Abstract
Aims
Whether pulmonary artery (PA) dimension and coronary artery calcium (CAC) score, as assessed by chest computed tomography (CT), are associated with myocardial injury in patients with coronavirus disease 2019 (COVID-19) is not known. The aim of this study was to explore the risk factors for myocardial injury and death and to investigate whether myocardial injury has an independent association with all-cause mortality in patients with COVID-19.
Methods and Results
This is a single-centre cohort study including consecutive patients with laboratory-confirmed COVID-19 undergoing chest CT on admission. Myocardial injury was defined as high-sensitivity troponin I >20 ng/L on admission. A total of 332 patients with a median follow-up of 12 days were included. There were 68 (20.5%) deaths; 123 (37%) patients had myocardial injury. PA diameter was higher in patients with myocardial injury compared with patients without myocardial injury [29.0 (25th–75th percentile, 27–32) mm vs. 27.7 (25–30) mm, P < 0.001). PA diameter was independently associated with an increased risk of myocardial injury [adjusted odds ratio 1.10, 95% confidence interval (CI) 1.02–1.19, P = 0.01] and death [adjusted hazard ratio (HR) 1.09, 95% CI 1.02–1.17, P = 0.01]. Compared with patients without myocardial injury, patients with myocardial injury had a lower prevalence of a CAC score of zero (25% vs. 55%, P < 0.001); however, the CAC score did not emerge as a predictor of myocardial injury by multivariable logistic regression. Myocardial injury was independently associated with an increased risk of death by multivariable Cox regression (adjusted HR 2.25, 95% CI 1.27–3.96, P = 0.005). Older age, lower estimated glomerular filtration rate, and lower PaO2/FiO2 ratio on admission were other independent predictors for both myocardial injury and death.
Conclusions
An increased PA diameter, as assessed by chest CT, is an independent risk factor for myocardial injury and mortality in patients with COVID-19. Myocardial injury is independently associated with an approximately two-fold increased risk of death.
Keywords: Myocardial injury, Coronavirus disease 2019, Mortality, Risk, Pulmonary artery
Introduction
Myocardial injury, as defined by cardiac troponin elevations above the normal values, has been reported in 19.7% and 27.8% of patients hospitalized for coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in two recent Chinese studies.1,2 Of note, patients with myocardial injury were older and presented with more comorbidities, including hypertension, diabetes, and cardiovascular disease, in comparison with patients without myocardial injury.1,2 Guo et al. also found an association of myocardial injury with systemic inflammatory indices, such as C-reactive protein and N-terminal probrain natriuretic peptide (NT-proBNP).1 In both studies, patients with myocardial injury had a higher short-term mortality than patients without myocardial injury.2 Nevertheless, whether these findings are generalizable to other countries during the COVID-19 pandemic is largely unknown.
Further, the identification of patients at high risk of myocardial injury may have clinical implications, and a better understanding of the factors associated with myocardial injury is of paramount importance. Several putative mechanisms underlying myocardial injury have been hypothesized, including inflammatory damage of the heart, a direct viral effect, the trigger of acute coronary events, and the onset of acute decompensated heart failure precipitated by the COVID-19 infection.3,4
Chest computed tomography (CT), without contrast administration, is the reference imaging tool routinely used for the diagnosis of COVID-19 pneumonia and may provide additional cardiovascular parameters of potential clinical relevance, such as the pulmonary artery (PA) dimension5 and the coronary artery calcium (CAC) score.6 PA dimension has been shown to be associated with cardiac troponin elevations in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease and has been related to pulmonary vascular damage.7 COVID-19 may lead to respiratory failure,8–11 and pulmonary endothelial damage usually affects patients with acute respiratory distress syndrome (ARDS).12 It is not known whether PA dimension is associated with myocardial injury. The CAC score is an established marker of atherosclerotic burden that has been shown to improve cardiovascular risk prediction in asymptomatic individuals.13 No study has assessed whether the CAC score may improve the identification of patients at risk of myocardial injury, beyond the assessment of the clinical risk profile in COVID-19 patients.
The aim of this study was to provide insights into the factors associated with myocardial injury in patients hospitalized with COVID-19 by integrating clinical data, biochemical markers, and CT data, and to investigate the impact of myocardial injury on mortality as well as the predictors of mortality.
Methods
Study population
This is a single-centre cohort study enrolling consecutive patients with a diagnosis of confirmed COVID-19, admitted to an academic tertiary hospital (Humanitas Research Hospital) undergoing non-gated chest CT for the assessment of COVID-19-related pneumonia, between 25 February and 2 April 2020. This time window was considered sufficient for the enrolment of >300 patients, given the COVID-19 outbreak. Laboratory confirmation for SARS-CoV-2 required a positive result of a real-time reverse transcription–polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swabs or a positive result of RT–PCR assay from lower respiratory tract aspirates or bronchoalveolar lavage fluid. The study was approved by the institutional Ethics Committee which waived the requirement to obtain an informed consent from individual patients due to retrospective chart reviews. The investigation conformed to the principles outlined in the Declaration of Helsinki.
