Abstract
Background
The high rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spreading represents a challenge to haemodialysis (HD) units. While fast isolation of suspected cases plays an essential role to avoid disease outbreaks, significant rates of asymptomatic cases have recently been described. After detecting an outbreak in one of our HD clinics, wide SARS-CoV-2 screening and segregation of confirmed cases were performed.
Methods
The entire clinic population, 192 patients, underwent testing for SARS-CoV-2 detection by real-time reverse-transcriptase polymerase chain reaction . We used univariate and multivariate logistic regression to define variables involved in SARS-CoV-2 infection in our dialysis unit. Later, we analysed differences between symptomatic and asymptomatic SARS-CoV-2-positive patients.
Results
In total, 22 symptomatic and 14 of the 170 asymptomatic patients had a SARS-CoV-2-positive result. Living in a nursing home/homeless [odds ratio (OR) 3.54; P = 0.026], having been admitted to the reference hospital within the previous 2 weeks (OR 5.19; P = 0.002) and sharing health-care transportation with future symptomatic (OR 3.33; P = 0.013) and asymptomatic (OR 4.73; P = 0.002) positive patients were independent risk factors for a positive test. Nine positive patients (25.7%) remained asymptomatic after a 3-week follow-up. We found no significant differences between symptomatic and asymptomatic SARS-CoV-2-positive patients.
Conclusions
Detection of asymptomatic SARS-CoV-2-positive patients is probably one of the key points to controlling an outbreak in an HD unit. Sharing health-care transportation to the dialysis unit, living in a nursing home and having been admitted to the reference hospital within the previous 2 weeks, are major risk factors for SARS-CoV-2 infection.
Keywords: asymptomatic carriers, COVID-19, dialysis, haemodialysis, outbreak, outcomes, risk factors
INTRODUCTION
The World Health Organization (WHO) characterized coronavirus disease 2019 (COVID-19), the disease caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], as a pandemic on 11 March. International guidelines [2, 3] and general and local recommendations [4–6] for dialysis patients focus on the importance of hygiene measurements and rapid identification and isolation of COVID-19-positive patients for preventing infection spread. Dialysis patients usually have a high burden of associated comorbidities and are more prone to develop severe complications of the disease. Measures of isolation are of special importance in dialysis units, since many patients need to be repeatedly treated at the same dialysis area and require transportation for at least thrice-weekly treatment. Thus, early identification of COVID-19 cases is highly necessary to cohort them.
The WHO-China Joint Mission on Coronavirus Disease 2019 [7] found that asymptomatic cases were relatively rare on the date of identification and most of them went on to develop the disease. Truly asymptomatic infections were not frequent and did not appear to be a major driver of transmission. However, significant rates of asymptomatic cases have been described by other authors [8–11], and some recent papers [12–17] propose transmission from pre-symptomatic or asymptomatic cases. Although the pre-symptomatic infectious period is not well defined, some preliminary data [18–20] suggest that it might be around 2 days before the onset of symptoms. The presence of asymptomatic or pre-symptomatic contagious patients can be a major epidemiological drawback for COVID-19 spread prevention in dialysis units.
However, scarce information is available about the infection in dialysis patients, and the rate of asymptomatic cases has not been well characterized. Based on data from Wuhan [21] and the first outbreak in Lombardy [5], COVID-19 infection could affect up to 10–30% of dialysis patients.
On 25 February 2020, to minimize the COVID-19 cross-infection risks, strict protocol measures based on international recommendations [4, 22, 23] were implemented in all Fresenius Medical Care (FMC) clinics in Spain as well as in 28 different countries included in the FMC EMEA (Europe, Middle East and Africa) region. Any person with suspected infection was isolated to be explored by the nephrologist and transferred to the hospital before starting haemodialysis (HD) treatment.
Despite previous prevention measures, during the second half of March, there was a COVID-19 outbreak in one of our HD clinics. Based on general recommendations, a nasopharyngeal swab SARS-CoV-2 polymerase chain reaction (PCR) testing was performed in all patients of this clinic.
The aim of this study was to analyse possible variables involved in SARS-CoV-2 transmission and the differences between symptomatic and asymptomatic SARS-CoV-2-positive dialysis patients.
