Summary
Background
The COVID-19 pandemic continues to have a significant impact on the provision of medical care. Planning to ensure there is capability to treat those that become ill with the virus has led to an almost complete moratorium on elective work. This study evaluates the impact of COVID-19 on cancer, in particular surgical intervention, in patients with esophago-gastric cancer at a high-volume tertiary center.
Methods
All patients undergoing potential management for esophago-gastric cancer from 12 March to 22 May 2020 had their outcomes reviewed. Multi-disciplinary team (MDT) decisions, volume of cases, and outcomes following resection were evaluated.
Results
Overall 191 patients were discussed by the MDT, with a 12% fall from the same period in 2019, including a fall in new referrals from 120 to 83 (P = 0.0322). The majority of patients (80%) had no deviation from the pre-COVID-19 pathway. Sixteen patients had reduced staging investigations, 4 had potential changes to their treatment only, and 10 had a deviation from both investigation and potential treatment. Only one patient had palliation rather than potentially curative treatment. Overall 19 patients underwent surgical resection. Eight patients (41%) developed complications with two (11%) graded Clavien-Dindo 3 or greater. Two patients developed COVID-19 within a month of surgery, one spending 4 weeks in critical care due to respiratory complications; both recovered. Twelve patients underwent endoscopic resections with no complications.
Conclusion
Care must be taken not to compromise cancer treatment and outcomes during the COVID-19 pandemic. Excellent results can be achieved through meticulous logistical planning, good communication, and maintaining high-level clinical care.
Keywords: Oesophageal Cancer, Oesophagectomy, COVID-19, Cancer, Pandemic
INTRODUCTION
Esophageal and gastric cancer affects approximately 1.5 million people each year across the world.1 The cornerstone for treatment of these diseases is surgery, and their aggressive nature means that treatment cannot be delayed due to concerns around disease progression, which may lead to potentially curable disease becoming metastatic.2,3
The COVID-19 (coronavirus disease SARS-CoV-2) pandemic has had a global impact on the provision of health care. A moratorium on elective surgery, and rationalization of investigations was instituted by many health-care providers in order to ensure sufficient capacity was available to manage patients that became critically ill with COVID-19.4 Additionally, changes were implemented to reduce the spread of the disease and because of concerns that contracting COVID-19 peri-operatively may have a major impact on morbidity and mortality.5 The emergency plans implemented will affect all patients requiring health-care input. This is particularly important in those patients being investigated and treated for cancer.
The aim of this review of care over the initial days of the trust’s COVID-19 pandemic plan was to identify and highlight changes in practice and to evaluate outcomes of those patients undergoing resection.
METHODS
Patient population
All patients discussed by the Northern Oesophago-Gastric Unit (NOGU) multidisciplinary team (MDT) for the treatment of cancer, and those who underwent an elective esophago-gastric cancer surgery or endoscopic resection between 12 March and 22 May 2020 were included.
The Newcastle upon Tyne Hospitals NHS Trust is one of the largest trusts in the UK and based across two main hospital sites. It initiated its pandemic plan on 12 March 2020 with the UK Government implementing the nationwide lockdown on 23 March 2020. As part of the hospital pandemic plan, strategic decisions were made regarding the continuation of elective work, the deployment of staff and the positioning of services.
The NOGU performs the highest volume of esophago-gastric resections in the UK6 and takes referrals from a population of 2.1 million spread over the North East of England and Cumbria.7 The unit was relocated to the designated non-COVID-19 receiving hospital within the trust during the early weeks of the period evaluated.
Those that were planned for surgery were to undergo a standard two phase, two-stage transthoracic esophagectomy for esophageal and esophago-gastric junction tumors (Siewert Types 1 and 2), open total gastrectomy for proximal gastric tumors and subtotal gastrectomy for distal gastric cancers where at least 5 cm of proximal clearance could be achieved. Patients who had early disease (T1) amenable to endoscopic resection that had their procedure during this time period were included.
