Abstract
Background
Currently, there is no direct evidence to prove the active replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the intestinal tract and relevant pathological changes in the colon and rectum. We investigated the presence of virions and pathological changes in surgical rectal tissues of a patient with clinically confirmed coronavirus disease 2019 (COVID-19) with rectal adenocarcinoma.
Methods
The clinical data were collected during hospitalization and follow-up of this patient. Quantitative reverse transcriptase–polymerasechain reaction (RT-PCR) was performed on the rectal tissue specimens obtained from surgical resection, succus entericus and intestinal mucosa of ileostomy, and rectal mucosa during follow-up after recovery. Ultrathin sections of surgical samples were observed for SARS-CoV-2 virions using electron microscopy. Histopathological examination was performed using hematoxylin-eosin stain. Immunohistochemical analysis and immunofluorescence were carried out on rectal tissues to evaluate the distribution of SARS-CoV-2 antigen and immune cell infiltrations.
Results
The patient had fever and cough on day 3 postoperatively, was diagnosed with COVID-19 on day 7, and was discharged from the hospital on day 41. RNA of SARS-CoV-2 was detected in surgically resected rectal specimens but not in samples collected 37 days after discharge. Notably, coincident with rectal tissues of surgical specimens testing nucleic acid positive for SARS-CoV-2, typical coronavirus virions in rectal tissue were observed under electron microscopy. Moreover, abundant lymphocytes and macrophages (some were SARS-CoV-2 positive) infiltrating the lamina propria were found with no significant mucosal damage.
Conclusions
We first report the direct evidence of active SARS-CoV-2 replication in a patient’s rectum during the incubation period, which might explain SARS-CoV-2 fecal–oral transmission.
Keywords: SARS-CoV-2, coronavirus disease 2019, COVID-19, intestinal infection rectal cancer
During the incubation period, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can be detected in the rectum of patients. Typical SARS-CoV-2 virions in the intestinal epithelial cells under electron microscopy were observed, which provided direct evidence of active SARS-CoV-2 replication in the intestine.
The current coronavirus disease 2019 (COVID-19) identified in 2019, caused by a novel coronavirus, has become a global public health problem [1, 2]. As of 27 May 2020, a total of 5 451 532 cases of COVID-19 have been confirmed globally, including 345 752 deaths [3]. There are many reports suggesting that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA can be detected and identified in anal/rectal swabs [4, 5] and stool specimens [6, 7]. In fact, 1 recent small-sample study found that RNA was consistently detected in rectal swabs, even after viral clearance from the upper respiratory tract, indicating extended duration of viral shedding in fecal samples and raising the possibility of fecal–oral transmission of SARS-CoV-2 [5]. Similar results were reported in another study with more cases involved, raising the possibility of prolonged presence of SARS-CoV-2 in stools. Notably, fecal samples remained positive for SARS-CoV-2 RNA nearly 5 weeks after the viral clearance from the upper respiratory tract in patients with COVID-19 [8]. Considering a high degree of sequence homology between the SARS-CoV-2 and SARS-CoV, angiotensin-converting enzyme 2 (ACE2) has been identified as the entry receptor of SARS-CoV-2. Since this receptor is highly expressed on the epithelial cells from the ileum and colon [9], the intestinal tract may be a potential route for SARS-CoV-2 infection and transmission.
Patients with cancer are considered to be more susceptible to SARS-CoV-2 [10, 11]. One patient with rectal cancer was admitted to Zhongnan Hospital of Wuhan University for radical surgery. On postoperative day 3, the patient began to develop cough and fever; chest computed tomography (CT) revealed radiologic features characteristic of viral pneumonia. On postoperative day 7, the patient was confirmed to be infected with SARS-CoV-2. Although live SARS-CoV-2 had been successfully isolated from the fecal sample of a patient with laboratory-confirmed SARS-CoV-2 [12], until now there has been no direct evidence to prove active SARS-CoV-2 viral replication in the intestinal tract. It remains unknown whether there are pathological changes related to SARS-CoV-2 infection existing in colorectal mucosa in patients with COVID-19. To clarify the above questions, we performed a retrospective study to detect the presence of SARS-CoV-2 virions and determine the pathological changes in rectal tissues of this patient.