Data collection
Data were collected using electronic heath records by staff who were not aware of the study hypothesis in order to reduce the risk of bias. Data included demographic characteristics (age and sex), clinical data {cardiovascular risk factors and comorbidities including prior history of coronary artery disease, history of cerebrovascular disease defined as prior stroke or carotid artery disease, peripheral artery disease, chronic obstructive pulmonary disease, cancer, respiratory metrics [i.e. fraction of inspired oxygen (FiO2), arterial partial pressure of oxygen (PaO2), PaO2/FiO2 ratio on admission], mode of respiratory support (invasive mechanical ventilation, non-invasive mechanical ventilation, oxygen mask)}, laboratory data [creatinine, C-reactive protein, systemic inflammatory biomarkers, D-dimer, cardiac biomarkers such as high-sensitivity troponin (hsTn)-I and BNP], treatments, and outcomes.
Hypertension was defined as a history of systolic blood pressure of ≥140 mmHg, or a diastolic blood pressure of ≥90 mmHg, or taking antihypertensive medications. Dyslipidaemia was defined as a history of elevated total or LDL cholesterol levels, low levels of HDL cholesterol, elevated triglycerides, or taking lipid-lowering medications. Current smokers were defined as having smoked 100 cigarettes in their lifetime and currently smoking, former smokers as having smoked at least 100 cigarettes in their lifetime but who had quit smoking in the last 28 days. Diabetes mellitus was defined as a history of diabetes or taking antidiabetic medications. A new diagnosis for each condition during hospitalization was also considered. Cardiac biomarkers were measured within 48 h from admission. Patients were categorized according to the presence or absence of myocardial injury, which was defined as blood levels of hsTn-I above the 99th percentile upper reference limit (20 ng/L), regardless of new abnormalities on electrocardiography and echocardiography. The last date of follow-up was 10 April 2020. The clinical outcomes included discharge, length of stay, intensive care unit admission, and mortality.
CT assessment
Patients were imaged on a dedicated 64-slice CT scanner (Philips Ingenuity Core) on admission. All CT scans were acquired without electrocardiographic gating and without contrast administration. Images were reconstructed at 2 mm thick slices on three orthogonal planes. The CT scans were read independently by two experienced readers, who were blinded for all clinical data to reduce the risk of bias, for the pulmonary and aortic dimensions, and the CAC score using a dedicated offline cardiac workstation (HeartBeat-CS IntelliSpace Portal, Philips). The transverse axial diameter of the main PA and the ascending aorta at the level of the bifurcation of the right PA were measured as previously reported.5 The ratio of PA to aorta was calculated as the ratio of the main PA to the ascending aorta diameter. CAC was assessed as quantitative CAC score (Agatston scoring), which has been shown to have a high correlation with gated CT studies and cardiovascular disease outcomes.6 Traditional CAC score groups (0, 1–100, 101–400, and >400) were obtained. Patients were categorized according to the presence or absence of a CAC score of zero.6
Outcomes
Two co-primary endpoints were defined: the occurrence of myocardial injury and death from any cause. Secondary outcomes were the duration of hospitalization and admission to an intensive care unit.
Statistical analysis
Baseline and procedural characteristics were summarized according to myocardial injury. Continuous variables were summarized as medians with interquartile ranges, and counts with proportions for categorical variables. Continuous variables were compared using the Wilcoxon rank-sum test, and categorical variables were compared using the Pearson χ2 test or Fisher exact test, as appropriate. Study outcomes were compared using the same tests, as appropriate. A multivariable logistic regression model was used to assess the predictors of myocardial injury. Variable selection was done based on prior knowledge from the literature and was confirmed by using a backward selection method maintaining the number of events per variable at >10 (Model 1). An additional model was used as a sensitivity analysis (Supplementary material online, Appendix).14 The results are presented as odds ratios (ORs) and 95% confidence intervals (CIs). A multivariable Cox proportional hazards model was used to assess the adjusted association between myocardial injury and mortality at the longest follow-up and to assess predictors of mortality. The results are presented as hazard ratios (HRs) and 95% CIs. Mortality was displayed by using cumulative incidence curves and compared between patients with and without myocardial injury, after adjusting for the multivariable Cox regression model. The statistical level of significance was a two-tailed P-value <0.05. STATA 16 statistical software (Statacorp LP, College Station, TX, USA) was used for all calculations. Details of the statistical analysis are reported in the Supplementary material online, Appendix.
Results
Study population and baseline characteristics
Of 445 patients with confirmed COVID-19 admitted to our Institution from 25 February 2020 to 2 April 2020, a total of 349 patients underwent chest CT; of these, 17 patients with missing core results of laboratory examination (hsTn-I) were excluded. Finally, the overall study population consisted of 332 patients with availability of CT data and hsTn-I levels. The main clinical characteristics of the patients initially screened, of those who were excluded, and of those who entered the study are reported in Supplementary material online, Table 1.
Baseline detailed characteristics of patients finally included in the study according to the presence of myocardial injury are displayed in Table 1. A total of 123 out of 332 (37%) patients had myocardial injury and 209 (63%) had no myocardial injury. Patients with myocardial injury were significantly older [74 (68–80) vs. 61 (51–70) years; P < 0.001], and had a higher prevalence of comorbidities including hypertension (76% vs. 41%; P < 0.001), diabetes mellitus (29% vs. 17%; P = 0.014), prior coronary artery disease (28% vs. 7%; P < 0.001), history of stroke or carotid artery disease (24% vs. 3%; P < 0.001), and peripheral arterial disease (11% vs. 2%; P < 0.001) than patients without myocardial injury.
Table 1.