In addition, the number of patients discussed at the MDT meeting and any potential deviation from the standard MDT decisions were identified and explored.
Pre-treatment staging
All patients diagnosed with esophago-gastric cancers in the catchment area are discussed at the regional weekly MDT meeting. Prior to the COVID-19 pandemic, patients were staged according to standardized protocols which include endoscopy with biopsy, a thoracoabdominal computed tomography (CT) scan and a positron emission tomography (PET)-CT scan. If no evidence of metastatic disease was identified, an endoscopic ultrasound (EUS) was routinely used for all esophageal cancer patients, and selectively for those with gastric cancers. Patients with locally advanced gastric cancer or esophago-gastric junction cancer with a significant abdominal component underwent staging laparoscopy with washings for cytology. Patients who were found to have potentially resectable disease then underwent cardiopulmonary exercise testing (CPET).
The concerns around the COVID-19 pandemic altered this pathway in the following way:
1) In an attempt to minimize aerosol generating procedures the use of endoscopy was selective and a MDT decision to stop EUS was carried out as per national guidelines.8
2) CPET was not undertaken due to the concern of the associated risk of aerosol generation.9
3) Initial avoidance of laparoscopy as per guidance during the early part of the time period.10
Comparison was made with the number of patients discussed over during the time period analyzed and the equivalent time period in 2019. Further, a case by case review was undertaken to determine what influence the COVID-19 pandemic had on patients. This was grouped into two main changes: (1) a deviation in staging; (2) a potential deviation in management.
Treatment
Concerns were identified regarding the risk to patients of receiving neoadjuvant treatment during the COVID-19 pandemic. A cohort of patients were currently receiving neoadjuvant chemo (radio) therapy, which is the standard of care for patients with locally advanced disease (>T2 or N+) or had recently completed this treatment and were awaiting surgery. MDT discussion for new patients weighed the potential risks for those with locally advanced disease of receiving neoadjuvant treatment versus progressing straight to surgery versus radical oncological treatment if this was a viable option.
For those patients that underwent radical surgery, short-term outcomes and complications were reviewed, and any delay to treatment that took them outside the normal pathway for surgery was identified. Surgery was relocated to the non-acute hospital within the trust due to the anticipated surge in COVID-19 beds that would require critical care input.
Patients awaiting surgical or endoscopic procedures were asked to self-isolate for 14 days and were then screened with a COVID-19 RNA test 48 hours pre-surgery and in patients planned for esophagectomy a CT thorax the day prior to surgery to exclude potential changes of COVID-19 in asymptomatic patients. The pre-operative CT thorax was stopped after analysis of over 100 pre-operative CT chests in our own hospital and the updated guidance from the Royal Colleges of Radiologists and Surgeons.11
After relocation, all surgeries were carried out as an open procedure, as has previously been described.12,13 Prior to the pandemic, approximately 30–40% of esophageal procedures were performed using a thoracoscopic chest phase and procedures were carried out by a consultant surgeon and senior trainee. A team decision was made that all resections should involve dual consultant input and a trainee.
Patients staged with early cancer had an endoscopic resection as per the unit’s set protocol. It is performed under intravenous sedation in the endoscopy department using the suction cap technique14 and following endoscopic resection, biopsies are taken from the residual circumferential margin. Short-term outcomes of these patients were reviewed.
Statistical analysis
Data concerning patient demographics, clinical stage, neoadjuvant treatment, type of resection, length of hospital stay, complications, re-admissions, and contraction of COVID-19 were analyzed. Data are presented as median (range). A Mann–Whitney test was used to compare non-parametric data.
Ethical approval was obtained from Newcastle University Ref: 4163/2020.
RESULTS
Between 12 March and 22May 2020, a total of 274 MDT discussions were performed on 191 different patients. The median age of patients was 70 (34–93). There was a non-significant drop of 12% in discussion episodes with the equivalent time period in 2019 (312 patients P = 0.106). There was a significant decline in the number of new referrals to the MDT over the same period in 2019 (83 versus 120, P = 0.0322).
