Abstract
Background
Understanding nosocomial acquisition, outbreaks, and transmission chains in real time will be fundamental to ensuring infection-prevention measures are effective in controlling coronavirus disease 2019 (COVID-19) in healthcare. We report the design and implementation of a hospital-onset COVID-19 infection (HOCI) surveillance system for an acute healthcare setting to target prevention interventions.
Methods
The study took place in a large teaching hospital group in London, United Kingdom. All patients tested for SARS-CoV-2 between 4 March and 14 April 2020 were included. Utilizing data routinely collected through electronic healthcare systems we developed a novel surveillance system for determining and reporting HOCI incidence and providing real-time network analysis. We provided daily reports on incidence and trends over time to support HOCI investigation and generated geotemporal reports using network analysis to interrogate admission pathways for common epidemiological links to infer transmission chains. By working with stakeholders the reports were co-designed for end users.
Results
Real-time surveillance reports revealed changing rates of HOCI throughout the course of the COVID-19 epidemic, key wards fueling probable transmission events, HOCIs overrepresented in particular specialties managing high-risk patients, the importance of integrating analysis of individual prior pathways, and the value of co-design in producing data visualization. Our surveillance system can effectively support national surveillance.
Conclusions
Through early analysis of the novel surveillance system we have provided a description of HOCI rates and trends over time using real-time shifting denominator data. We demonstrate the importance of including the analysis of patient pathways and networks in characterizing risk of transmission and targeting infection-control interventions.
Keywords: surveillance, hospital-onset infection, COVID-19, SARS-Cov-2, network analysis
We implemented a novel surveillance system to reveal the epidemiology of hospital-onset COVID-19 infections over time. Hospital-wide patient pathways need to be taken into account to effectively inform infection-prevention practices. Local surveillance systems can effectively support national surveillance methods.
Within 5 months of its recognition, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread globally, causing 6.8 million reported cases of coronavirus disease 2019 (COVID-19) [1]. Dramatic differences in country-specific mortality rates (0.5–84 per 100 000) are reported [2] and the World Health Organization estimates that 397 000 (5.8%) have died of COVID-19 globally [1]. Mortality rates in hospitalized patients exceed 26%, with higher rates observed in persons over 65 years old [3, 4]. Worryingly, COVID-19–associated hospital admissions continue to increase, causing unparalleled clinical and economic challenges to healthcare settings worldwide.
Nosocomial SARS-CoV-2 acquisition has been reported [5, 6]; yet, our understanding of transmission dynamics is imperfect. Extensive and sustained community transmission, presymptomatic transmission, and likely long tail of infection suggest unique transmission potential compared with other respiratory pathogens [7, 8]. Furthermore, transmission risk is amplified by overstretched healthcare capacity, lack of isolation facilities, complex patient pathways, fluctuating supplies of personal protective equipment, and use of aerosol-generating procedures [9]. It is plausible that uncontrolled nosocomial transmissions will challenge pandemic control and fuel peaks of infection. Unsurprisingly healthcare worker anxiety around healthcare-acquired COVID-19 infection is sizable [10].
Understanding healthcare acquisition and transmission chains will be critical for any recovery strategy. The challenges of capturing dynamic and complex individual patient pathways and establishing a pragmatic case definition for surveillance must be addressed. Furthermore, the number of COVID-19–susceptible inpatients will vary within and between healthcare institutions and will depend upon the stage of the epidemic.
International bodies have introduced surveillance programs to monitor trends in incidence, prevalence, rates of hospitalization, and deaths associated with COVID-19 [11, 12]. Recently, the UK government mandated surveillance of COVID-19 infections identified in hospitals [13]. While these data are likely to provide valuable insights into national epidemiological trends, the absence of ward-level data will limit impact on local infection-prevention and -control (IPC) interventions. To address this, surveillance incorporating factors such as the clinical setting and patient pathways is essential.