METHODS
Patient and Associated Procedures
The patient’s clinical information is described in Supplementary Table 1. The small pieces of stored rectal tissues obtained from surgical specimens during the operation on 16 January 2020 were used for retrospective detection. This study was approved by the Ethics Committee of Chinese Clinical Trial Registry (reference number: ChiECRCT20200116).
Real-time Reverse Transcriptase–Polymerase Chain Reaction
Samples of rectal tissues, succus entericus and intestinal mucosa of ileostomy, and rectal mucosa were tested for SARS-CoV-2 nucleic acid using quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR). The qRT-PCR analyses were performed following a previously described method [13]. The qRT-PCR test kits (BioGerm) were recommended by the Chinese Center for Disease Control and Prevention.
Electron Microscopy
The rectal tissue obtained by resection was soaked in RNAlater solution Ambion company overnight, the solution was discarded, and the tissue was frozen at −80°C. The rectal tissue was cut into 1-mm-thick sections and fixed in 2.5% glutaraldehyde and 1% osmium tetroxide in a biosafety cabinet with level 2 protection, and subsequently dehydrated using different ascending concentrations of alcohol (30% to 100%), and immersed and embedded in epoxy resin. Ultrathin sections (80–100 nm) were prepared on formvar-coated copper grids (200 mesh) Yasheng Electronic Technology Co., Ltd. The virions were observed with a Tecnai G2 20 Twin electron microscope (FEI Company) under 200 kV.
Immunochemistry and Immunofluorescence Assay
Immunohistochemical staining was performed on formalin-fixed and paraffin-embedded tissue sections (4 μm). Sections of rectal tissues were immunostained to evaluate the expression and distribution of the SARS-CoV-2 antigen. Briefly, sections were deparaffinized with xylene and alcohol and subsequently heated in citrate buffer (pH 6.0) for antigen retrieval. After blockage with 3% bovine serum albumin (BSA) in PBS for 30 minutes, a rabbit antibody against Rp3 NP protein (kindly provided by Dr Zhengli Shi, Wuhan Institute of Virology, Chinese Academy of Science [14]) was incubated with the sections overnight at 4°C. For the immunochemistry study, the slides were subsequently incubated with horseradish peroxidase (HRP)–conjugated goat anti-rabbit immunoglobulin G (IgG) (Promoter Biotech, China) for 1 hour at 37°C. Then, sections were stained with 3, 3 -diaminobenzidine (DAB) and hematoxylin. For immunofluorescence assay, Cy3-conjugated goat anti-rabbit IgG (Abcam, USA) was used as a secondary antibody. Images were acquired using a Panoramic scanner (3D-Histech, Hungary) or a fluorescence microscope (Olympus IX51). CD117 (rabbit polyclonal anti-human) and CD20(L26) were stained on the Leica Bond-Max autostainer (Lecia Microsystems, Chicago, IL), and CD3(P7.2.28), CD4(4B12), CD5(4C7), CD8(C814423), CD38(SP149), and CD68(KP1) were stained on a DAKO Autostainer Link48.
RESULTS
The timeline of SARS-CoV-2 infection after rectal cancer surgery is shown in Figure 1. The patient’s clinical information is shown in Supplementary Table 1. On 16 January 2020, the patient underwent rectal surgery. The surgically removed tissue was used for pathological diagnosis. A small portion of the remaining tissue was soaked with RNAlater solution for 24 hours and transferred to a −80°C freezer. On day 3 postoperatively, the patient presented with fever and cough. On day 7 postoperatively, chest CT scan showed typical viral pneumonia with ground-glass opacity (Figure 2). At the same time, the SARS-CoV-2 infection was confirmed by real-time qRT-PCR assay using throat-swab samples, and the patient was transferred to an isolation ward for treatment. The patient was discharged on day 41 after 2 consecutive negative qRT-PCR test results plus absence of clinical symptoms and radiological abnormalities. In the middle of March, we conducted a retrospective study on the patient’s surgically removed tissue. First, SARS-CoV-2 nucleic acid detection was performed on the surgical specimens of rectal tissues, which were positive for SARS-CoV-2. In addition, throat swab, rectal swab, terminal ileum mucosa, and succus entericus samples were collected on day 72 during follow-up and tested for SARS-CoV-2 nucleic acid; all of these were found to be negative for SARS-CoV-2.