Deviations in patient management from MDT
Of the 191 patients discussed over the time period, 153 (80.1%) had no discernible deviation from what would have been their normal staging pathway (Table 1) and 38 patients had deviations to either investigations, management or both (Fig. 1). These patients were largely a mix of patients who had early determination of metastatic disease, clear evidence of frailty that would preclude them from oncological interventions (either curative or palliative) and were therefore subject to symptom support (best supportive care), patients under surveillance (patients with gastrointestinal tumors, or were under endoscopic surveillance) and patients that completed investigations or neoadjuvant treatment close to the start of the time period.
Table 1.
Patients where no deviation to investigations or treatments occurred
| n | 153 |
|---|---|
| Median age (range) | 66 (38–93) |
| Male (%) | 106 (67%) |
| Curative treatment | 43 |
| Unimodality surgery | 6 |
| Surgery post NA | 8 |
| NA | 11 |
| Chemoradiotherapy | 2 |
| Radiotherapy | 12 |
| Adjuvant treatment | 2 |
| EMR | 2 |
| Palliative treatment | 59 |
| Chemotherapy | 14 |
| Radiotherapy | 13 |
| Best supportive care | 32 |
| Other | 51 |
| Surveillance | 33 |
| Epeat EMR | 8 |
| Radiofrequency ablation | 1 |
| Discharge | 9 |
NA: neoadjuvant treatment followed by surgery; best supportive care: symptomatic control of the disease including stent placement; EMR: endoscopic mucosal resection.
Fig. 1.
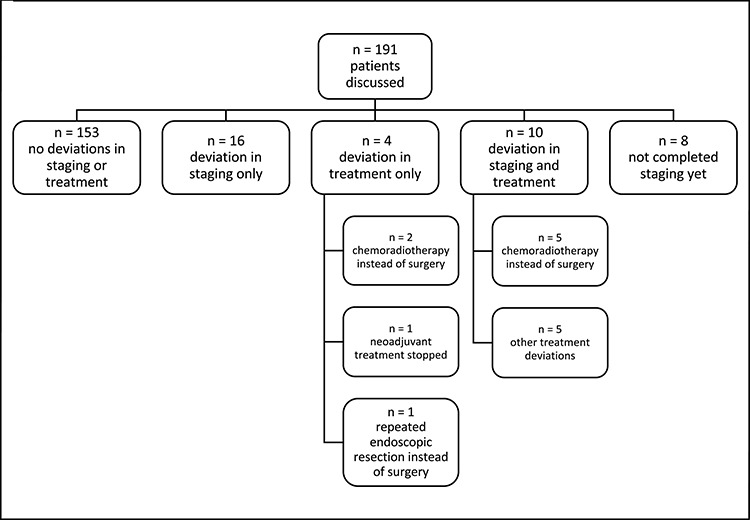
Deviations in patient management from the MDT.
Deviation to investigation
Overall 16 patients had a deviation to their investigation pathway with nine of these not having CPET testing, 11 not having an EUS and 6 no staging laparoscopy (some patients had multiple investigations omitted).
Deviation to treatment
Four patients had potential deviations to their treatment, with two patients opting for chemoradiotherapy rather than surgery, one patient had neoadjuvant treatment stopped due to their wife developing COVID-19 and one person eligible for resection opted to have a repeat endoscopic resection at an interval.
Deviation to investigation and treatment
Ten patients had a deviation to both their investigations and potential treatment pathway. Eight of these patients did not have an EUS, although staging laparoscopies were not performed in two patients and CPET in three. Five of these patients opted for radical chemoradiotherapy rather than surgery due to the COVID-19 pandemic. A further patient did not receive neoadjuvant treatment (for T3 N0 disease). In the remaining four, treatment deviation involved: extending chemotherapy due to concerns of invasion of local structures, a patient opting out of chemotherapy and requesting surgery after one cycle, a patient progressing to surgery without a staging laparoscopy, and a patient being offered palliative radiotherapy rather than potentially surgery.