Baseline clinical characteristics, laboratory values, and medications
| Characteristic | All (n = 332) | Myocardial injury (n = 123) | No myocardial injury (n = 209) | P-value |
|---|---|---|---|---|
| Age, years | 66.9 (55.4–75.5) | 74.2 (67.8–80.1) | 60.7 (51.4–70.5) | <0.001 |
| Female sex, n (%) | 95 (28.6) | 28 (22.8) | 67 (32.1) | 0.07 |
| Body mass index | 26.7 (24.2–30.1) | 26.4 (23.9–29.7) | 26.8 (24.2–30.4) | 0.60 |
| Risk factors | ||||
| Hypertension, n (%) | 179 (54.1) | 93 (76.2) | 86 (41.1) | <0.001 |
| Diabetes mellitus, n (%) | 71 (21.4) | 35 (28.7) | 36 (17.2) | 0.014 |
| Current or recent smoker, n (%) | 31 (9.4) | 19 (15.6) | 12 (5.7) | 0.003 |
| Dyslipidaemia, n (%) | 85 (25.7) | 47 (38.5) | 38 (18.2) | <0.001 |
| Comorbidities | ||||
| Known CAD, n (%) | 49 (14.5) | 34 (27.6) | 15 (7.2) | <0.001 |
| Cerebrovascular disease, n (%) | 36 (10.9) | 29 (23.8) | 7 (3.35) | <0.001 |
| PAD, n (%) | 18 (5.4) | 14 (11.5) | 4 (1.9) | <0.001 |
| COPD, n (%) | 33 (9.9) | 18 (14.7) | 15 (7.2) | 0.026 |
| Cancer, n (%) | 37 (11.2) | 21 (17.2) | 16 (7.7) | 0.008 |
| Laboratory findings | ||||
| Creatinine, mg/dL | 0.94 (0.74–1.18) | 1.11 (0.79–1.60) | 0.88 (0.72–1.06) | <0.001 |
| eGFR, mL/min | 81.9 (59.8–96.4) | 64.4 (40.1–87.4) | 88.2 (72.1–102.22) | <0.001 |
| Leucocytes, count/mm3 | 6420 (5020–9390) | 6800 (5000–9880) | 6090 (5020–8870) | 0.19 |
| Lymphocytes count/mm3 | 898 (634–1287) | 817 (516–1183) | 967 (682–1315) | 0.006 |
| Haemoglobin, g/dL | 13.9 (12.6–15.0) | 13.6 (12.0–14.5) | 14.2 (13.0–15.2) | 0.004 |
| C-reactive protein, mg/dL | 9.39 (3.98–16.7) | 11.8 (6.84–18.3) | 7.69 (2.85–15.5) | 0.002 |
| D-dimera, ng/mL | 483 (297–890) | 654 (353–1711) | 394 (281–642) | <0.001 |
| hs-Troponin I, mg/L | 11.4 (4.65–37.3) | 52 (33.6–143) | 6 (3.2–10.5) | <0.001 |
| BNPb, pg/mL | 72.5 (34.5–198) | 202 (94–501) | 45 (27–91) | <0.001 |
| BNP >100 pg/mL, n (%) | 121 (39.8) | 80 (70.2) | 41 (21.2) | <0.001 |
| Baseline medical therapy | ||||
| ACEI/ARB, n (%) | 117 (35.3) | 58 (47.5) | 59 (28.2) | <0.001 |
| Beta-blockers, n (%) | 96 (29) | 54 (44.3) | 42 (20.1) | <0.001 |
| Statin, n (%) | 72 (21.7) | 42 (34.4) | 30 (14.3) | <0.001 |
| In-hospital medical therapy | ||||
| Hydroxychloroquine, n (%) | 283 (85.2) | 109 (88.6) | 174 (83.2) | 0.18 |
| Lopinavir/ritonavir, n (%) | 175 (52.7) | 74 (60.2) | 101 (48.3) | 0.037 |
| Darunavir/cobicistat, n (%) | 103 (31.0) | 32 (26.0) | 71 (33.9) | 0.13 |
| Remdesivir, n (%) | 2 (0.6) | 2 (1.63) | 0 (0) | 0.14 |
Continuous variables are reported as median and 25th–75th percentiles.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; PAD, peripheral arterial disease.
D-dimer was available in 270 (81%) out of 332 patients.
BNP values were available in 310 (93.4%) out of 332 patients.
P-value refers to the comparison between patients with myocardial injury and patients without myocardial injury.
Laboratory findings
Creatinine levels were higher [1.11 (0.79–1.60) vs. 0.88 (0.72–1.06) mg/dL, P < 0.001] and estimated glomerular filtration rate (eGFR) was lower [64 (40–87) vs. 88 (72–102) mL/min, P < 0.001] in patients with myocardial injury as compared with patients without myocardial injury (Table 1). Patients with myocardial injury had higher levels of C-reactive protein, D-dimer, and BNP in comparison with patients without myocardial injury (Table 1). White blood count was similar, and lymphocyte count and haemoglobin levels were lower in patients with myocardial injury as compared with patients without myocardial injury (Table 1).
CT findings
Table 2 summarizes CT findings. PA diameter was higher in patients with as compared with patients without myocardial injury [29.0 mm (27–32) vs. 27.7 (25–30) mm, P < 0.001]. No significant differences were found in the PA/aorta ratio between the two groups (Table 2). Patients with myocardial injury had a lower prevalence of a CAC score of zero (25% vs. 55%, P < 0.001), and higher median values of CAC score in comparison with patients without myocardial injury [108 (0–389) vs. 0 (0–78), P < 0.001]. The prevalence of a CAC score of 101–400 and >400 was higher in patients with cardiac injury as compared with those without (Table 2).