Figure 1.
Timeline of SARS-CoV-2 infection after rectal cancer surgery. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 2.
Chest CT of the patient. A, On day 2 before the operation, no abnormalities in the lung. B, On day 3 after the operation, infection was found in the bilateral lower lung fields but without radiologic characteristics of the SARS-CoV-2 infection. C, On day 7 after the operation, the blue arrows show the ground-glass opacities affecting bilateral, subpleural lung parenchyma, which are radiologic characteristics of the SARS-CoV-2 infection. D, On day 14 after the operation, the blue arrow shows the ground-glass opacities and the consolidation dissipated into irregular linear fibrosis and irregular fibrosis foci. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
To explore the direct evidence of SARS-CoV-2 infection and replication in the rectal tissues of surgical specimens, ultrathin sections of rectal tissues were prepared, and virus particles were found in the cytoplasm of intestinal epithelial cells. Under electron microscopy, the virions showed typical morphology of coronavirus (Figure 3).
Figure 3.
SARS-CoV-2 virions in the intestinal tissue. The intestinal tissue was used to prepare ultrathin sections. A and B, The viral particles were observed under electron microscope at 200 kV. The arrows in A indicate the viral particles. The insert in B means the magnification of virus particle, which shows the typical morphology of coronavirus. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
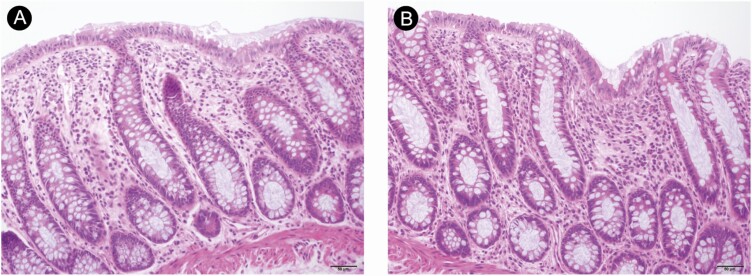
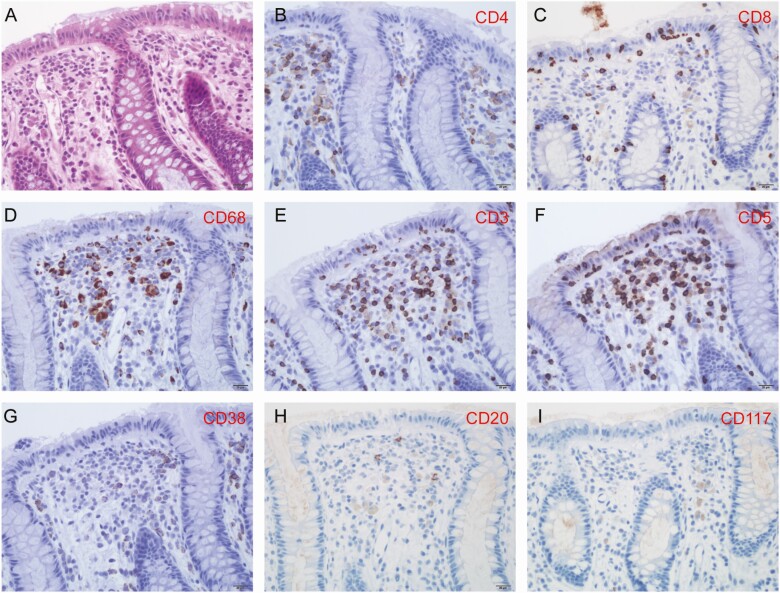
Pathological changes of rectal mucosa with SARS-CoV-2 infection and replication are shown in Figure 4. Hematoxylin-and-eosin–stained rectal mucosa showed prominent lymphocytes and macrophages infiltrating the lamina propria without significant mucosal damage (Figure 5). T lymphocytes and macrophages were found to be more numerous than B lymphocytes in the lamina propria, as demonstrated on immunostaining. No viral inclusion body were observed in the tissues.