Eight patients had not completed their staging programme, so it is too early in the pathway to determine if a departure from normal investigation or treatment pathways was likely.
Surgical management
There were 19 patients treated with surgical resections over the time period.
The surgical resections included 11 subtotal esophagectomies (including one robotic/thoracoscopic), six subtotal gastrectomies (including one robotic), and two total gastrectomies. The demographics for patients who had surgery are shown in Table 2. The median age of patients having surgery was 70 (43–81), 79% were male and 89% had at least one other comorbid condition. Two patients were diagnosed with pulmonary emboli during neoadjuvant treatment and had an inferior vena cava filter fitted prior to surgery.
Table 2.
Demographics of patients undergoing esophago-gastric surgery during the trust COVID-19 pandemic plan
| Median age/years (range) | 70 (43–81) | |
|---|---|---|
| Gender | Male | 15 |
| Female | 4 | |
| Comorbidity | Yes | 17 |
| No | 2 | |
| Respiratory disease | Yes | 7 |
| No | 11 | |
| Cardiovascular disease | Yes | 12 |
| No | 6 | |
| Diabetes | Yes | 3 |
| No | 16 | |
| ASA | 2 | 10 |
| 3 | 9 | |
| Median BMI/kg m−2 (range) | 27.1 (21.1–41.9) | |
| Caucasian ethnicity | 19 | |
Patients were screened for COVID-19 as described above. No patient was found preoperatively to be COVID-19 positive. Two patients had surgery delayed at short notice: one with a negative COVID-19 screening swab but CT thorax findings that potentially may have signified COVID-19, infection, and another with a minor cough. Bother patients proceeded on to surgery after a 2-week delay and repeat negative COVID-19 swabs and CT thorax.
Two patients with locally advanced disease did not receive neoadjuvant treatment due to concurrent morbidities, and as is local practice went directly to surgery due to concerns regarding further deconditioning and not being able to undergo surgery. One further patient with locally advanced disease opted to proceed straight to surgery without neoadjuvant chemotherapy.
One gastric cancer patient proceeded to resection not having had a staging laparoscopy but no other patients undergoing surgery in the time frame review had a deviation from what would be regarded normal clinical management.
Table 3 shows clinical data and outcomes related to surgery. There was one return to theatre due to small bowel obstruction, 1 week after discharge, and another patient required a pyloric dilatation. Two patients developed COVID-19. One was the patient that developed small bowel obstruction. They had a prolonged hospital stay due to respiratory complications on a background of COPD. The second patient that was found to be COVID-19 positive presented after feeling faint to their local hospital. This patient was high-risk for respiratory complications (BMI 38 and sleep apnoea) but had no recognized stigmata for COVID-19 and was found to be COVID-19 positive on routine testing.
Table 3.
Histology, neoadjuvant treatment and surgical outcomes of patients undergoing esophago-gastric surgery during the trust COVID-19 pandemic plan
| Surgery | Subtotal esophagectomy | 11 |
|---|---|---|
| Subtotal gastrectomy | 6 | |
| Total gastrectomy | 2 | |
| Histology | Esophageal/esophago-gastric junction AC | 10 |
| Esophageal SCC | 2 | |
| Gastric AC | 6 | |
| Gastric GIST | 1 | |
| Neoadjuvant treatment | Yes | 12 |
| No | 7 | |
| Neoadjuvant treatment type | FLOT | 6 |
| ECX | 4 | |
| CROSS | 2 | |
| Median length of stay†/days (range) | 7 (6–11) | |
| Complications† | Yes | 7 |
| No | 10 | |
| Clavien-Dindo Grade† | 2 | 5 |
| 3a | 1 | |
| 4b | 1 | |
| Re-operations† | Yes | 1 |
| No | 16 | |
| Return to critical care† | Yes | 1 |
| No | 16 | |
| R0 resection† | 17 | |
| Median lymph node harvest† (range) | 35 (15–72)* | |
| In-hospital mortality† | 0 | |
AC, adenocarcinoma; SCC, squamous cell carcinoma; GIST, gastrointestinal stromal tumor; FLOT, 5-fluorouracil, leucovorin, oxaliplatin, docetaxel; ECX, epirubicin, cisplatin, capecitabine; CROSS, paclitaxel, carboplatin, 41.4Gy radiotherapy in 23 fractions.
