Abstract
On human lung parenchymal explants, chloroquine concentration clinically achievable in the lung (100 µM) inhibited the lipopolysaccharide-induced release of TNF-ɑ (by 76%), IL-6 (by 68%), CCL2 (by 72%), and CCL3 (by 67%). Besides its antiviral activity, chloroquine might also mitigate the cytokine storm associated with severe pneumonia caused by coronaviruses.
Keywords: chloroquine, lung explant, tumor necrosis factor-alpha, interleukin-6, chemokine
The severe pneumonia caused by severe acute respiratory syndrome coronaviruses (SARS-CoVs) features rapid viral replication followed by an intense, prolonged cytokine/chemokine response known as a “cytokine storm.” This extreme response involves the release of proinflammatory cytokines, such as interleukin (IL)-6, tumor necrosis factor α (TNF-α), macrophage inflammatory protein 1α (CCL3), and monocyte chemoattractant protein 1 (CCL2) [1–4]. Reports that plasma cytokine levels are higher in patients requiring intensive care than those not requiring intensive care suggest that the cytokine storm is linked to disease severity [3]. Hence, dysregulated and/or exaggerated cytokine and chemokine responses to SARS-CoV infection might have a major influence on the pathogenesis of coronavirus disease and the associated morbidity and mortality [1–4].
The antimalarial drugs chloroquine and hydroxychloroquine have been used to treat patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Given the efficacy observed in trials conducted in China, chloroquine is to be included in the forthcoming version of the Chinese national guidelines for the prevention, diagnosis, and treatment of pneumonia caused by SARS-CoV-2 [5]. Chloroquine will probably be the first compound to be widely used in China and worldwide as the first-line treatment of severe SARS-CoV-2 infections due to its broad availability, low cost, and low frequency of adverse drug reactions [6]. The US Food and Drug Administration also encourages the use of chloroquine and hydroxychloroquine in clinical trials and authorizes their emergency use for the treatment of patients with SARS-CoV-2 for whom a clinical trial is not available or participation is not feasible.
Micromolar concentrations of chloroquine are effective in preventing the spread of SARS-CoVs in primate cell cultures [7, 8]. At 100-fold higher concentrations (~100 µM), chloroquine additionally exerts anti-inflammatory activities, such as a reduction in the production of inflammatory cytokines by various cell types—including human monocytes and lung macrophages [9, 10]. In the rat, chloroquine is distributed extensively into the tissues: a peak/plasma concentration ratio of more than 1000 is obtained in the lung [11]. In a very recent study [12], the simulated human lung-to-plasma concentration ratio was as high as 400, with lung concentrations of30 000 ng/mL or greater (ie, close to or greater than 100 µM) [12]. The objective of the present study of human lung explants (in which the in situ parenchymal architecture and cell–cell communications are maintained) was to assess the potential anti-inflammatory effects of chloroquine on the lipopolysaccharide (LPS)-induced release of cytokines and chemokines involved in the SARS-CoV-2–induced cytokine storm.
METHODS
Drugs and Chemicals
Chloroquine diphosphate was obtained from Sigma-Aldrich (Saint-Quentin Fallavier, France). Chloroquine was solubilized and diluted in sterile water. Antibiotics, l-glutamine, and LPS (from Escherichia coli serotype 0111:B4) were purchased from Sigma (St Louis, MO). Roswell Park Memorial Institute (RPMI) medium was purchased from Eurobio Biotechnology (Les Ulis, France).
Preparation and Treatment of Human Lung Explants
The use of resected lung tissue for in vitro experiments was approved by the regional independent ethics committee (Comité de Protection des Personnes Île-de-France VIII, Boulogne-Billancourt, France), and all patients provided their informed consents. Lung tissue samples were obtained from 7 patients (4 males and 3 females; smokers:ex-smokers, 1:6; mean ± standard deviation [SD] age, 63.2 ± 7.4 years; forced expiratory volume in 1 second [FEV1], 82.4% ± 14.1%; pack-years, 41 ± 21; FEV1:forced vital capacity [FVC], 0.78 ± 0.08; FEV1:FVC, <0.7:1; severity of airflow limitation, mild) undergoing surgical resection for lung carcinoma and who had not received prior chemotherapy. The procedure for the preparation of lung explants in our laboratory has been described previously [13]. Briefly, all tissue specimens were taken at some distance from the tumor; dissected free of pleura, visible airways, and blood vessels; and then finely chopped into ~3–5-mm3 fragments. The parenchymal fragments were washed in complete culture medium (RPMI supplemented with 2 mM l-glutamine, 100 µg/mL streptomycin and 100 U/mL penicillin) to prevent contamination by blood and then maintained overnight at 4°C.
On the day after isolation, the parenchymal fragments were washed in the culture medium. Five fragments were distributed into 6-well plates (3 mL of medium per well) and incubated with chloroquine or vehicle (medium) for 1 hour prior to stimulation with LPS (1 µg/mL). After a 24-hour incubation at 37°C in a 5% CO2 humidified atmosphere, supernatants were collected and stored at −80°C for subsequent cytokine assays.