Table 2.
Chest computed tomography findings
| Characteristic | All (n = 332) | Myocardial injury (n = 123) | No myocardial injury (n = 209) | P-value |
|---|---|---|---|---|
| CAC scorea | 3.74 (0–191.7) | 108.4 (0–388.7) | 0 (0–78.5) | <0.001 |
| CAC score of 0, n (%) | 146 (43.9) | 31 (25.2) | 115 (55.0) | <0.001 |
| CAC scorea, n (%) | <0.001 | |||
| 0 | 146 out of 295 (44.5) | 31 out of 98 (31.6) | 115 out of 197 (58.4) | |
| 1–100 | 52 out of 295 (17.6) | 15 out of 98 (15.3) | 37 out of 197 (18.8) | |
| 101–400 | 56 out of 295 (18.9) | 28 out of 98 (28.6) | 28 out of 197 (14.2) | |
| >400 | 41 out of 295 (13.9) | 24 out of 98 (24.5) | 17 out of 197 (8.6) | |
| PA diameter, mm | 28.2 (25.8–30.8) | 29 (27–32) | 27.7 (25–30) | <0.001 |
| AO diameter, mm | 35 (32.1–38) | 36 (34–40) | 34 (31.5–37) | <0.001 |
| PA/AO diameter ratio | 0.81 (0.73–0.89) | 0.82 (0.73–0.89) | 0.81 (0.74–0.89) | 0.84 |
Continuous variables are reported as median and 25th–75th percentiles.
CAC score was not calculated in 37 patients with prior coronary artery disease and stent implantation or coronary artery bypass grafts. These patients were considered as having a CAC score >0.
PA, pulmonary artery; AO, aorta.
P-value refers to the comparison between patients with and without myocardial injury.
Respiratory metrics
The PaO2/FiO2 ratio was lower in patients with myocardial injury in comparison with patients without myocardial injury [252 (171–329) vs. 300 (214–374), P = 0.005]. The prevalence of a PaO2/FiO2 ratio of 0–100, 101–200, and 201–300, and the need for mechanical ventilation was significantly higher in patients with myocardial injury than in patients without myocardial injury (Table 3).
Table 3.
Respiratory metrics and clinical outcomes
| Characteristic | All (n = 332) | Myocardial injury (n = 123) | No myocardial injury (n = 209) | P-value |
|---|---|---|---|---|
| PaO2/FiO2 ratio | 285.7 (185.7–357.1) | 252.4 (171.4–328.6) | 300.0 (214.3–373.8) | 0.005 |
| PaO2/FiO2 ratio, n (%) | 0.014 | |||
| 0–100 | 34 (10.2) | 15 (12.1) | 19 (9.1) | |
| 101–200 | 57 (17.2) | 27 (21.9) | 30 (14.3) | |
| 201–300 | 95 (28.6) | 41 (33.3) | 54 (25.8) | |
| >300 | 146 (43.9) | 40 (32.5) | 106 (50.7) | |
| Mode of ventilation | <0.001 | |||
| Ambient air, n (%) | 65 (19.8) | 8 (6.45) | 57 (27.7) | |
| Oxygen inhalation, n (%) | 159 (48.3) | 63 (51.2) | 96 (46.6) | |
| Non-invasive ventilation, n (%) | 40 (12.2) | 13 (10.6) | 27 (13.1) | |
| Invasive mechanical ventilation, n (%) | 65 (19.8) | 39 (31.7) | 26 (12.6) | |
| Clinical outcomes | ||||
| Admission to ICU, n (%) | 72 (21.7) | 38 (30.9) | 34 (16.3) | 0.002 |
| Length of hospital stay, days | 12 (8–17) | 13 (9–19) | 12 (7–17) | 0.028 |
| Alive, n (%) | 264 (79.5) | 73 (59.4) | 191 (91.4) | <0.001 |
| Remained in hospital, n (%) | 68 out of 264 (25.8) | 34 out of 73 (46.6) | 34 out of 191 (17.8) | |
| Discharged, n (%) | 196 out of 264 (74.2) | 39 out of 73 (53.4) | 157 out of 191 (82.2) | |
| Died, n (%) | 68 (20.5) | 50 (40.6) | 18 (8.61) | <0.001 |
Continuous variables are reported as median and 25th–75th percentiles.
ICU, intensive care unit; FiO2, fraction of inspired oxygen; PaO2, arterial partial pressure of oxygen.
P-value refers to the comparison between patients with and without myocardial injury.
Echocardiographic data
A small subset of 21 patients underwent echocardiography during hospitalization. The main echocardiographic data are reported for descriptive purposes in Supplementary material online, Table 2. Patients with myocardial injury presented a larger right ventricular dimension compared with patients without myocardial injury.