*Excludes patient with GIST.
†Excludes 2 patients still in hospital at time of submission operated after 20 May 2020.
Endoscopic management
A total of 12 patients underwent endoscopic resection over the time period including 8 new patients and 4 patients who were under surveillance. The median age of patients was 68.5 years (58–78). Figure 2 shows the histology outcomes from endoscopic resections. One patient with an esophageal endoscopic resection had pT1a (m3) adenocarcinoma with a clear deep margin but one positive circumferential margin biopsy and has opted for initial further endoscopic resection over an esophagectomy. A further patient who is a high-risk candidate for surgical resection has been referred for radical radiotherapy for a pT1a m3 esophageal adenocarcinoma with positive deep margins and circumferential biopsies. Three patients had endoscopic resection postponed.
Fig. 2.
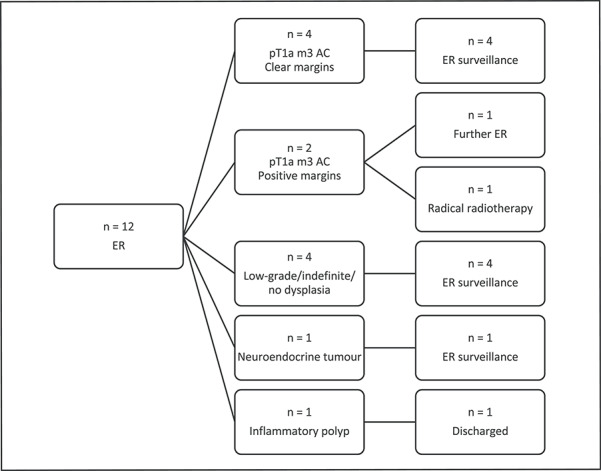
Histology and follow up for patients undergoing ER.
DISCUSSION
One of the major concerns regarding the COVID-19 pandemic is its impact on unrelated conditions that may have delayed or altered treatment. The results of this study indicate that esophago-gastric surgery with curative intent can be carried out without compromising patient outcomes, despite significant changes to staging, changes to provision of neoadjuvant oncological support, and rapidly evolving guidance. The cohort who underwent surgery had a complication rate that compares favorably with what has been previously reported from this unit.15 Although two patients developed COVID-19 in the post-operative period, and both could be regarded as potentially high risk for poor outcomes due to underlying co-morbidities, only one of these had a prolonged critical care stay. Similarly, there were no adverse outcomes from performing endoscopic resections. The strategic move to the COVID-19 ‘cold’ hospital correlates with subsequent recommendations to create a ‘clean’ pathway.16 Although the whole surgical team including surgeons, specialist anesthetists and theatre teams moved to the new site, the high level of post-operative care was provided by critical care and ward teams unfamiliar with managing esophago-gastric patients. This rapid-learning curve required engagement of staff from multiple disciplines.
Another major concern regarding the pandemic is its impact on delayed diagnosis and thus delayed treatment. The concern regarding those harboring undiagnosed cancer is gaining increased attention. Within the esophago-gastric field, where the majority of patients present at a very advanced stage that prohibits curative treatment, COVID-19 may further delay diagnosis and lead to a decline in cure rates. This is potentially further exacerbated by the fact that the majority of patients who develop an esophago-gastric malignancy are men who are known to be less likely to seek help for medical problems. The additional concern regarding COVID-19 is likely to deter those with worrying symptoms further.