MATERIALS AND METHODS
Study population
We present an analytical observational study where a population of 192 end-stage kidney disease patients in HD treatment were tested for SARS-CoV-2 infection. All of them received regular HD treatment in a single centre placed within L’Hospitalet de Llobregat, Barcelona, Spain.
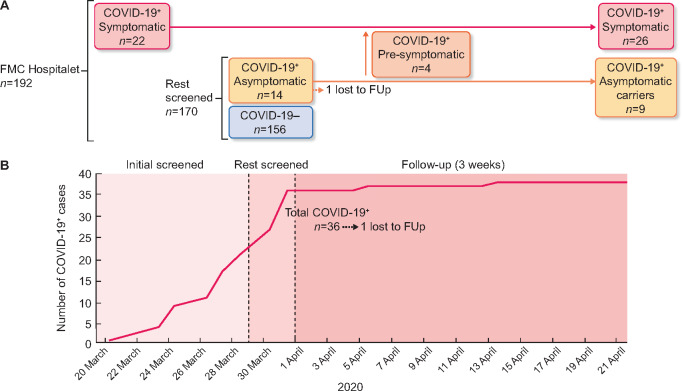
Between 20 and 28 March 2020, a total of 22 (11.5%) patients reported COVID-19-compatible symptoms to the clinical staff and were tested and diagnosed with COVID-19 disease (‘initial screened patients’). After the confirmation of positive results and as part of containment measures, from 30 March to 31 March, the rest of the patients from the centre—170 asymptomatic patients—were also tested by real-time reverse-transcriptase PCR (RT-PCR). Fourteen out of the 170 asymptomatic patients had a SARS-CoV-2-positive result (Figure 1A) and an isolated COVID-19-specific room was created in a different clinic, to which asymptomatic COVID-19-positive patients were transferred. All cases were followed up within 3 weeks after testing.
FIGURE 1:
(A) Between 20 and 28 March 2020, a total of 192 nasopharyngeal samples from HD patients were collected and screened for SARS-CoV-2 detection by RT-PCR. A first group of symptomatic patients (n = 22) were initially tested and confirmed as positive for COVID-19 disease. After this finding, the rest of the patients from the clinic, asymptomatic at that moment, were also screened. Fourteen out of 170 asymptomatic patients had a SARS-CoV-2-positive result. One patient was considered as lost to follow-up. Since early April, four patients COVID-positive who remained asymptomatic communicated the appearance of COVID-19 symptoms. However, a group of nine patients remained totally asymptomatic after a follow-up of 21 days. +, positive population; −, negative population; FUp, follow-up. (B) Cumulative incidence of the number of COVID-19-positive cases along the study.
All patients had previously been informed about data privacy and had provided written informed consent for the use of their data to conduct scientific research. Clinical data were extracted from the European clinical database from FMC (EuCliD).
SARS-CoV-2 tests
Nasopharyngeal swabs were collected by the clinical staff, stored between 2°C and 8°C and processed within 24 h. The specimens corresponding to the initial screened patients were processed, according to their protocol, by the Central Laboratory of Hospital Universitari Bellvitge (Barcelona, Spain). Furthermore, those samples from the rest of the screened patients were managed by Synlab Diagnósticos Globales S.A. (Barcelona, Spain). According to supplier’s availability, two kits were used: TaqMan™ 2019-nCoV Assay Kit v2 from Applied Biosystems (ThermoFisher Scientific, Pleasanton, CA, USA) and VIASURE SARS-CoV-2 Real Time PCR Detection Kit (Certest Biotec, Zaragoza, Spain).
Study variables
Age, gender, Charlson Comorbidity Index, basal (February 2020) laboratory parameters (leucocytes, lymphocytes, sodium, C-reactive protein, ferritin and albumin), concomitant medication [angiotensin-converting enzymes (ACEs), angiotensin II receptor blockers (ARBs) and non-steroidal anti-inflammatory drugs (NSAIDs)], dialysis session details, hydration status measured by Body Composition Monitor ( Fresenius Medical Care), symptoms and patients’ outcomes were all extracted from EuCliD.
Kt/V is measured in every dialysis session through the OCM (Online Clearance Monitor). The previous month (February) mean value was calculated. The average of relative overhydration (AvROH) (pre-dialysis minus normohydrated weight and adjusted per extracellular water) was calculated.
