Abstract
Background
Internationally, key workers such as healthcare staff are advised to stay at home if they or household members experience coronavirus disease 2019 (COVID-19)–like symptoms. This potentially isolates/quarantines many staff without SARS-CoV-2, while not preventing transmission from staff with asymptomatic infection. We explored the impact of testing staff on absence durations from work and transmission risks to others.
Methods
We used a decision-analytic model for 1000 key workers to compare the baseline strategy of (S0) no RT-PCR testing of workers to testing workers (S1) with COVID-19–like symptoms in isolation, (S2) without COVID-19–like symptoms but in household quarantine, and (S3) all staff. We explored confirmatory re-testing scenarios of repeating all initial tests, initially positive tests, initially negative tests, or no re-testing. We varied all parameters, including the infection rate (0.1–20%), proportion asymptomatic (10–80%), sensitivity (60–95%), and specificity (90–100%).
Results
Testing all staff (S3) changes the risk of workplace transmission by −56.9 to +1.0 workers/1000 tests (with reductions throughout at RT-PCR sensitivity ≥65%), and absences by −0.5 to +3.6 days/test but at heightened testing needs of 989.6–1995.9 tests/1000 workers. Testing workers in household quarantine (S2) reduces absences the most by 3.0–6.9 days/test (at 47.0–210.4 tests/1000 workers), while increasing risk of workplace transmission by 0.02–49.5 infected workers/1000 tests (which can be minimized when re-testing initially negative tests).
Conclusions
Based on optimizing absence durations or transmission risk, our modeling suggests testing staff in household quarantine or all staff, depending on infection levels and testing capacities.
Keywords: testing, RT-PCR, isolation, SARS-CoV-2, COVID-19
RT-PCR testing of all staff reduces the risk of workplace transmission the most, but at increased staff shortages and testing resource needs. Testing staff in quarantine allows some staff to return to work but at slightly increased risk of workplace transmission.
Since the first reports in December 2019, the newly emerged respiratory coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a pandemic with widespread community transmission in many countries. Because of the global spread of a newly emerged virus, and no antivirals or vaccines being available, the World Health Organization and many public health agencies internationally advise individuals to stay at home if either they or a household member experiences symptoms of COVID-19, to mitigate the community spread of the pandemic SARS-CoV-2 virus [1–4].
As COVID-19 symptoms are nonspecific, not all individuals staying at home will be infected, and testing workers for SARS-CoV-2 would enable uninfected individuals to remain available for work [5–7]. This is particularly important for workers whose occupational roles are critical to the functioning of society and the COVID-19 response (so-called “key workers”), including in health and social care, transport, education, public safety, government, utilities, and food production and delivery [8, 9]. Additionally, asymptomatic SARS-CoV-2 likely contributes to transmission [10], which could be reduced by testing. Capacity for (extensive) testing may be limited [11, 12], however, and is also required by patients. Targeted testing and optimizing testing strategies are thus important internationally.
For key workers, the crucial question faced is whether to concentrate on testing staff for infection with SARS-CoV-2 who present as cases with COVID-19–like symptoms, staff who are asymptomatic but quarantining at home, or all staff regardless of symptom status or quarantine. We used a decision-analytic model to explore the impact of different testing strategies for SARS-CoV-2 infection by swabbing and reverse transcriptase–polymerase chain reaction (RT-PCR) on (1) the duration key workers such as healthcare staff spend in household isolation, (2) the numbers of staff at work who may spread SARS-CoV-2, (3) the testing accuracy (ie, the proportion of true positive and true negative results among all tests), and (4) the required numbers of tests.
METHODS
Epidemiological Data Informing the Decision-Analytic Model
We varied the proportion of key workers and their households with infectious and detectable SARS-CoV-2 infection at any given time between 0.1% and 20% (base value: 2% [13]), reflecting different levels of mitigation and including the expected prevalence of 5.8% when an assumed 80% of the workers and household members become infected over the course of a 3-month epidemic, with a mean duration of infectiousness of 6.5 days [14] (0.80 × 6.5 days/90 days = 0.058). We considered between 10% and 80% of SARS-CoV-2 infections to be asymptomatic or subclinical (base value: 18%) [10], between 10% and 80% of cases to involve COVID-19–like symptoms of a high fever (base value: 47%) [11], and between 0% and 20% of infections to be too severe for isolation at home and to require hospitalization (base value: 4.4%) [14]. Another 10% of workers are assumed to experience COVID-19–like symptoms from other respiratory illnesses [6], of whom 2% are assumed to become hospitalized [15]. Furthermore, key workers without symptoms but with symptomatic household contacts need to self-isolate (49% of key workers in the United Kingdom live with children and a partner [9]; the rates of illness in household members and key workers were assumed to be the same). RT-PCR test sensitivity (proportion of infected individuals testing positive) was varied between 60% and 95% (with an assumed base value of 75%), and specificity (proportion of uninfected individuals testing negative) was varied between 90% and 100% (base value: 90%) [16, 17]. All input parameters of the model were varied in sensitivity analyses.