Assays
The supernatant’s cytokine concentrations (TNF-ɑ, CCL2, CCL3, IL-6, and IL-8 [CXCL8]) were measured with an enzyme-linked immunoassay (R&D Systems Europe, Lille, France), according to the manufacturer’s instructions. The assay detection limits were 4 pg/mL for CCL3, 8 pg/mL for TNF-α and CCL2, 10 pg/mL for IL-6, and 16 pg/mL for CXCL8. Cytokine concentrations were expressed in picograms or nanograms per 100-mg explants (wet weight). Lactate dehydrogenase (LDH) activity in the supernatants was measured using a cytotoxicity assay (CytoTox 96; Promega Corporation, Madison, WI).
Statistical Analysis
The data are presented as the mean ± standard error of the mean (SEM) obtained in experiments with explants from independent donors. To compare chloroquine experiments with LPS-only (control) experiments, we applied a 1-way analysis of variance for repeated measures (Friedman test) and then Dunn’s post-hoc multiple comparison test. The threshold for statistical significance was set to P < .05.
RESULTS
Effects of Chloroquine on LPS-Induced Cytokine Production by Human Lung Explants
Lipopolysaccharide induced a marked increase in the cytokine release: 118.6-fold for TNF-α, 26.4-fold for CCL3, 8.9-fold for IL-6, 6.0-fold for CCL2, and 4.3-fold for CXCL8 (n = 6–7, paired preparations). These findings are in agreement with our previous results [13].
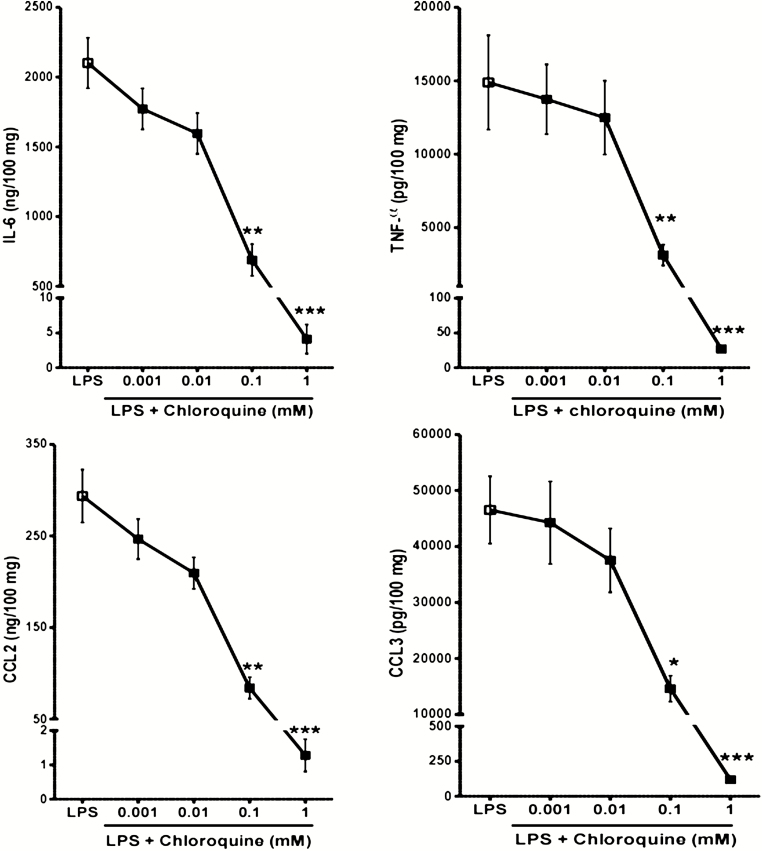
In a concentration-dependent manner, chloroquine inhibited the LPS-induced release of 4 of the cytokines known to be involved in the SARS-CoV cytokine storm (Figure 1), together with CXCL8 (data not shown). At a chloroquine concentration of 100 µM, the relative reduction in release was 76% ± 7% for TNF-α, 68% ± 6% for IL-6, 72% ± 5% for CCL2, 67% ± 5% for CCL3, and 43% ± 5% for CXCL8. Exposure to chloroquine concentrations of up to 100 µM induced small, nonsignificant changes in LDH release. However, chloroquine is known to reduce cell viability at concentrations above 100 µM and a significant increase in LDH release was observed at the highest concentration, suggesting that the near maximal inhibition for all analytes at 1 mM was related (at least in part) to cell toxicity.
Figure 1.
Concentration–response curves for chloroquine’s effect on the LPS-triggered production of TNF-α, IL-6, CCL2, and CCL3. Human lung explants from 6 to 7 patients were stimulated with LPS in the absence or presence of chloroquine (0.001–1 mM). *P < .05, **P < .01, ***P < .001 compared with LPS alone. Abbreviations: CCL2, monocyte chemoattractant protein 1; CCL3, macrophage inflammatory protein 1α; IL-6, interleukin-6; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor α.