The prevalence of COVID-19 infection associated with the patients’ residence area was calculated. We used the data published by the Catalonian Quality and Assessment Health Agency [Agència de Qualitat i Avaluació Sanitàries de Catalunya (AQUAS)] [24]. The zip code from each patient’s address was obtained from EuCliD and then matched to the prevalence (rate per 10 000 residents) of COVID-19 infection within that zip code area.
At no point were COVID-19-diagnosed patients receiving dialysis treatment together with the rest of patients in the clinic. However, based on a recent publication [25], we assumed a pre-symptomatic infectious period of 6 days. We registered the patients who had been dialysed by the same nurse, in the same room and in adjacent dialysis machines as a patient who developed symptoms within 6 days and was diagnosed with COVID-19.
Information about patients living in nursing homes, those who were attended at the reference hospital for any cause within the previous 2 weeks and patient’s health-care transportation was collected.
All these data were saved in an independent database where the subjects’ identities were anonymized.
Statistical analysis
Data are presented as mean values with standard deviation (SD) for normally distributed variables, medians with interquartile range (25th–75th percentile) for non-normally distributed variables and percentages for categorical variables. Chi-square test, Student’s t-test or Mann–Whitney U test were used for univariate analyses, based on variable characteristics.
First, variables involved in SARS-CoV-2 transmission in our centre were analysed using a univariate approach. Variables that statistically related to transmission in univariate analyses were included in a multivariate logistic regression. Linear relationship between continuous predictors and the logit of the outcome variable and no multi-collinearity (tolerance and variance inflation factor statistics) assumptions were checked.
Later, we analysed the differences between symptomatic and asymptomatic SARS-CoV-2-positive patients to look for variables associated with an asymptomatic infection.
All P-values are two-sided. Statistical significance was set at P < 0.05. Statistical analyses were performed using IBM SPSS statistics version 19 (IBM Corp., Armonk, NY, USA).
RESULTS
This study included 192 chronic dialysis patients from a single FMC centre in Spain. The mean age was 74.3 ± 12.6 years.
A first group of 22 symptomatic patients was initially tested for SARS-CoV-2 infection. All of them obtained positive PCR results for COVID-19 disease. After this confirmation, the rest of the patients, 170 subjects, were tested without evidence of clinical symptoms at the time of testing. From this group of asymptomatic patients, 14 were found positive for SARS-CoV-2 infection (Figure 1A). Thus, a total of 36 patients [18.7%; 95% confidence interval (95% CI) 16.6–20.1] were confirmed as infected by SARS-CoV-2 virus, of whom 22 patients (61.1%) presented initially clinical symptoms and 14 (38.9%) were asymptomatic. One of the asymptomatic patients stopped dialysis treatment and left the clinic by family decision, being then considered as lost to follow-up.
Differences between SARS-CoV-2-positive and -negative patients
Regarding demographic data, we found no significant differences in age, sex or dialysis vintage between negative and positive SARS-CoV-2 patients. However, while Charlson Comorbidity Index median values resulted identical between both groups of patients, we found that 8.3% of the positive cases presented moderated/severe hepatic disease compared with negative patients, among which only 0.6% presented this comorbidity (P = 0.02). Sodium levels were lower in SARS-CoV-2-positive patients (135.5 ± 3.38 versus 136.89 ± 2.8 mmol/L; P = 0.01) and they were significantly more overhydrated (13.35 ± 11.77 versus 9.58 ± 8.99; P = 0.03) (Table 1).
Table 1.