Predictors of myocardial injury
By multivariable regression analysis, age (OR 1.05, P = 0.001), hypertension (OR 2.72, P = 0.009), history of cerebrovascular disease (OR 3.33, P = 0.01), eGFR (OR 0.98, P = 0.006), the PaO2/FiO2 ratio, as categorical variable (OR 1.35, P = 0.03), and PA diameter (OR 1.10, P = 0.012) emerged as independent predictors of myocardial injury (Model 1, Table 4). Coronary artery disease showed an association of borderline significance with myocardial injury (OR 2.14, P = 0.053) (Model 1, Table 4). The performance of the predictive model was high (Supplementary material online, Table 3). In a sensitivity analysis investigating the stability of Model 1, the results were unchanged (Supplementary material online, Table 4). In Model 2, the effect of hypertension (OR 1.83, P = 0.055) and of the PaO2/FiO2 ratio (OR 1.23, P = 0.18) on myocardial injury was attenuated, while the strength of evidence for an association between coronary artery disease and myocardial injury increased (OR 2.17, P = 0.048) (Supplementary material online, Table 5). Consistent effects with the main analysis were found for the remaining predictors. CAC score was not selected as a predictor in any multivariable model. C-reactive protein did not emerge as a predictor of myocardial injury (Supplementary material online, Table 5). The performance of Model 1 was slightly better than that of Model 2 (Supplementary material online, Tables 3 and 6).
Table 4.
Multivariable logistic regression model for myocardial injury (Model 1)
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Age, years | 1.05 (1.02–1.07) | 0.001 |
| Hypertension | 2.72 (1.28–5.75) | 0.009 |
| Cerebrovascular disease | 3.33 (1.29–8.62) | 0.013 |
| CAD | 2.14 (0.99–4.63) | 0.053 |
| Cancer | 2.12 (0.93–4.88) | 0.075 |
| eGFR, mL/min | 0.98 (0.97–0.99) | 0.006 |
| PaO2/FiO2 ratioa | 1.35 (1.03–1.77) | 0.029 |
| PA diameter, mm | 1.10 (1.02–1.19) | 0.012 |
| Baseline ACEI/ARB use | 0.52 (0.26–1.06) | 0.07 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; PA, pulmonary artery; CAD, coronary artery disease; CI, confidence interval; eGFR, estimated glomerular filtration rate; FiO2, fraction of inspired oxygen; PaO2, arterial partial pressure of oxygen; OR, odds ratio.
PaO2/FiO2 ratio ratio is expressed as a categorical variable of >300, 201–300, 101–200, and 0–100.
In another sensitivity analysis using sex-specific cut-off values of hsTn-I (i.e. 19.8 ng/L for males and 11.6 ng/L for females), the predictors of the main analysis were confirmed, and female sex emerged as an additional predictor of myocardial injury (Supplementary material online, Table 7).
Clinical outcomes
Patients with myocardial injury had a longer duration of hospitalization [13 (9–19) vs. 12 (7–17) days, P = 0.028) and a higher frequency of intensive care unit admission (31% vs. 16%, P = 0.002) (Table 3). Among patients who remained alive (n = 264), the prevalence of discharged patients was lower in patients with myocardial injury in comparison with patients without myocardial injury (53% vs. 82%, P < 0.001) (Table 3).
Myocardial injury and mortality
Median time to follow-up was 12 days (8–17). As of 10 April 2020, no patient was lost to follow-up. Of 332 patients, 68 (20.5%) died; all deaths occurred in the hospital; 50 (40.6%) deaths occurred in patients with myocardial injury compared with 18 (8.61%) in patients without myocardial injury (P < 0.001). The results from univariable Cox regression analysis for death are displayed in Supplementary material online, Table 8. By multivariable Cox regression analysis, myocardial injury was an independent predictor of death (HR 2.25, 95% CI 1.27–3.96, P = 0.005, Model 1, Table 5) and the adjusted cumulative incidence of death at 30 days was computed to 32.4% (95% CI 19.9–46.6%) in patients with myocardial injury, and to 16.1% (95% CI 8.6–26.2%) in patients without myocardial injury (Figure 1). Age (HR 1.06, P < 0.001), eGFR (HR 0.98, P = 0.002), PaO2/FiO2 ratio, treated as categorical variable (HR 1.49, P = 0.002), and PA diameter (HR 1.09, P = 0.01) were other independent predictors of death (Model 1). The performance of the predictive model was high (Supplementary material online, Table 9). In a sensitivity analysis, we performed a bootstrap stability investigation of Model 1, and the results of the predictive model were unchanged (Supplementary material online, Table 10). In another sensitivity analysis, the addition of BNP to Model 1 did not substantially affect the prognostic value of the identified predictors, nor did BNP emerge as a predictor (Supplementary material online, Table 11).
Table 5.
Multivariable Cox regression model for mortality
| Variable | HR (95% CI) | P-value |
|---|---|---|
| Myocardial injury | 2.25 (1.27–3.96) | 0.005 |
| Age, years | 1.06 (1.03–1.09) | <0.001 |
| eGFR, mL/min | 0.98 (0.97–0.99) | 0.002 |
| PaO2/FiO2 ratioa | 1.49 (1.16–1.92) | 0.002 |
| PA diameter, mm | 1.09 (1.02–1.17) | 0.012 |
| Baseline ACEI/ARB use | 0.68 (0.41–1.14) | 0.14 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; PA, pulmonary artery; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
PaO2/FiO2 ratio is expressed as a categorical variable of >300, 201–300, 101–200, and 0–100.
Figure 1.