We designed and implemented a pragmatic, real-time surveillance system for hospital-onset COVID-19 infection (HOCI) within our acute healthcare institute prior to the UK government mandate. We present the early analysis of the data generated, based on mapping patient pathways and transmission networks, including the identification of healthcare transmission, and accounting for epidemiological trends in COVID-19 infection. Furthermore, we highlight how these systems can be effectively integrated into national surveillance systems.
METHODS
Setting
The surveillance system was developed and utilized at a large London teaching hospital group serving a diverse population, comprising 5 hospitals across 4 sites. Our institute has 1200 inpatient beds, employs 12 000 staff, and undertakes 1.2 million episodes of patient contact per year.
The surveillance system was applied between 4 March 2020 and 14 April 2020. During this time frame 907 patient admissions were identified as COVID-19 positive. The median number of new cases per day was 37 (interquartile range [IQR], 36.3; range, 12.5–48.8). Total daily inpatient admission rates varied throughout this period, reflecting bed closures and dynamic ward reconfigurations.
Surveillance Case Criteria
Any patient admission tested for SARS-CoV-2 with their first positive sample 7 days or more after admission to the hospital was included. Patients were tested for SARS-CoV-2 based on national recommendations, which at the time of writing were any patients requiring admission to a hospital with clinical or radiological evidence of pneumonia or acute respiratory distress syndrome or an influenza-like illness [14]. All patients were tested using throat and nasal or nasopharyngeal swabs. Diagnosis was made using commercially available real-time polymerase chain reaction (PCR) assays.
Definitions
“Daily incidence rate” was assessed by determining patient admissions with and without COVID-19–positive tests. Because the incubation period for COVID-19 is fewer than 14 days [7] it is plausible that development of symptoms within 14 days of hospital admission could be attributed to community acquisition. With a median time to symptoms of 5 days [7], we made the pragmatic assumption that symptom development at fewer than 7 days was likely to reflect community acquisition, and symptom development at days 7–13 more likely reflected healthcare acquisition. Consequently, we developed 2 definitions: “HOCI” was defined as any patient with a SARS-CoV-2–positive test sample 14 days or more after admission and who did not have any symptoms of COVID-19 (fever, shortness of breath, cough, malaise) on admission. “Possible HOCI” (pHOCI) was defined as any patient with a SARS-CoV-2–positive sample between 7 and 14 days after admission and who did not have symptoms of COVID-19 on admission as determined by review of electronic patient records. Since our development of HOCI classifications, the UK government has provided specific definitions based on timing between healthcare admission and positive test [13] consistent with the definitions used by Meredith et al [14]. The definitions we developed parallel these but include patient-level information, which may provide higher-resolution case identification.
“HOCI clusters” were defined as 2 or more cases of HOCI or pHOCI occurring on the same ward within 14 days of each other.
Surveillance System Development and Implementation
Details are found in Supplementary Appendix 1. This system was developed with input from end-users (IPC teams) and clinical leads. To provide clear and usable outputs, unique data and visual reports were developed. Data outputs included daily patient-admissions rates, daily new confirmed cases of COVID-19, daily incidence of HOCI, and cumulative HOCI cases over time and space.
Network Analysis
Electronic health records were interrogated to produce visual reports of inpatient pathways by incorporating into a network analysis. The network analysis utilizes directed patient ward movements within the preceding 14 days (or up to 14 days in cases of pHOCI) from positive COVID-19 results to establish 2 key inputs: (1) cumulative number of days spent on individual wards and (2) number of incoming and outgoing ward transfers. These inputs were used to create a graphical representation of wards as network nodes, sized to reflect cumulative days spent on the ward and number of HOCI and pHOCI cases. Nodes are connected by arrows, or directed edges, with a width reflecting the cumulative number of movements from one node to another, and their direction. Together, these networks visualized patient flows associated with HOCI and pHOCI, and highlighted epidemiological links within patient pathways.
RESULTS
Here we report on the early implementation and outputs of our HOCI surveillance system.
Prevalence Data
Of 907 confirmed COVID-19–positive patient admissions during the analysis period, 90 (9.9%) met our criteria for HOCI across 39 wards; 28 were positive 7 to less than 14 days after admission (pHOCI) and 62 were positive 14 days or more after admission (HOCI). The median number of daily cases was 1 (IQR, 0–3). By including patient-level data we excluded 15 cases where patients had symptoms of COVID-19 on admission.