Baseline characteristics of the entire study population (n = 192)
| Variables | SARS-CoV-2 negative | SARS-CoV-2 positive | P-value |
|---|---|---|---|
| n = 156 | n = 36 | ||
| Demographics | |||
| Age, years | 74.46 ± 12.66 | 73.61 ± 12.9 | 0.71 |
| Gender, female | 46 (29.5) | 7 (19.4) | 0.22 |
| Dialysis vintage, months | 40.5 (16–63) | 28.5 (11.25–42.75) | 0.14 |
| Dry weight, kg | 71.1 ± 15.92 | 72.45 ± 15.52 | 0.64 |
| AvROH, % | 9.58 ± 8.99 | 13.35 ± 11.77 | 0.03 |
| Charlson Comorbidity Index | 4 (3–6) | 4 (2.25–6) | 0.96 |
| Diabetes | 70 (44.9) | 19 (52.8) | 0.39 |
| Hepatic disease | 1 (0.6) | 3 (8.3) | 0.02 |
| ACEIs/ARBs treatment | 14 (9) | 2 (5.6) | 0.74 |
| NSAIDs treatment | 68 (43.6) | 12 (33.3) | 0.26 |
| Laboratory parameters | |||
| Leucocytes, no./mm3 | 6800 (5400–8000) | 6600 (5300–8575) | 0.91 |
| Lymphocytes, % | 18 (13.3–23.38) | 15.8 (11.1–24.05) | 0.48 |
| Lymphocytes, no./mm3 | 1175.6 (869.88–1504.38) | 1057.15 (728.65–1471.33) | 0.40 |
| Ferritin, µg/L | 375 (225–477) | 347 (204.25–441.75) | 0.66 |
| C-reactive protein, mg/L | 6.9 (2.93–14.37) | 7.32 (2.56–14.79) | 0.98 |
| Albumin, g/dL | 3.9 (3.7–4.2) | 4 (3.6–4.2) | 0.94 |
| Sodium (mmol/L) | 136.89 ± 2.8 | 135.5 ± 3.38 | 0.01 |
| Social environment | |||
| Nursing home/homeless | 12 (7.7) | 9 (25) | 0.006 |
| Prior visit(s) to hospital | 14 (9) | 11 (30.6) | 0.002 |
| SARS-CoV-2 prevalence/area | 0.49 (0.39–0.58) | 0.39 (0.36–0.58) | 0.63 |
| Contact(s) with future positive patient(s) | |||
| Health-care transport shared (future symptomatic patients) | 21 (13.5) | 15 (41.7) | <0.001 |
| Health-care transport shared (future asymptomatic patients) | 16 (10.3) | 13 (36.1) | <0.001 |
| Dialysis room | 123 (78.8) | 28 (77.8) | 0.88 |
| Adjacent monitor(s) | 35 (22.4) | 7 (19.4) | 0.69 |
| Nurse shared | 123 (78.8) | 27 (75.0) | 0.61 |
Baseline characteristics: last available data before PCR performance. Laboratory parameters correspond to the previous month measurement. Values are represented as mean ± SD, medians with interquartile range (25th–75th percentile) or n (%).
Regarding the factors directly related to a social environment shared and/or contact interactions, we found a higher incidence of positive cases in homeless patients or living in nursing homes (25% versus 7.7%; P = 0.006), in patients that had visited a hospital at least 2 weeks prior to being tested (30.6% versus 9%; P = 0.002) and/or had shared health-care transportation with a future symptomatic (41.7% versus 13.5%; P ≤ 0.001) or asymptomatic (36.1% versus 10.3%; P ≤ 0.001) positive patient. On the other hand, the location of the normal area of residence did not determine any significant difference between the positive and negative patients (Table 1).
We found no differences in receiving dialysis in the same room, sharing the same nurse or receiving treatment in adjacent monitors as a future positive (Table 1).
Multivariate logistic regression confirms that sharing health-care transportation with future symptomatic and asymptomatic positive patients, living in a nursing home/homeless and having attended at the hospital in the prior 2 weeks were independent risk factors for getting a positive PCR test (Table 2).
Table 2.
Multivariate logistic regression for a positive SARS-CoV-2 result
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| Contact(s) in health-care transport shared with a future symptomatic positive | 3.33 | 1.3–8.55 | 0.013 |
| Contact(s) in health-care transport shared with a future asymptomatic positive | 4.73 | 1.74–12.87 | 0.002 |
| Nursing home/homeless | 3.54 | 1.16–10.81 | 0.026 |
| Prior visit(s) to hospital | 5.19 | 1. 84–14.66 | 0.002 |
| Overhydration, % | 1.02 | 0.98–1.07 | 0.331 |
| Na+, mmol/L | 0.91 | 0.79–1.05 | 0.209 |
Differences between symptomatic and asymptomatic SARS-CoV-2-positive patients
A total of four patients who were initially asymptomatic developed clinical symptoms related to COVID-19 disease a few days after performance of PCR (0, 2, 2 and 11 days, respectively). These pre-symptomatic patients were then classified as symptomatic for the following analysis. In total, we found that 26 (74.3%) of the positive cases were symptomatic and 9 (25.7%) remained asymptomatic until the end of the 3-week follow-up period (Figure 1A). The frequencies of each symptom are described in Table 3.
