1991; 78:691–2. [Google Scholar]
- 28. Feng Y, Ling Y, Bai T, et al. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med 2020; 201:1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li G, Hu R, Zhang X. Antihypertensive treatment with ACEI/ARB of patients with COVID-19 complicated by hypertension. Hypertens Res 2020; 43:588–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen L, Liu W, Zhang Q, et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect 2020; 9:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 2020; 116:1097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics: tissue-based map of the human proteome. Science 2015; 347:1260419. [DOI] [PubMed] [Google Scholar]
- 33. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 2000; 87:E1–9. [DOI] [PubMed] [Google Scholar]
- 34. Husain-Syed F, Slutsky AS, Ronco C. Lung-kidney cross-talk in the critically ill patient. Am J Respir Crit Care Med 2016; 194:402–14. [DOI] [PubMed] [Google Scholar]
- 35. Liu KD, Thompson BT, Ancukiewicz M, et al. ; National Institutes of Health National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med 2011; 39:2665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Joannidis M, Forni LG, Klein SJ, et al. Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med 2020; 46:654–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965; 58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang Y, Shen C, Li J, et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol 2020;S0091-6749(20)30576-5. doi:10.1016/j.jaci.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020; 382:1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.