Incidence Data
Structured reports of daily incidence were generated and distributed to IPC teams and management boards on a daily basis. Three key metrics on SARS-CoV-2–positive patient admissions were reported: (1) numbers and locations of new cases identified in the preceding 24 hours, (2) daily incidence trends, and (3) identification of cases meeting HOCI criteria.
Daily reports highlighted a reduction in hospital inpatient admissions coupled with a proportional rise in COVID-19–positive inpatients (Figure 1A). Strikingly, the proportion of HOCI cases remained low. Evaluation of the daily incidence of HOCI (Figure 1B) revealed a peak of cases in the third week of March, with 73 of 90 (81%) cases identified prior to 1 April 2020.
Figure 1.
An example of daily surveillance metrics. A, Total daily patient admissions without a positive COVID-19 test (gray), those with a positive COVID-19 test (light gray), and those classified as HOCI and possible HOCI (dark gray). B, The daily incident rate of 90 HOCI and possible HOCI cases identified during the analytical period. C, The geotemporal relationship of 90 HOCI and possible HOCI cases during the study period. Each circle represents case(s) of HOCI (gray) and possible HOCI (white) identified on wards across the 5 hospitals (wards 1–39) during the analytic period. Each circle is sized to reflect the frequency of cases. Abbreviations: COVID-19, coronavirus disease 2019; HOCI, hospital-onset COVID-19 infection.
Epidemiological Investigation
To support IPC responses, daily reports were augmented with ward-level data and 2 visual representations were created as discussed in the following sections.
Clustering in Time and Space
Prospective data integration allowed real-time HOCI identification. Figure 1C provides an example of a geotemporal chart generated for individual wards, which can be assessed for clusters. Our cluster definition was met 19 times across 19 different wards (involving 70/90 [78%] HOCI and pHOCI cases), including ward areas managing patients at higher risk of complications (renal and hematology).
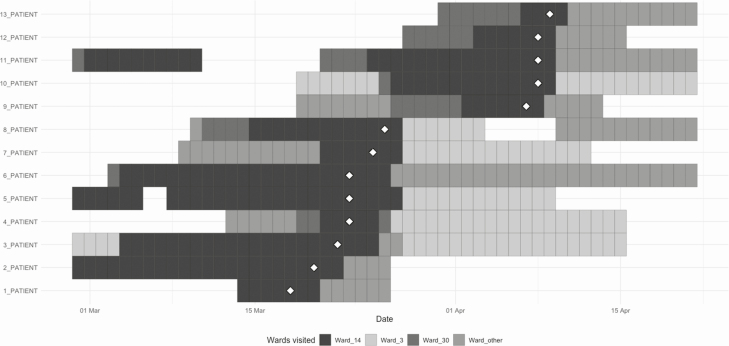
Where clusters were identified, patient pathways were analyzed to assess for epidemiological links. The largest cluster (ward 14 in Figure 1C) consisted of 13 HOCI cases identified on a single medical ward (at times, managing COVID-19–positive patients in side rooms and cohort bays) over 23 days. Figure 2 provides the patient pathway report for this cluster and reveals that, while ward 14 was the common location where the positive result was identified, many cases shared links to other clinical areas. Seven of 13 (54%) patients spent time on ward 30, another medical ward, prior to COVID-19 identification. Furthermore, other medical areas (including ward 3) were common to several patient admission pathways and, while most patients were admitted to these areas after the positive results, this may reflect broader pathway involvement.
Figure 2.