Table 3.
Frequencies of symptoms attending to its presence in COVID-19-positive patients
| Variables | Patients | % |
|---|---|---|
| Asymptomatic | 9/35 | 25.7 |
| Symptomatic | 26/35 | 74.3 |
| Hospitalization | 23/26 | 88.5 |
| Pneumonia | 21/26 | 80.8 |
| Fever ≥37.5°C | 19/26 | 73.1 |
| Cough | 17/26 | 65.4 |
| General malaise | 13/26 | 50.0 |
| Dyspnea | 11/26 | 42.3 |
| Feverishness | 5/26 | 19.2 |
| Gastrointestinal discomfort | 3/26 | 11.5 |
| ICU requirement | 1/26 | 3.8 |
| Exitus | 7/26 | 26.9 |
Asymptomatic and symptomatic percentages were calculated referred to total COVID-19-positive population. Each symptom percentage represents its frequency referred to the total of COVID-19-positive symptomatic population. ICU, intensive care unit.
We did not find any statistically significant difference among the demographic characteristics, the laboratory parameters or the social environment-related variables analysed between symptomatic and asymptomatic patients (Table 4).
Table 4.
Baseline characteristics of COVID-19-positive population
| Symptomatic | Asymptomatic | P-value | ||
|---|---|---|---|---|
| Variables | n = 26 | n = 9 | ||
| Age, years | 72.35 ± 12.86 | 76.67 ± 13.86 | 0.40 | |
| Gender, female | 5 (19.2) | 2 (22.2) | 1.00 | |
| Dialysis vintage, months | 29 (9.75–45.75) | 21 (12.5–35.5) | 0.54 | |
| Body mass index, kg/m2 | 26 (23–28.25) | 26 (24–32) | 0.38 | |
| Charlson Comorbidity Index | 3.5 (2–6.25) | 4 (3.5–6.5) | 0.31 | |
| Co-existing conditions (%) | ||||
| Coronary artery disease | 0 (0) | 2 (22.2) | 0.06 | |
| Congestive heart disease | 7 (26.9) | 2 (22.2) | 1.00 | |
| Peripheral vascular disease | 4 (15.4) | 2 (22.2) | 0.64 | |
| Cerebrovascular disease | 4 (15.4) | 0 (0) | 0.55 | |
| Chronic pulmonary disease | 6 (23.1) | 2 (22.2) | 1.00 | |
| Diabetes | 12 (46.2) | 6 (66.7) | 0.44 | |
| Hepatic disease | 2 (7.7) | 1 (11.1) | 1.00 | |
| Treatments | ||||
| ACEI/ARB | 0 (0) | 1 (11.1) | 0.26 | |
| Calcimimetics | 4 (15.4) | 1 (11.1) | 1.00 | |
| Vitamin D | 13 (50) | 5 (55.6) | 1.00 | |
| NSAIDs | 6 (23.1) | 5 (55.6) | 0.10 | |
| Corticoids | 5 (19.2) | 2 (22.2) | 1.00 | |
| Statins | 12 (46.2) | 5 (55.6) | 0.71 | |
| Body composition | ||||
| AvROH (%) | 12.27 ± 9.09 | 13.51 ± 16.02 | 0.78 | |
| Lean Tissue Index, kg/m2 | 12.38 ± 3.27 | 12.63 ± 3.3 | 0.84 | |
| Fat Tissue Index, kg/m2 | 12.55 ± 4.26 | 14.77 ± 7.06 | 0.27 | |
| Laboratory values | ||||
| Na+, mmol/L | 135.23 ± 3.57 | 136.78 ± 2.33 | 0.24 | |
| K+, mmol/L | 5.1 ± 0.73 | 4.9 ± 0.6 | 0.45 | |
| Leucocytes, no./mm3 | 6600 (5300–8625) | 6100 (4500–8400) | 0.47 | |
| Lymphocytes, no./mm3 | 1057.15 (555.35–1467.18) | 992.2 (857.2–1697) | 0.54 | |
| Haemoglobin, g/dL | 11.18 ± 1.52 | 10.91 ± 0.86 | 0.62 | |
| 25-hydroxyvitamin D, ng/mL | 23.43 ± 10.55 | 23.99 ± 12.21 | 0.90 | |
| Ferritin, µg/L | 324.5 (164.75–449.75) | 377 (288.5–590.5) | 0.34 | |
| C-reactive protein, mg/L | 6.52 (2.73–14.92) | 8.35 (1.66–14.58) | 0.99 | |
| Albumin, g/dL | 4.05 (3.6–4.3) | 3.8 (3.55–4) | 0.18 | |
| Alanine aminotransferase, UI/mL | 15 (10–21) | 13 (10.5–20.5) | 0.81 | |
| Dialysis parameters | ||||
| Pre-dialysis systolic blood pressure, mmHg | 142.08 ± 17.76 | 140.56 ± 13.33 | 0.82 | |
| Online haemodiafiltration (%) | 24 (92.3) | 9 (100) | 1.00 | |
| Kt/V | 1.71 (1.55–1.99) | 1.62 (1.42–1.87) | 0.32 | |
Baseline characteristics: last available data before PCR performance. All laboratory parameters correspond to the previous month measurement, excepting 25-hydroxyvitamin D (6 months). Values are represented as mean ± SD, medians with interquartile range (25th–75th percentile) or n (%).