Despite the 12% drop in MDT discussions, 80% of patients had no deviation in what would have been expected prior to the pandemic. Whilst this fall in discussed episodes did not prove to be statistically significant this is probably due to the short time period studied and thus represents a type 2 error. As further time progresses this impact may become more apparent. As described, few of these patients were being considered for curative surgical treatment. In total 14 patients had a potential change to their treatment plan with half of these opting for radical oncological treatment in the form of chemoradiotherapy rather than surgery, decisions strongly influenced by the COVID-19 pandemic. However, a recent study based on the US National Cancer Database has suggested that whilst radical chemoradiotherapy is inferior to chemoradiotherapy and surgery for long-term outcomes, those who subsequently had salvage surgery had comparable outcomes to the neoadjuvant group.17 The remaining patients had adjustments to their therapeutic treatment which was tailored to their situation which included opting not to have neoadjuvant treatment or to reduce its length by some, and extend chemotherapy where concerns still existed about resectability. Importantly, only one patient was recommended to proceed down a palliative route, where a curative option may have existed. This patient did not have the full complement of staging investigations but was regarded to be of borderline fitness and decided upon palliative radiotherapy.
Twenty-six patients did not receive the full complement of staging investigations with omissions most frequently to EUS, staging laparoscopy and CPET. Each of these three were withdrawn early in the pandemic course due to concerns about the unnecessary risk presented by aerosol generating procedures.18 CPET has been indefinitely withdrawn as part of the service, with patients being fully assessed by members of the anesthetic perioperative team as required. EUS has been selectively employed and staging laparoscopy gradually reintroduced as more confidence about the safety of laparoscopic procedures has formed. Initial concerns were expressed regarding the use of laparoscopy due to the fear of virus spread from smoke plumes and the potential aerosolization at laparoscopy.10 Further guidance as time progressed now suggests that the use of laparoscopy with appropriate precautions was reasonable.19 For those patients that did not receive a full initial staging, many may potentially have this performed after neoadjuvant therapy.
The preoperative assessment of patients’ COVID-19 status evolved during the course of this study. There were initial concerns regarding the sensitivity of the swab-testing and early suggestions that CT scans could provide further information regarding pulmonary infiltrates that were frequently found with COVID-19 infections.
There were frequent changes to guidelines produced by many organizations regarding the management of patients during the pandemic which potentially led to some inconsistency in management. This highlights the importance of an experienced team making pragmatic decisions based on the application of common-sense throughout. The fluid state of patient guidelines reflected how little was known or understood about the virus. An international survey of esophagogastric surgeons highlighted the variation in practices around the world with limitations on the availability of COVID-19 tests and uncertainty on how to prioritize patients.5
One of the most important considerations is how to counsel patients throughout the crisis. Although there is a paucity of data within this patient cohort there is emerging evidence that that mortality rates are over 20% in those that develop COVID-19 post-surgery.20 Within this cohort there was a 10% post-operative COVID-19 infection rate, but no mortality. There are several factors that will have contributed towards these good outcomes, including meticulous planning and strong team-working, provision of a ‘cold’ site to operate that would hopefully minimize the risk of in-hospital virus exposure to patients, good communication with patients and families to ensure vigilance pre-operatively to ensure testing and delay if required, and maintaining excellent post-operative care. The COVIDSURG collaborative indicated that mortality was 24% and pulmonary complications 51% in patients that developed COVID-19 between 7 days prior to surgery and the 30 days after surgery. Part of their recommendation was to have higher thresholds for surgery during the pandemic.21 In cancer patients where surgery offers the best chance of cure this recommendation must be treated cautiously so that patients are not deprived of the optimum treatment, although they must be informed of the increased risk.
The integration of a two-consultant operating policy may be controversial and is not something that would normally be advocated from this unit with good prior evidence that trainees performing these major resections do not compromise outcomes.12,13 However, a team decision was made at the start of this process to move to dual consultant operating to minimize the stress and fatigue of operating within a new environment at a time of increased pressure. Although one patient at the beginning of this time frame underwent a robotic gastric mobilization all patients operated at the ‘cold’ site underwent completely open operations. Approximately 40% of patients have undergone a thoracoscopic chest phase for Ivor Lewis esophagectomy in the past 2 years, thus a deviation in surgical technique may have occurred to a proportion of patients which is difficult to quantify.