Reconstructed patient pathways of the largest cluster of HOCI involving 13 cases during the analytical period. The chart represents ward movements up to 14 days prior to positive sample of the 13 patients (patients 1–13) diagnosed with HOCI or possible HOCI on the same ward (ward 14). The date of first positive SARS-CoV-2 sample is depicted by white diamonds. Abbreviations: HOCI, hospital-onset COVID-19 infection; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Network Analysis
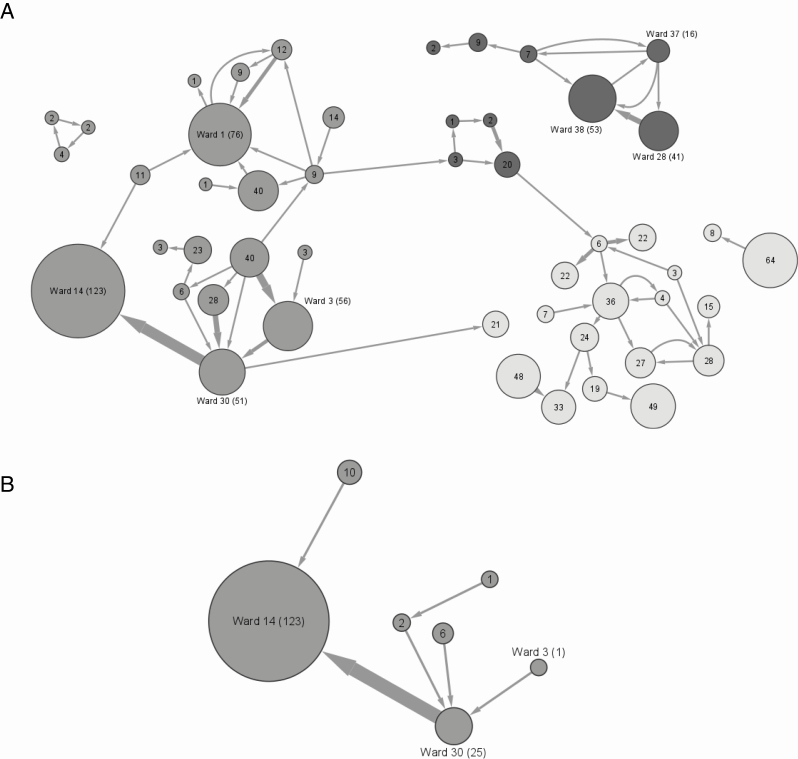
To further assess common epidemiological links, all HOCI cases were incorporated into a network analysis. Figure 3A provides an overview of networks within hospital sites, revealing a complete network of all patient movements prior to the identification of HOCI cases structured by hospital site. The network comprises 49 nodes and 69 edges representing directed transfers. The total patient days spent on each ward varied from less than 1 day to 123 days, and directed numbers of ward transfers ranged from 0 to 6. Three single points of transfers between sites were observed.
Figure 3.
Reconstructed network of patient movement up to 14 days prior to the positive SARS-CoV-2 sample that met the HOCI surveillance definition. Numbers of patient-days spent on wards (circular nodes) are annotated within each node and represented by the size of the node, which reflects the total cumulative inpatient stay on the ward, thus combining information on the number of HOCI and possible HOCI cases who passed through the ward and the time they spent there. Selected wards have been annotated with ward numbers. Arrows (or edges) represent movement between wards and the width reflects the number of patient transfers. Wards are colored by site: hospital 1 (dark gray), hospital 2 (gray), hospital 3 (light gray). A, A reconstructed network of all HOCIs and possible cases. B, A subnetwork analysis of the largest cluster of 13 HOCI cases. Abbreviations: HOCI, hospital-onset COVID-19 infection; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
At hospital 1, 10 ward areas were identified. The largest node (ward 38 in Figure 3A) represents a renal ward that is highly connected, in terms of patient ingress, to 2 other renal wards (ward 28 and ward 37 in Figure 3A). These wards collectively had 8 HOCIs; 6 on ward 38 and 1 each on wards 28 and 37. This suggests dynamics within specialty areas, possibly relating to accessing hemodialysis facilities.