Evolution of the outbreak after the screening
After wide screening and segregation of asymptomatic SARS-CoV-2-positive patients, there was a reduction in the incidence of new cases (18.7%, 95% CI 16.6–20.9 versus 1.3%, 95% CI 1.1–1.5). Only two new patients had been diagnosed with COVID-19 infection, one was a homeless and the other living in a nursing home (Figure 1B).
Seven patients from the COVID-19-positive population died during their hospitalization period [median age 80 (75–85) years; six males and one female].
DISCUSSION
In the present study, we described the incidence, clinical symptoms, risks factors and management of an outbreak of SARS-CoV-2 infection in ambulatory HD patients receiving regular treatment in a dialysis facility located in the metropolitan area of Barcelona (Spain). The outbreak involved 18% of patients receiving treatment in this facility and diagnosed during a very short time frame. Initially, 22 patients were diagnosed at the referring hospital after presenting clinical symptoms. This outbreak led to screening of the remaining patients by real-time PCR of nasopharyngeal swabs. This screening was performed on two consecutive days and we obtained lab results 24 h later. Then, 14 additional asymptomatic positive cases from 170 screened patients were diagnosed (8.2%). To properly isolate infected patients, they were transferred to another HD unit to continue regular ambulatory HD treatment in an isolated and fully dedicated COVID-19-positive ward area. Preventive measures to the control spread of infection were reinforced. Additionally, patients receiving treatment on consecutive dialysis shifts no longer shared the waiting hall of the dialysis ward. We worked with the transport providers to minimize cross-infection between patients with known COVID-19 and other patients.
Until now, there have been very few reports of SARS-CoV-2 outbreaks in dialysis units to allow us to better manage this situation. The first outbreak in an HD unit in Europe was described in Lombardy (Italy) [5]. In a satellite HD centre, 18 out of 60 treated patients (30%) were diagnosed over 1 week. During this time, the nephrology unit was transformed into an isolation unit and the 18 patients were treated in a small, dedicated dialysis ward set up to deal with the emergency, separated from the main dialysis ward. Despite no universal screening being done, the rigidly implemented isolation measures were effective and no other patient developed a clinical picture thereafter. However, the lack of precise knowledge about the natural history of the disease and the awareness that even non-symptomatic or oligo-symptomatic cases may spread the infection led us to perform screening for all patients once the outbreak appeared.
We investigated risk factors for SARS-CoV-2 infection and inferred that neither demographic nor lab data were associated with risk. Interestingly, positive SARS-CoV-2 patients had lower basal sodium levels and were more overhydrated previously to being infected. The hypothesis that these two factors, aside from social distancing, may in part explain and facilitate the infection has not been reported before, and should be interpreted with caution due to the small sample size.
As expected, by univariate and multivariate regression analysis we found that (i) sharing health-care transportation to the dialysis unit, (ii) living in a nursing home and (iii) having been admitted to the reference hospital within the previous 2 weeks are major risk factors for SARS-CoV-2 infection.