Figure 4.
Pathological changes of the rectal mucosa. A and B, The abundant lympho-plasma cells in the lamina propria with intact mucosal architecture.
Figure 5.
H&E staining and immunostaining of rectal mucosa. The field in the tissue shown is the same as in Figure 4. A, H&E staining shows abundant lympho-plasma cells in the lamina propria. B, CD4-positive T cells in the lamina propria. C, CD8-positive T cells in the epithelium and the lamina propria. D, CD68-positive macrophages in the lamina propria. E, CD3-positive T cells in the epithelium and the lamina propria. F, CD5-positive T cells in the epithelium and the lamina propria. G, CD38-positive plasma cell. H, Minimal CD20-positive B cells in the lamina propria. I, Minimal CD117-positive mast cells in the lamina propria. Abbreviation: H&E, hematoxylin and eosin.
To further confirm the SARS-CoV-2–specific infection and replication in the rectum, we conducted immunohistochemistry and immunofluorescence using the rabbit anti–SARS-CoV-2 NP antibody. SARS-CoV-2 antigens were confirmed to be expressed on intestinal epithelial cells, lymphocytes, and macrophages in the lamina propria (Figure 6).
Figure 6.
SARS-CoV-2 infection in the intestinal tissue. A and B, Immunochemistry image with the SARS-CoV-2 antigen. C, Immunofluorescence image with the SARS-CoV-2 antigen. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
In this study, we found, for the first time, that SARS-CoV-2 had already replicated in the patient’s rectum during the incubation period, with no obvious intestinal pathological damage. In this case, the patient developed a dry cough and fever (39.2°C) in the early stage after the operation (postoperative day 3). At the same time, an atypical pulmonary inflammation was revealed by the chest CT. Fever and pulmonary infection are common complications after surgery. However, numerous possible causes of these clinical manifestations to some extent led to a challenge in the differential diagnosis between SARS-CoV-2–related fever and postoperative fever. Initially, when the patient started developing fever, the throat swab was not tested for SARS-CoV-2.
Here, we observed typical SARS-CoV-2 virus particles in the intestinal epithelial cells of a patient under electron microscopy and obtained direct evidence of active SARS-CoV-2 viral replication in the intestine. Meanwhile, we detected the viral components of SARS-CoV-2 in the intestine. It is worth mentioning that virus particles were found in intestinal epithelial cells, but the results of immunofluorescence and immunohistochemistry showed that the viral components were mainly present in intestinal lymphocytes and macrophages, in addition to the intestinal epithelial cells. To explain this seemingly self-contradictory phenomenon, we need to understand the scientific question of how the virus spreads from the lungs to other nonpulmonary tissues. With regard to this issue, some research has been carried out in the influenza virus and several studies have shown that influenza viruses can be transported by immune cells and can spread from the lungs to other tissues [15–17]. These “virus-carrying” immune cells contain professional antigen-presenting cells, such as macrophage, monocytes, and dendritic cells [18–21]. Influenza virus RNA carried by immune cells includes viral RNA fragments (majority; these cannot infect other cells) and whole RNA virions (minority; these can infect other cells). These are jointly called the influenza virus transcriptome. Several studies have strongly supported the concept that the influenza virus transcriptome can be transported by the immune cells within the systematic circulation, following which the viral transcriptome could spread to distant organs such as the intestine [21]. We speculate that the SARS-CoV-2 virus uses the same strategy as the influenza virus to spread from the lung to the distant organs. Therefore, a large number of noninfectious components of SARS-CoV-2 in intestinal lymphocytes and macrophages were found, with few components in the intestinal epithelial cells. Of course, further experiments are needed to verify this hypothesis.