At hospital 2, 21 ward areas were identified in network analysis (Figure 3A). Wide variations in patient days on wards were observed across these ward areas. The largest node (ward 14 in Figure 3A) was highly connected to 2 nodes (ward 30 and ward 3 in Figure 3A). Wards 14, 30, and 3 are surgical wards located within hospital 2 that were being used as medical wards during the analytical period, including managing patients with COVID-19 in side rooms or cohort bays. In addition to the 13 HOCIs identified on ward 14, an additional 7 HOCIs were identified on highly connected ward 30 (n = 3) and ward 3 (n = 3), representing 22% of 90 HOCIs identified. In turn, this suggested that IPC interventions were required across all of these medical wards and consolidates findings from the geotemporal cluster analysis (Figure 1C and Figure 2).
At hospital 3, 18 ward areas were identified in network analysis. All wards had comparable nodal sizes and, while patient transfers connected nodes, there was no dominant node or overrepresentation of directional transfers.
Figure 3B provides the network subanalysis of the largest cluster on ward 14. Overall, 13 patients with HOCI spent 123 patient days on ward 14 prior to their positive test for COVID-19. The analysis highlights that ward 14 was highly connected with another medical ward (ward 30), as revealed in Figure 2 and Figure 3A. Although overlaying patient pathways (Figure 2) and mapping these to a network offer complementary information of exposure to prior clinical areas where transmissions could have taken place, this subanalysis in Figure 3B does not uncover the connectivity of ward 3 (as seen in Figure 3A).
Supporting National Surveillance Systems
Following the analysis period, the UK government mandated institutes to report HOCIs on a weekly basis. In response, outputs from our established surveillance system were directly linked to national surveillance system databases, permitting rapid and seamless uploads. This was combined with established infrastructure to support the rapid identification of HOCI clusters and epidemiological investigation of individual HOCIs with rapid cluster analysis.
DISCUSSION
We have developed and implemented a surveillance system that applies network analysis to inform on possible hospital acquisition and guide IPC interventions. From initial surveillance data analysis we have:
-
•
provided a description of HOCI rates and trends throughout the COVID-19 epidemic curve using real-time shifting denominator data. We have shown that HOCI and pHOCI cases were more common during the early phase of the epidemic curve, prior to the peak. Monitoring epidemiological trends in real-time provides awareness of the institution’s trajectory within the pandemic which, in turn, provides a platform for future planning,
-
•
highlighted key wards common to patient pathways prior to developing HOCI, which may represent hubs of transmission, likely due to frequency of movement through the wards, time spent on them, and patient movements to these wards;
-
•
demonstrated the importance of including patient pathway network analysis; and
-
•
shown that linkage to national surveillance systems is seamless.
Healthcare-associated infections are frequently defined as development of disease more than 48 hours after admission (used for methicillin-resistant Staphylococcus aureus and gram-negative bloodstream infection and Clostridioides difficile). Due to the prolonged incubation period, application of this to HOCI is unsuitable. In March 2020 we developed a clinically relevant definition. We proposed that development of infection more than 14 days after admission reflects healthcare acquisition, while infection identified within the incubation period is less certain. Based on the current literature, infections presenting between 7 and 14 days after admission are more likely to be healthcare associated than community acquired, which was reflected in our definition. Recently, the UK government announced the introduction of a national surveillance system, providing epidemiological definitions of hospital-onset infections [13]. While these pragmatic definitions are likely to be valuable in national surveillance, the absence of patient-level data means that it is plausible that there will be overreporting of cases.
At the time of writing, no official figures have been published on healthcare-acquired COVID-19, and low-quality data coupled with heterogenous definitions across clinical settings mean that our understanding of HOCI rates is imprecise. HOCI rates at our institute have varied considerably depending on the epidemic stage. Figure 1B reveals that rates were positively skewed towards the increase in the epidemic curve when physical distancing and visitor restrictions had not yet been nationally recommended nor implemented locally. The impact of these measures on the epidemiology of this disease remains unclear, but this association gives reason to speculate that they may have, at least in part, had an impact.
Surveillance systems are widely used to prospectively monitor nosocomial infection trends and provide early warning signals for outbreaks. These systems commonly reflect static ward areas of known patient populations. Adapting these to COVID-19 has required flexibility to unique and changing circumstances. They must address the rapidly shifting nature of inpatients and case mixes alongside mapping major patient pathway changes, while also encompassing complexities of case definitions, the challenge of appropriate denominator data, and diagnostic testing capacity.