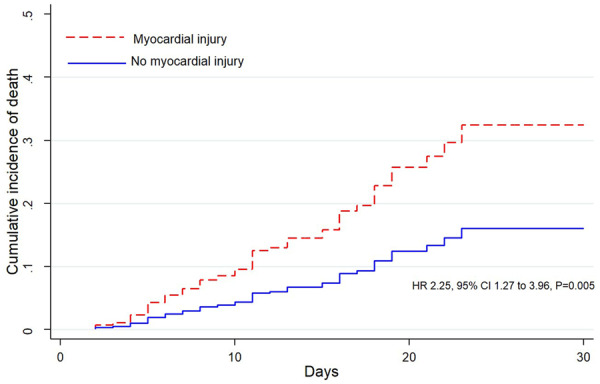
Adjusted cumulative incidence of death, as calculated from a multivariable Cox regression model, in patients with and without myocardial injury. HR, adjusted hazard ratio; CI, confidence interval. P = 0.005.
In a post-hoc exploratory analysis, a cut-off value of 32 mm for PA diameter, selected on the basis of the optimal threshold for sensitivity and specificity, replaced PA diameter as a continuous variable in the multivariable Cox regression model and was associated with an increased adjusted HR of mortality of 1.78 (P = 0.04) (Supplementary material online, Table 12).
In another sensitivity analysis, using sex-specific cut-off values of hsTn-I (i.e. 19.8 ng/L for males and 11.6 ng/L for females), the multivariable Cox regression analysis confirmed the predictors of the main analysis (Supplementary material online, Table 13).
Discussion
In this study including 332 patients, admitted to a single-centre academic institution, with laboratory-confirmed COVID-19 undergoing chest CT, myocardial injury occurred in approximately one-third of cases and was independently associated with an approximately two-fold increased risk of death at 30 days by multivariable Cox regression (adjusted HR 2.2, P = 0.005). The predictive value of myocardial injury was consistently confirmed in a number of sensitivity analyses.
Recent studies have reported myocardial injury in patients with COVID-19 and suggested an association between myocardial injury and mortality.1,2 Guo et al. reported a higher in-hospital crude mortality in patients with myocardial injury than in patients without myocardial injury (59.6% vs. 8.9%) in a study of 187 patients with COVID-191, and found a stepwise increase in mortality when they split the study population into subgroups of patients according to the presence of pre-existing cardiovascular disease alone, myocardial injury alone, or their combination (13.3%, 37.5%, and 69.4%, respectively).1 Shi et al. also found that myocardial injury was independently associated with mortality, with an adjusted HR of 4.26 (P < 0.001), in a study of 416 patients with COVID-19.2
Our findings provide strong evidence of a link between myocardial injury and the adjusted risk of death in COVID-19 patients in a different patient population and support the recommendation of a systematic assessment of cardiac troponins for risk stratification of COVID-19 patients regardless of prior comorbidities and the severity of clinical presentation. These findings also underscore the need for future studies assessing whether early initiation of tailored therapies to patients with myocardial injury may improve clinical outcomes. In this regard we found weak evidence that prior use of an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) at the time of admission could be associated with a reduced risk of myocardial injury (adjusted OR 0.52, P = 0.07), as well as of death (adjusted HR 0.68, P = 0.14), by multivariable regression. Recently, inpatient use of ACEIs or ARBs has been shown to be associated with lower risk of all-cause mortality compared with ACEI or ARB non-users in a propensity score-matched analysis of patients hospitalized with COVID-19 (adjusted HR 0.37, P = 0.03).15
This is the first study to identify an increased PA diameter, as assessed by CT, as an independent predictor of both myocardial injury and mortality in COVID-19 patients. PA diameter has been shown to correlate with the presence of pulmonary hypertension16 and has been associated with increased mortality in patients with chronic obstructive pulmonary disease.17 COVID-19 pneumonia is characterized by a systemic inflammatory disorder with a hypercoagulation state, endothelial dysfunction, hypoxic vasoconstriction leading to a hypoxia-inducible transcription factor-dependent signalling pathway, that may predispose to acute endothelial damage of the pulmonary circulation, the occurrence of thrombo-embolic phenomena, and/or small vessel thrombosis.18–20 Although we do not have availability of systematic echocardiographic data, we may hypothesize that the PA diameter may reflect the ongoing damage to the pulmonary circulation leading to right ventricular overload and/or dysfunction and myocardial injury. Of note, a recent systematic echocardiographic study of COVID-19 has shown that patients with elevated Tn-I above the 99th percentile upper reference limit have worse right ventricular function, but do not have any significant difference in left ventricular systolic function compared with patients with normal troponin.21 Future studies are needed to provide a better understanding of the mechanisms underlying the relationship between pulmonary arterial circulation, right ventricular dysfunction, and myocardial injury, and assess whether early identification and treatment of right-sided heart failure may lead to improved outcomes and reduced mortality in patients with COVID-19. Whether the use of cut-off values of PA diameter, such as the one we identified in our post-hoc exploratory analysis, in conjunction with myocardial injury could provide a useful adjunct to short-term risk estimation of death needs external validation in prospective studies. For example, a patient with both a PA diameter ≥32 mm and myocardial injury has an estimated 30-day adjusted risk for death, by multivariable Cox regression, of 47.2%, compared with an estimated risk of 14.6% in a patient with none of these in our cohort.