These results are from a single high-volume center but many of the principles will be applicable to other specialities and cancer institutions. It provides reassurance to those who have a new diagnosis of esophago-gastric cancer that good short-term outcomes can be achieved despite the COVID-19 pandemic. It is important not to disadvantage cancer patients by denying them potentially curative therapy if the clinician and patient feel the risk of developing COVID-19 is relatively low and the appropriate precautions are taken. An explicit an honest consent process is vital in allowing patients to make the correct decision for them, at a time of uncertainty.
There are a number of limitations with the current study. These results come from a high-volume unit with extensive experience in managing patients with esophago-gastric cancer. Thus, it could be argued that these results may not be generalizable to other units. Further, the option to relocate to a designated ‘cold hospital’ may not be a viable option for many departments. Potentially one solution to this is further centralization, in the short term, to try and minimize the risks of patients being exposed. Further, whilst data have been provided regarding potential changes to patient management, exact data on delays for patients having palliative treatment are not available. This paper only focuses on short-term outcomes and has no comparison group. As such it provides a snapshot of care at a single unit and it is difficult to fully explain the impact of COVID-19 on longer term outcomes, both in those that were treated with curative intent and also those that were palliated. The results indicate that a full range of treatment options were offered to patients following the MDT discussion. However, it is difficult to ascertain whether discussions made between physicians and patients may have been biased by the ongoing pandemic and whether this would have influenced patient choice.
This study supports the continued delivery of esophago-gastric cancer surgery at designated ‘cold’ sites rather than deferring to potentially inferior oncological interventions during the COVID-19 pandemic. Whilst this is the experience of a single center it helps to illustrate that despite referrals being decimated, those being treated may not have the bleak outcomes suggested by others, particularly if they do contract COVID-19.
One of the major challenges for hospitals is how to deploy resources given the requirement to deal with the influx of COVID-19 patients that may require admission and the provision of other emergency and urgent elective services.22 The ability to isolate acute COVID-19 patients at an acute site allowing cancer provision elsewhere may have the potential of reducing nosocomial spread of the virus.
ACKNOWLEDGMENTS
Thanks, must go to Helen Jaretzke, the NOGU data manager, for helping collate the data. In addition, we would like to thank all the staff involved in looking after esophago-gastric patients during the COVID-19 redeployment, particularly from the Freeman Hospital, Newcastle upon Tyne the staff of Wards 37 (Critical Care), Ward 21 (Cardiothoracic Critical Care) and ward 30.
Contributor Information
S Wahed, Northern Oesophago-Gastric Unit, Royal Victoria Infirmary, Newcastle upon Tyne, UK.
J Chmelo, Northern Oesophago-Gastric Unit, Royal Victoria Infirmary, Newcastle upon Tyne, UK.
M Navidi, Northern Oesophago-Gastric Unit, Royal Victoria Infirmary, Newcastle upon Tyne, UK.
N Hayes, Northern Oesophago-Gastric Unit, Royal Victoria Infirmary, Newcastle upon Tyne, UK.
A W Phillips, Northern Oesophago-Gastric Unit, Royal Victoria Infirmary, Newcastle upon Tyne, UK; School of Medical Education, Newcastle University, Newcastle upon Tyne, UK.
A Immanuel, Northern Oesophago-Gastric Unit, Royal Victoria Infirmary, Newcastle upon Tyne, UK.
Authors contribution
S.W. and A.W.P. collected and analyzed data, A.W.P. wrote the manuscript, S.W., M.N., J.C., N.H., and A.I. contributed to the writing process. All authors reviewed the final manuscript.
Funding source
None declared.