(i) The first result confirmed the recommendation of maintaining social distancing. The CDC Community Mitigation Framework (CDC COVID-19 Response Team) recommends a phased approach to be implemented at the community level, according to its incidence and its severity [26]. This measure means not only a big challenge for our patients, who are unable to ‘stay at home’ because of the need for thrice-weekly treatments, but also for the transportation providers, who had to take extreme precautions to keep at least 2 m distanct from patients. The small size of the waiting hall (20 m2) of the dialysis ward probably also contributed to the spread of the infection. Therefore, measures directed at reducing the use of shared transport to the dialysis units (e.g. transfer by families, individual transport by ambulance or taxi) will reduce the spread of infection. Moreover, the Spanish government declared a total lockdown in the country on 15 March, helping to control the spread of infection in the general population.
(ii) Of noteworthy importance is the risk of transmitting infection to the elderly, particularly those over the age of 60 years [27]. One of the most vulnerable and affected populations for the COVID-19 infection in the reported papers [25] has been people living in nursing homes. A high number of deaths among this vulnerable population has been described, related to small outbreaks despite the strict measures implemented by the government [28].
(iii) Patients who visited the reference hospital during the 2 weeks before the PCR test were more likely to become infected, suggesting the importance of considering the hospital as a potential hot spot for COVID-19 [29].
When new respiratory infectious diseases become widespread, such as during the COVID-19 pandemic, health-care workers’ adherence to infection prevention and control (IPC) guidelines becomes even more important. Houghton et al. [30] highlighted the importance of including all facility staff when implementing the IPC guidelines. Interestingly, there was no transmission from health professionals working at the dialysis unit to patients, since we were not able to find any association between nurses treating positive patients and the spread of infection.
In this outbreak, we observed that 25% of patients on dialysis were asymptomatic carriers of SARS-CoV-2. Despite the prevalence of asymptomatic carriers of SARS-CoV-2 infection has not been well-characterized until now; this figure is much larger than the 1.2% reported from the Chinese CDC [31] in more than 44 000 confirmed cases and it is close to the estimated asymptomatic proportion of 17.9% (95% CI 15.5–20.2) among SARS-CoV-2 cases aboard the Diamond Princess cruise ship in Japan [10]. In a large study to trace close contacts of confirmed cases (206 confirmed cases) in two centres from China, the prevalence of the silent infection of COVID-19 was 5.8% (95% CI 3.4–9.9), and was more likely to occur in young adults without chronic diseases [9].
We did not observe any demographic or clinical data that differentiate asymptomatic from symptomatic patients. In fact, asymptomatic carriers of SARS-CoV-2 infection were old patients (mean age of 76 years) with high comorbidities (median Charlson Comorbidity Index of 4). Thus, there will be individual factors not controlled in this study that will explain why some patients did not experience symptoms.
In summary, we described an outbreak of SARS-CoV-2 infection in an HD unit located in the metropolitan area of Barcelona (Spain). Testing by PCR of nasopharyngeal swabs from all the remaining patients allowed detection of asymptomatic carriers and enabled them to be properly isolated, leading to control of the spread of infection. The main risk factors for SARS-CoV-2 infection were sharing health-care transportation, living in a nursing home and having been admitted to the reference hospital within the previous 2 weeks. Thus, we recommend screening for SARS-CoV-2 infection for all patients treated in dialysis clinics that suffer an outbreak, in order to mitigate the spread of infection. Of course, accurate and strict implementation of recommended general practices is mandatory to reduce the risk of outbreaks in the first place.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (13 April 2020, date last accessed)
- 2.European Centre for Disease Prevention and Control. Infection prevention and control and preparedness for COVID-19 in healthcare settings - second update. https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-and-preparedness-covid-19-healthcare-settings. Published 31 March 2020 (9 April 2020, date last accessed)
- 3.CDC. Interim Additional Guidance for Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed COVID-19 in Outpatient Hemodialysis Facilities. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/dialysis.html. Published 11 February 2020 (9 April 2020, date last accessed)