We also identified other factors that were associated with an increased risk of myocardial injury. One possible explanation for the independent association of prior cerebrovascular disease with myocardial injury is that patients with prior cerebrovascular disease may have clinically undetected obstructive coronary artery disease and may be at high risk of myocardial ischaemia and of type 2 myocardial infarction. Indeed, silent myocardial ischaemia on non-invasive tests has been detected in 25–60% of stroke patients without any clinical coronary artery disease, and about a third of patients before carotid surgery had one or more coronary artery stenoses ≥70%.22,23 History of coronary artery disease was found to have an independent association, albeit weak, with increased risk of myocardial injury in our study. We also explored the predictive value of the CAC score given its correlation with the presence of obstructive angiographic coronary lesions.24 Nevertheless, the CAC score showed an association with myocardial injury by univariable analysis only, thus indicating that it does not provide incremental value for risk stratification of myocardial injury in patients with COVID-19. We report an independent association of hypertension with an increased risk of myocardial injury that is in agreement with previous evidence.25
Nevertheless, owing to the lack of systematic use of electrocardiography and echocardiography or cardiovascular magnetic resonance imaging, we could not assess the proportion of patients with myocardial injury who experienced type 2 myocardial infarction, or had direct cardiac involvement due to inflammation including myocarditis, cardiomyopathy, or new ventricular dysfunction. Indeed, cases of myocarditis have been previously reported in COVID-19 patients.26–28
We also show that a lower PaO2/FiaO2 ratio on admission, older age, and a lower eGFR were common risk factors for both myocardial injury and death. Shi et al. reported a higher incidence of ARDS in patients with myocardial injury than in those without (58.5% vs. 14.7%; P < 0.001); they found that ARDS was independently associated with mortality, with an adjusted HR of 7.89 (P < 0.001).2 Taken together, these findings indicate that hypoxic myocardial injury is a prognostically relevant characteristic of cardiac involvement in patients with COVID-19.
Zhou et al. reported older age as an important risk factor for death in patients with COVID-19.29 An age-dependent increase in the proinflammatory response to viral infection owing to impairment in T-cell and B-cell function has been hypothesized, that may lead to worse outcomes.30
Finally, the independent association of eGFR with myocardial injury and mortality is in line with the findings from previous studies showing that poor renal function is associated with higher troponin concentrations and that there is an inverse relationship between eGFR and mortality across different patient populations.31–34
Limitations
The retrospective design of the study conducted at a hot-spot academic centre of SARS-COV-2 infection with selective inclusion of patients undergoing chest CT scan for the assessment of COVID-19 pneumonia may lead to the selection of a high-risk population, which is reflected by the high mortality rate observed in the case series. It is also difficult to ascertain the causal role of myocardial injury in the occurrence of death in an individual case. We could not assess the right ventricular dimension by CT owing to the lack of contrast administration. Chest CT imaging abnormalities such as focal unilateral or diffuse bilateral ground-glass opacities were not reported in this study.
The absence of haemodynamic and echocardiographic data in the overall study population does not allow us to provide definitive explanations for the observed increased PA diameter in patients with myocardial injury. Indeed, an increased PA diameter might reflect the severity of respiratory compromise and lung involvement, as known to occur in other ARDS conditions. Myocardial injury might reflect the severity of COVID-19 pneumonia.
From a methodological standpoint, strengths of this study are the inclusion of participants who were still in hospital and have neither recovered nor died within the study time period, with the use of appropriate time-to-event analysis to allow for administrative censoring and the absence of loss to follow-up. Further, the performance of the prediction models for cardiac injury and mortality, including discrimination and calibration, was systematically assessed and was high. However, we did not perform an independent external validation of these models; therefore, their generalizability and implementation remain to be addressed in individual participant data from other prospective cohort studies.
Conclusions
This study of hospitalized patients with COVID-19 undergoing chest CT scan shows that myocardial injury, as assessed by cardiac troponins, occurs in approximately one-third of cases, and is associated with an adjusted two-fold increased risk of mortality. An increased PA diameter is an independent predictor of both myocardial injury and mortality. Future studies are needed to elucidate the underlying mechanisms that link a higher pulmonary artery size to myocardial injury and mortality.
Supplementary material
Supplementary material is avilable at Cardiovascular Research online.
Author contributions
G.F., G.C., and L.M. have made substantial contributions to the conception of the work; F.F., O.C., M.M., and L.M. have made substantial contributions to the acquisition of data; R.B. and F.F. performed the echocardiographic analyses; G.F. analysed and interpreted the data, and drafted the work. L.M. and C.G. have revised the work critically. All authors have given their final approval of the version to be published. The corresponding authors take responsibility for all aspects regarding the accuracy or integrity of any part of the work.
Translational perspective
The present study identifies myocardial injury as a clinically relevant independent risk factor for death in the short term in a population of hospitalized patients with laboratory-confirmed COVID-19 outside of China undergoing chest computed tomography for suspected pneumonia on admission.
The study also provides novel insights into the risk factors for myocardial injury, showing that an increased pulmonary artery diameter, assessed by chest computed tomography, is an independent predictor of myocardial injury as well as of mortality, suggesting that pulmonary circulation dysfunction is a pivotal pathological event with cardiac implications in COVID-19.
Data availability
The data underlying this article can be shared with researchers who provide a methodologically sound proposal to the corresponding authors for future collaborative research.
Conflict of interest: none declared.
Funding
This study received COVID-19 funds from Lombardy Region, CARIPLO, Fondazione Umberto Veronesi.