DISCUSSION
Here, we used a validated human lung explant model to investigate the pulmonary anti-inflammatory effect of chloroquine in vitro. The lung explants contain the full set of lung cells with a normal spatial configuration and normal cell-to-cell ratios; as such, they approximate well in vivo conditions in the lung [13]. Lipopolysaccharide is a widely used inflammatory stimulus that acts on a number of cells within the lung. The LPS concentration used in the present study was clinically relevant because it was estimated from the levels measured in bronchoalveolar lavage fluids from patients with acute respiratory distress syndrome (ARDS) [13]. In the lung explant model, LPS causes a marked release of the cytokines involved in the cytokine storm associated with SARS-CoV-induced severe pneumonia and ARDS. We found that clinically relevant concentrations of chloroquine reduced the LPS-induced release of TNF-α, IL-6, CCL2, CCL3, and CXCL8. At a concentration of 100 µM, chloroquine more than halved the release of the 4 cytokines reportedly involved in the cytokine storm. In this concentration range, chloroquine has been found to inhibit the LPS-induced release of various cytokines by human monocytes, monocyte/macrophage cell lines (U937, THP-1, and RAW 264.7), and more recently, human lung macrophages [9, 10]. In lung explants, cells other than macrophages contribute to the cytokine release; unfortunately, we cannot determine the exact source of the cytokines released in response to LPS or the various lung cells relative involvement in chloroquine’s inhibitory effect.
The high tissue levels of chloroquine are consistent with its large volume of distribution (~3000 l) [14]. Exposure to chloroquine in the lungs, plasma, and blood was recently simulated in a validated physiologically based pharmacokinetic model [12]. With a high daily dose level (500 mg twice daily), the simulated free chloroquine concentration was much higher in the lung than in plasma; the lung-to-plasma ratio increased over time and reached a value of ~400, with lung levels of more than 30 000 ng/mL (ie, close to or higher than 100 µM) after 3 or 4 days of treatment [12]. After 600-mg and 1500-mg oral doses of chloroquine, the blood concentrations reached 1.8 µM and 3.4 µM, respectively [14]. These blood levels would also be associated with lung concentrations in the range of 100 µM according to the lung-to-blood ratio in the pharmacokinetic model [12]. Hence, chloroquine treatment would preferably be initiated in the first days of lung symptoms caused by SARS-CoVs: first to reduce viral replication and then to mitigate the cytokine storm that typically occurs a few days later in last-stage disease. Given that chloroquine and hydroxychloroquine both appear to affect cytokine production by human monocytes and to accumulate in the lung in a similar way, they might exert the same effect on the cytokine storm [9, 12]. Although the in vitro effects on viral replication have yet to be confirmed in patients and translated into clinical benefits, a recent randomized clinical study suggested that hydroxychloroquine has the potential to control acute inflammation and prevent disease progression in patients infected with SARS-CoV-2 [15].
Notes
Author contributions. S. G.-D. conceived the study and analyzed the data. H. S. analyzed the data and helped to draft the manuscript. M. B. and E. N. performed the experiments and analyzed the data. E. S., E. C., and L.-J. C. revised the manuscript for critical content. P. D. conceived the study, analyzed the data, drafted the manuscript, and revised the manuscript for critical content. All authors read and approved the final manuscript.
Acknowledgments. The authors thank David Fraser (Biotech Communication SARL, Ploudalmézeau, France) for copyediting assistance.
Potential conflicts of interest. L-J. C. reports personal fees from AstraZeneca, Boehringer Ingelheim, and Novartis; grants from Air Liquide Lvl and Elivie; and nonfinancial support from AstraZeneca and Boehringer Ingelheim, outside the submitted work. P. D. reports personal fees for asthma expertise from Sanofi-Aventis, outside the submitted work. H. S. reports nonfinancial support from LVL Medical and Oxyvie, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020; 14:72–3. [DOI] [PubMed] [Google Scholar]
- 6. Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vincent MJ, Bergeron E, Benjannet S, et al. . Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005; 2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang M, Cao R, Zhang L, et al. . Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 2020; 16:155–66. [DOI] [PubMed] [Google Scholar]
- 10. Grassin-Delyle S, Salvator H, Mantov N, et al. . Bitter taste receptors (TAS2Rs) in human lung macrophages: receptor expression and inhibitory effects of TAS2R agonists. Front Physiol 2019; 10:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McChesney EW, Banks WF Jr, Fabian RJ. Tissue distribution of chloroquine, hydroxychloroquine, and desethylchloroquine in the rat. Toxicol Appl Pharmacol 1967; 10:501–13. [DOI] [PubMed] [Google Scholar]
- 12. Yao X, Ye F, Zhang M, et al. . In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buenestado A, Chaumais MC, Grassin-Delyle S, et al. . Roflumilast inhibits lipopolysaccharide-induced tumor necrosis factor-α and chemokine production by human lung parenchyma. PLoS One 2013; 8:e74640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mzayek F, Deng H, Mather FJ, et al. . Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin Trials 2007; 2:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang W, Cao Z, Han M, et al. . Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. medRxiv. doi: 10.1101/2020.04.10.20060558 [DOI] [Google Scholar]