Traditionally, surveillance systems identify the ward area (or preceding ward area), where the sample was taken, as the geographical target where IPC activity should focus. Yet, due to long incubation periods and presymptomatic infection, involvement of the patient pathway for up to 14 days prior to the positive test needs to be considered. Network analyses have been previously employed to understand infectious-disease transmission and dynamics [15]. To assess geotemporal associations of individuals and HOCI clusters we employed network analysis at a local hospital level. Mapping patient pathways to networks reveals clinical areas common to HOCI patients’ pathways, offering evidence of exposure. We found wards in key specialities overrepresented in the HOCI and pHOCI case numbers, suggesting a need to analyze patient pathway routes across hospital sites and potentially focus IPC interventions on wards earlier in the patient pathway. Furthermore, we found that, while network subanalyses identify common pathways, the exclusion of patients who test positive on a different ward but share pathways underestimates additional key ward exposures. This suggests that evaluation of entire networks, rather than subanalyses, is likely to provide the most valuable data to inform IPC interventions.
Our work has a number of limitations. First, we used the date of the first positive sample to reflect the date of infection and have not included preceding SARS-CoV-2–negative samples. It is possible we have overestimated the incidence of HOCI. Second, disease prevalence will affect the positive-predictive value (PPV) of the assay; as COVID-19 prevalence decreases, the PPV will decrease, which, in turn, can lead to higher false-positive rates. Third, healthcare worker samples were not included, so inferences about the role of staff in HOCI are not available. Fourth, it is possible that secondary COVID-19 infections could occur in patients who have already been diagnosed with COVID-19. Our data do not account for these. Fifth, healthcare exposure within the 14 days prior to admission, which may contribute towards nosocomial acquisition, was not included. Sixth, routine testing for SARS-CoV-2 at discharge is not performed at our institution. Seventh, we screened electronic records of patients to determine symptoms on admission. Although this method is fast, it could have resulted in overinclusion of patients. Yet, using this method we were able to rule out 15 patients who were symptomatic on admission. Eighth, while network analysis incorporates patient-admission journeys, the risk of transmission on wards is not inferred. Furthermore, in response to increasing rates, many wards changed to COVID-19–cohorting wards at short notice, which was challenging to disentangle.
Sharing surveillance strategies for adoption by local, national, and international healthcare groups is essential. We developed a customizable surveillance system using platforms that are globally available and can be integrated with other clinical data platforms and national surveillance systems. Further work, incorporating healthcare worker sampling, is required to understand the role of staff in HOCI. It is likely that the higher resolution offered by whole-genome sequencing will be required to answer this [16].
In conclusion, we present the adoption of a network-analysis surveillance system to prospectively identify and characterize COVID-19 infections in hospitalized patients. We identified varying rates of HOCIs associated with epidemiological changes observed through the stages of the pandemic. Through network analysis we revealed that examining entire patient pathways is fundamental to understand common epidemiological links in cases, which, in turn, helps target IPC activities. As we move into the recovery phase of the pandemic, more COVID-naive patients will be admitted to the hospital. While the opportunity to target hospital interventions may be limited at the height of the pandemic, these options will be more achievable in the recovery phase and the importance of appropriate triage of patients along pathways will increase. Preventing transmission and acquisition is going to be fundamental for an effective recovery, and robust surveillance systems will be key to achieving this.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. J. R. P. and S. M. contributed equally and share first authorship. Study conception and design: J. R. P., S. M., F. D., E. T. B., T. G., J. A. O., and A. H. H. Data curation: S. M., E. D., W. L., and Y. S. Funding acquisition: A. H. H. Analysis and data interpretation: J. R. P., S. M., A. M., A. Y. W., M. B., J. A. O., and A. H. H. Drafting of the manuscript: J. R. P., S. M., J. A. O., and A. H. H. Critical revision of the manuscript for important intellectual content: J. R. P., S. M., A. M., A. Y. W., E. T. B., T. G., P. R., F. D., F. B., M. B., J. A. O., and A. H. H. Administrative, technical, or material support: J. R. P., S. M., E. D., A. M., A. Y. W., Y. S., D. M., P. R., F.B., M. B., J. A. O., and A. H. J. R. P. is the guarantor of the study. The corresponding author attests that all listed authors meet the ICMJE criteria for authorship and that no other meeting the criteria have been omitted. The manuscript represents the original work of the authors. It is not currently under consideration for publication elsewhere.