Supplementary Material
References
- 1. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z.. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C.. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O.. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol 2020;doi:10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 4. Liu PP, Blet A, Smyth D, Li H.. The science underlying COVID-19: implications for the cardiovascular system. Circulation 2020;doi: 10.1161/CIRCULATIONAHA.120.047549 [DOI] [PubMed] [Google Scholar]
- 5. Truong QA, Massaro JM, Rogers IS, Mahabadi AA, Kriegel MF, Fox CS, O’Donnell CJ, Hoffmann U.. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging 2012;5:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leigh A, McEvoy JW, Garg P, Carr JJ, Sandfort V, Oelsner EC, Budoff M, Herrington D, Yeboah J.. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging 2019;12:852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wells JM, Morrison JB, Bhatt SP, Nath H, Dransfield MT.. Pulmonary artery enlargement is associated with cardiac injury during severe exacerbations of COPD. Chest 2016;149:1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B.. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L.. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z.. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maniatis NA, Orfanos SE.. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care 2008;14:22–30. [DOI] [PubMed] [Google Scholar]
- 13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE.. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol 2018;72:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zou H. The adaptive LASSO and its oracle properties. J Am Stat Assoc 2006;101:1418–1429. [Google Scholar]
- 15. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Rohit L, Liu PP, Li H.. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ratanawatkul P, Oh A, Richards JC, Swigris JJ.. Performance of pulmonary artery dimensions measured on high-resolution computed tomography scan for identifying pulmonary hypertension. ERJ Open Res 2020;6:00232–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de-Torres JP, Ezponda A, Alcaide AB, Campo A,, Berto J, Gonzalez J, Zulueta JJ, Casanova C, Rodriguez-Delgado LE, Celli BR, Bastarrika G.. Pulmonary arterial enlargement predicts long-term survival in COPD patients. PLoS One 2018;13:e0195640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S.. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta N, Zhao YY, Evans CE.. The stimulation of thrombosis by hypoxia. Thromb Res 2019;181:77–83. [DOI] [PubMed] [Google Scholar]
- 20. Ullah W, Saeed R, Sarwar U, Patel R, Fischman DL.. COVID-19 complicated by acute pulmonary embolism and right-sided heart failure. JACC Case Rep 2020;doi: 10.1016/j.jaccas.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, Oz Gal, Rothschild A, Baruch E, Peri G, Arbel Y, Topilsky Y. Y. The spectrum of cardiac manifestations in coronavirus disease 2019 (COVID-19)—a systematic echocardiographic study. Circulation 2020;doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adams RJ, Chimowitz MI,, Alpert JS, Awad IA, Cerqueria MD, Fayad P, Taubert KA.. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Circulation 2003;108:1278–1290. [DOI] [PubMed] [Google Scholar]
- 23. Hertzer NR, Young JR, Beven EG, Graor RA, O’Hara PJ,, Ruschhaupt WF, deWolfe VG, Maljovec LC.. Coronary angiography in 506 patients with extracranial cerebrovascular disease. Arch Intern Med 1985;145: 849–852. [PubMed] [Google Scholar]
- 24. O’Rourke RA, Brundage BH, Froelicher VF, Greenland P, Grundy SM, Hachamovitch R, Pohost GM, Shaw LJ, Weintraub WS, Winters WL Jr, Forrester JS, Douglas PS, Faxon DP, Fisher JD, Gregoratos G, Hochman JS, Hutter AM Jr, Kaul S, Wolk MJ.. American College of Cardiology/American Heart Association Expert Consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation 2000;102:126–140. [DOI] [PubMed] [Google Scholar]
- 25. Chapman AR, Adamson PD, Mills NL.. Assessment and classification of patients with myocardial injury and infarction in clinical practice. Heart 2017;103:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS.. Pathological findings of COVID-19 associated with acute respiratory distress syndrome Lancet Respir Med 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M.. Cardiac involvement in a patient with coronavirus 2019 (COVID-19) infection. JAMA Cardiol 2020;doi:10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu H, Ma F, Wei X, Fang Y.. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020;doi:10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Opal SM, Girard TD, Ely EW.. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis 2005;41Suppl 7:S504–S512. [DOI] [PubMed] [Google Scholar]
- 31. deFilippi C, Seliger SL, Kelley W, Duh SH, Hise M, Christenson RH, Wolf M, Gaggin H, Januzzi J.. Interpreting cardiac troponin results from high-sensitivity assays in chronic kidney disease without acute coronary syndrome. Clin Chem 2012;58:1342–1351. [DOI] [PubMed] [Google Scholar]
- 32. Cardinaels EP, Altintas S, Versteylen MO, Joosen IA, Jellema LJ, Wildberger JE, Das M, Crijns HJ, Bekers O, van Dieijen-Visser MP, Kietselaer BL, Mingels AM.. High-sensitivity cardiac troponin concentrations in patients with chest discomfort: is it the heart or the kidneys as well? PLoS One 2016;11:e0153300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Potter JM, Simpson AJ, Kerrigan J, Southcott E, Salib MM, Koerbin G, Hickman PE.. Cross-sectional study of high-sensitivity cardiac troponins T and I in a hospital and community outpatient setting. Clin Biochem 2017;50:105–109. [DOI] [PubMed] [Google Scholar]
- 34. Bjurman C, Petzold M, Venge P, Farbemo J, Fu ML, Hammarsten O.. High-sensitive cardiac troponin, NT-proBNP, hFABP and copeptin levels in relation to glomerular filtration rates and a medical record of cardiovascular disease. Clin Biochem 2015;48:302–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article can be shared with researchers who provide a methodologically sound proposal to the corresponding authors for future collaborative research.
Conflict of interest: none declared.