- 4. Basile C, Combe C, Pizzarelli F. et al. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant 2020; 35: 737–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rombolà G, Heidempergher M, Pedrini L. et al. Practical indications for the prevention and management of SARS-CoV-2 in ambulatory dialysis patients: lessons from the first phase of the epidemics in Lombardy. J Nephrol 2020; 33: 193–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li J, Xu G.. Lessons from the experience in Wuhan to reduce risk of COVID-19 infection in patients undergoing long-term hemodialysis. Clin J Am Soc Nephrol 2020; 15: 717–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) (9 April 2020, date last accessed)
- 8. Tian S, Hu N, Lou J. et al. Characteristics of COVID-19 infection in Beijing. J Infect 2020; 80: 401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He G, Sun W, Fang P. et al. The clinical feature of silent infections of novel coronavirus infection (COVID-19) in Wenzhou. J Med Virol 2020; doi: 10.1002/jmv.25861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mizumoto K, Kagaya K, Zarebski A. et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama. Euro Surveill 2020; doi: 10.2807/1560-7917.ES.2020.25.10.2000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiong F, Tang H, Liu L. et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China [published online ahead of print, 2020 May 8]. J Am Soc Nephrol 2020; doi: 10.1681/ASN.2020030354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan X, Chen D, Xia Y. et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis 2020; 20: 410–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song J-Y, Yun J-G, Noh J-Y. et al. Covid-19 in South Korea—challenges of subclinical manifestations. N Engl J Med 2020; 382: 1858–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ye F, Xu S, Rong Z. et al. Delivery of infection from asymptomatic carriers of COVID-19 in a familial cluster. Int J Infect Dis 2020; 94: 133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bai Y, Yao L, Wei T. et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020; 323: 1406–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qian G, Yang N, Ma AHY. et al. A COVID-19 transmission within a family cluster by presymptomatic infectors in China. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li R, Pei S, Chen B. et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). Science 2020; 368: 489–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson RM, Heesterbeek H, Klinkenberg D. et al. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet 2020; 395: 931–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei WE, Li Z, Chiew CJ. et al. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23-March 16, 2020. Morb Mortal Wkly Rep 2020; 69: 411–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He X, Lau EHY, Wu P. et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26: 672–675 [DOI] [PubMed] [Google Scholar]
- 21. Wang H. Maintenance hemodialysis and Coronavirus disease 2019 (COVID-19): saving lives with caution, care, and courage [published online ahead of print, 2020 Mar 26]. Kidney Med 2020; doi: 10.1016/j.xkme.2020.03.003 [DOI] [PMC free article] [PubMed]
- 22.Coronavirus disease (COVID-19) technical guidance: Infection prevention and control/WASH. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/infection-prevention-and-control (25 April 2020, date last accessed)
- 23. Kampf G, Todt D, Pfaender S. et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 2020; 104: 246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CORONAVIRUS SARS-CoV-2. Agència de Qualitat i Avaluació Sanitàries de Catalunya -AQUAS. [Catalonian Quality and Assessment Health Agency]. http://aquas.gencat.cat/.content/IntegradorServeis/mapa_covid/Taxes-estandarditzades-edat-i-sexe/atlas.html (6 April 2020, date last accessed)
- 25. Arons MM, Hatfield KM, Reddy SC. et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020. ; 382: 2081–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lasry A. Timing of community mitigation and changes in reported COVID-19 and community mobility ― four U.S. metropolitan areas, February 26–April 1, 2020. Morb Mortal Wkly Rep 2020; 69: 451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dong Y, Mo X, Hu Y. et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020; doi: 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 28.Guía de prevención y control frente al COVID-19 en residencias de mayores y otros centros de servicios sociales de carácter residencial 24.03.2020. Ministerio de Sanidad: Gobierno de España. [Prevention and control guidelines against COVID-19 in nursing homes and other residential social services centres. 20 March 2020. Ministry of Health. Government of Spain]. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Residencias_y_centros_sociosanitarios_COVID-19.pdf (26 April 2020, date last accessed)
- 29. Baracchini C, Pieroni A, Viaro F. et al. Acute stroke management pathway during Coronavirus-19 pandemic. Neurol Sci 2020; 41: 1003–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Houghton C, Meskell P, Delaney H. et al. Barriers and facilitators to healthcare workers’ adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: a rapid qualitative evidence synthesis. Cochrane Database Syst Rev 2020; 4: CD013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41: 145–15132064853 [Google Scholar]