Acknowledgments. The authors acknowledge the dedication and support of the Infection Prevention and Control Department at Imperial College Healthcare NHS Trust, London, United Kingdom. The support of the Imperial College Healthcare Trust NIHR Biomedical Research Centre (BRC) is also acknowledged. Professor Alison Holmes is a National Institute for Health Research (NIHR) Senior Investigator. Data were collected and analyzed as part of routine infection-control response to emerging infections and outbreaks, and as such were considered exempt from needed research ethics approval in line with Statutory Instrument 1438, UK Public Health Regulations (2002).
Disclaimer. The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Financial support. Surveillance systems were developed for patient care in the National Health Service. This work was supported by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in partnership with Public Health England (PHE), in collaboration with Imperial Healthcare Partners, University of Cambridge, University of Warwick, and the Medical Research Foundation National PhD Training Programme in AMR Research.
This report is independent research funded by the National Institute for Health Research. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, the Department of Health and Social Care, or Public Health England.
Potential conflicts of interest. J. A. O. reports personal fees from Gama 34 Healthcare Ltd and Pfizer in the past 3 years, outside the submitted work; there are no other relationships or activities that could appear to have influenced the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Coronavirus disease (COVID-19) situation report—139. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 6 June 2020.
- 2. European Centre for Disease Prevention and Control. COVID-19 situation update worldwide, as of 24 May 2020. Available at: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases. Accessed 24 May 2020.
- 3. Centers for Disease Control and Prevention. A weekly summary of U.S. COVID-19 hospitalization data: laboratory-confirmed COVID-19-associated hospitalizations (COVID-NET). Available at: https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html. Accessed 4 May 2020.
- 4. Docherty AB, Harrison EM, Green CA, et al. ; ISARIC4C Investigators Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwierzeck V, Konig JC, Kuhn J, et al. First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric dialysis unit. Clin Infect Dis 2020; ciaa491. doi: 10.1093/cid/ciaa491. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang X, Zhou Q, He Y, et al. Nosocomial outbreak of 2019 novel coronavirus pneumonia in Wuhan, China. Eur Respir J 2020; 55: 2000544. doi: 10.1183/13993003.00544-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020; 172: 577– 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Treibel TA, Manisty C, Burton M, et al. COVID-19: PCR screening of asymptomatic health-care workers at London hospital. Lancet 2020; 395:1608–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020; 8:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lacobucci G. Covid-19: doctors sound alarm over hospital transmissions. BMJ 2020; 369:m2013. [DOI] [PubMed] [Google Scholar]
- 11. European Centre for Disease Prevention and Control. Technical report: strategies for the surveillance of COVID-19.2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-surveillance-strategy-9-Apr-2020.pdf. Accessed 9 April 2020.
- 12. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. NHS England and NHS Improvement. Operating framework for urgent and planned services within hospitals. Available at: https://covidlawlab.org/wp-content/uploads/2020/06/Operating-framework-for-urgent-and-planned-services-within-hospitals.pdf. Accessed 15 June 2020. [Google Scholar]
- 14. Meredith LW, Hamilton WL, Warne B, et al. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: a prospective genomic surveillance study. Lancet Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Public Health England. COVID-19: investigation and initial clinical management of possible cases. 2020. Available at: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigation-and-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infection. Accessed 4 May 2020.. [Google Scholar]
- 16. van Kleef E, Robotham JV, Jit M, Deeny SR, Edmunds WJ. Modelling the transmission of healthcare associated infections: a systematic review. BMC Infect Dis 2013; 13:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife 2020; 9: e58728. doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.