Our study provides further evidence to support the fecal–oral transmission route of SARS-CoV-2 and that the intestine represents a target organ of SARS-CoV-2 [22]. A recent study indicated the persistent presence of SARS-CoV-2 RNA in stool samples of SARS-CoV-2–positive patients with moderate disease, even 5 weeks after onset of symptoms [8]. However, in this study, no SARS-CoV-2 RNA was detected in samples from the gastrointestinal tract in this case on day 37 after hospital discharge. To explore the mechanism of high viral load and persistence of SARS-CoV-2 RNA in the intestinal tract of some patients when SARS-CoV-2 nucleic acid tests on throat swabs are negative and clinical indicators are normal, further studies with large sample sizes are needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are grateful to the Center for Instrumental Analysis and Metrology, The Core Facility and Technical Support, Wuhan Institute of Virology, Chinese Academy of Sciences.
Financial support. This work was supported by the National Science and Technology Major Projects (grant number 2020YFC0846200).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res 2020; 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Coronavirus disease (COVID-2019) situation reports. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situationreports. Accessed 27 May 2020.
- 4. Liang W, Feng Z, Rao S, et al. Diarrhea may be underestimated: a missing link in 2019 novel coronavirus. Gut 2020;69(6): 1141–3. [DOI] [PubMed] [Google Scholar]
- 5. Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 2020; 26:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. Published online 2020. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- 7. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020; 323:1488–94. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020; 5:434–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang H, Kang Z, Gong H, et al. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. bioRxiv 927806 [Preprint]. 30 January 2020 [cited 2020 May 27]. Available at: https://www.biorxiv.org/content/10.1101/2020.01.30.927806v1. [Google Scholar]
- 10. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 2020; 6:1108–10. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Chen C, Zhu SL, et al. Isolation of 2019-nCoV from a stool specimen of a laboratory confirmed case of the coronavirus disease 2019 (COVID-19). China CDC Weekly 2020; 2(8): 123–4. [PMC free article] [PubMed] [Google Scholar]
- 13. Lan L, Xu D, Ye G, et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA 2020; 323(15): 1502–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korteweg C, Gu J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am J Pathol 2008; 172:1155–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Likos AM, Kelvin DJ, Cameron CM, Rowe T, Kuehnert MJ, Norris PJ; National Heart, Lung, Blood Institute Retrovirus Epidemiology Donor Study-II (REDS-II) . Influenza viremia and the potential for blood-borne transmission. Transfusion 2007; 47:1080–8. [DOI] [PubMed] [Google Scholar]
- 17. Choi SM, Xie H, Campbell AP, et al. Influenza viral RNA detection in blood as a marker to predict disease severity in hematopoietic cell transplant recipients. J Infect Dis 2012; 206:1872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Short KR, Brooks AG, Reading PC, Londrigan SL. The fate of influenza A virus after infection of human macrophages and dendritic cells. J Gen Virol 2012; 93:2315–25. [DOI] [PubMed] [Google Scholar]
- 19. Hou W, Gibbs JS, Lu X, et al. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood 2012; 119:3128–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoeve MA, Nash AA, Jackson D, Randall RE, Dransfield I. Influenza virus A infection of human monocyte and macrophage subpopulations reveals increased susceptibility associated with cell differentiation. PLoS One 2012; 7:e29443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang BM, Cootes T, McLean AS. From influenza-induced acute lung injury to multiorgan failure. Annual Update in Intensive Care and Emergency Medicine 2019; 449–58. Available at: https://www.springer.com/series/8901. Accessed 27 May 2020. [Google Scholar]
- 22. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020; 158:1831–3 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.