References
- 1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol 2020; 21: e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ueda M, Martins R, Hendrie P C et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Canc Netw 2020; 1–4. [DOI] [PubMed] [Google Scholar]
- 4. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA 2020; 323: 1545–6. [DOI] [PubMed] [Google Scholar]
- 5. SK K, SR M, P S et al. The influence of the SARS-CoV-2 pandemic on esophagogastric cancer services: an international survey of esophagogastric surgeons. Dis esophagus Off J Int Soc Dis Esophagus Epub ahead of print 2020. doi: 10.1093/DOTE/DOAA054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michalowski J, Salvador A, Napper R. Commissioned by healthcare quality improvement partnership National Oesophago-Gastric Cancer Audit 2018 an audit of the care received by people with Oesophago-gastric cancer in England and Wales. Annual Report 2018; 2018. [Google Scholar]
- 7. Upper GI Cancer Site Specific Group OG Cancer Clinical Guidelines. Northern England Strategic Clinical Networks, 2019. Epub ahead of print 2019. doi: http://www.northerncanceralliance.nhs.uk/wp-content/uploads/2018/11/OGCLinicalGuidelines.pdf. [Google Scholar]
- 8. Endoscopy activity and COVID-19 : BSG and JAG Guidance, British Society of Gastroenterology; Available from: https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/. 2020. [Google Scholar]
- 9. Guidance on Perioperative Cardiopulmonary Exercise Testing During the COVID-19 Pandemic, Perioperative Exercise Testing and Training Society; Available from: https://www.poetts.co.uk/Guidelines. 2020. [Google Scholar]
- 10. Intercollegiate General Surgery Guidance on COVID-19 Update. Royal College of Surgeons of England, 2020. [Google Scholar]
- 11. Radiologists RC of The Role of CT in Screening Elective Pre-operative Patients, Royal College of Radiologists; Available from: https://www.rcr.ac.uk/college/coronavirus-covid-19-what-rcr-doing/clinical-information/role-ct-chest/role-ct-screening. 2020. [Google Scholar]
- 12. Phillips A W, Dent B, Navidi M et al. Trainee involvement in Ivor Lewis esophagectomy does not negatively impact outcomes. Ann Surg 2018; 267: 94–8. [DOI] [PubMed] [Google Scholar]
- 13. Navidi M, Madhavan A, Griffin S M et al. Trainee performance in radical gastrectomy and its effect on outcomes. BJS Open 2020; 4: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pouw R E, Van Vilsteren F G I, Peters F P et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett’s neoplasia. Gastrointest Endosc 2011; 74: 35–43. [DOI] [PubMed] [Google Scholar]
- 15. Sinclair R C F, Phillips A W, Navidi M et al. Pre-operative variables including fitness associated with complications after oesophagectomy. Anaesthesia 2017; 72: 1501–7. [DOI] [PubMed] [Google Scholar]
- 16. Søreide K, Hallet J, Matthews J B et al. Immediate and long-term impact of the COVID-19 pandemic on delivery of surgical services. Br J Surg Epub ahead of printApril 30, 2020. doi: 10.1002/bjs.11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamarajah S, Phillips A W, Hanna G et al. Definitive chemoradiotherapy compared to neoadjuvant chemoradiotherapy with esophagectomy for locoregional esophageal cancer: National Population-Based Cohort Study. Ann Surg. Epub ahead of printMay 2020. [DOI] [PubMed] [Google Scholar]
- 18. Spinelli A, Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg 2020; 107(7): 785–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mowbray N G, Ansell J, Horwood J et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg Epub ahead of printMay 3, 2020. doi: 10.1002/bjs.11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lei S, Jiang F, Su W et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. E Clini Med 2020; 21: 100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Archer J E, Odeh A, Ereidge S et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 396. Epub ahead of print 2020 . doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhangu A, Lawani I, Ng-Kamstra J S et al. Global guidance for surgical care during the COVID-19 pandemic. Br J Surg Epub ahead of print April 152020. doi: 10.1002/bjs.11646. [DOI] [PMC free article] [PubMed] [Google Scholar]