Strategies of Testing for SARS-CoV-2 Infection in Key Workers
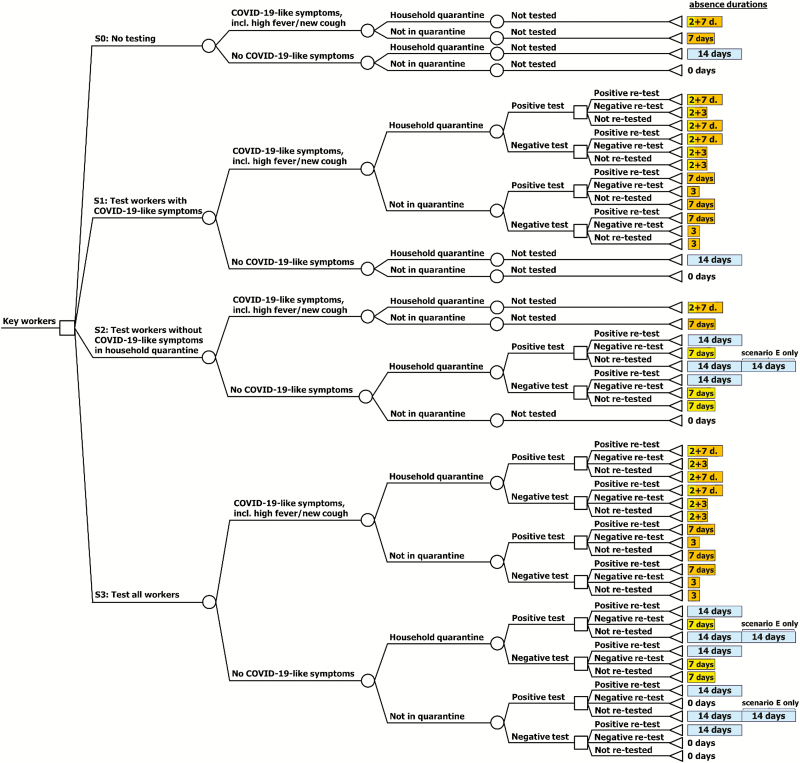
Our static decision-analytic model followed 1000 key workers to compare the baseline strategy of no testing and self-isolating based on COVID-19–like symptoms alone (S0) with 3 strategies of testing: (S1) key workers with COVID-19–like symptoms in isolation, (S2) key workers without COVID-19–like symptoms but in household quarantine due to exposure to symptomatic household contacts, and (S3) one-off testing all key workers, including those without COVID-19–like symptoms or household exposure.
In addition, we explored 5 confirmatory re-testing scenarios for each strategy: repeating (A) all initial tests, (B) initially positive tests [18], (C) initially negative tests, (D) no re-testing [5, 18, 19], or (E) no re-testing but additional isolation for 2 weeks for laboratory-confirmed cases without severe symptoms [1] (Table 1, Figure 1).
Table 1.
Strategies of Testing Key Workers for Infection With SARS-CoV-2 to Enable Them to Return to Work as Soon as Possible While Minimizing the Risk of Transmission
| Population of Key Workers Tested | (Re-)Testing Scenarios | |||||||
|---|---|---|---|---|---|---|---|---|
| Strategy | Workers With COVID-19–Like Symptoms in Isolation | Workers Without Symptoms in Household Quarantine | Workers Without Symptoms Not in Quarantine | Re-test of Positives and Negatives | Re-test of Positives but Not Negatives | Re-test of Negatives but Not Positives | No Re-tests | No Re-tests (+14 Days for Mild, Positive Cases) |
| 0 | No | No | No | n/a | n/a | n/a | n/a | n/a |
| 1 | Yes | No | No | 1A | 1B | 1C | 1D | 1E |
| 2 | No | Yes | No | 2A | 2B | 2C | 2D | 2E |
| 3 | Yes | Yes | Yes | 3A | 3B | 3C | 3D | 3E |
Strategy 0 assumes no testing is performed. In line with guidance [5, 11], strategy 1 explored testing key workers with COVID-19–like symptoms, including a new continuous cough and/or high fever [3]. Strategy 2 explored testing key workers without symptoms but household exposure to symptomatic contacts in household quarantine (note: we explored testing the symptomatic household contact of the key worker as the index case who required the household to quarantine in scenario analysis; see Supplementary Figure 2). Strategy 3 explored testing all key workers, including one-off testing of key workers without symptoms or household exposure and not in quarantine/isolation, to identify infections and then isolate infected workers. The letters correspond to the 5 confirmatory testing scenarios (A–E), including the WHO recommendation for mild laboratory-confirmed cases to isolate for an additional 2 weeks where confirmatory testing is not possible [1]. Note: “Isolation” refers to symptomatic cases, while “quarantine” refers to individuals who are not currently infectious and show no symptoms (yet) but may have been exposed to symptomatic household contacts and so might be infected and might become infectious. Where we need an umbrella term to refer to some people who are sick and others who are not we use the term isolation.
Abbreviations: COVID-19, coronavirus diseasea 2019; n/a, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization.
Figure 1.
Model decision tree of the explored testing strategies in key workers for infection with SARS-CoV-2, and the assumed absence durations (in days). For a technical description of the underlying model accounting for test sensitivity and specificity see Supplementary Figure 1. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
For strategy S2, we also explored in a separate scenario analysis testing the symptomatic household contact of the key worker in household quarantine (as the index case who required the household to quarantine).
Assumed Absence Durations Informing the Model
International guidance recommends key workers with COVID-19–like symptoms to self-isolate for 7 days, and workers in household quarantine for 14 days [1, 3]. We assumed symptomatic workers who tested negative to self-isolate for 3 days on account of their presumed non–COVID-19 acute respiratory illness [20]. Symptomatic workers in symptomatic households are assumed to stay an additional 2 days at home (for an equal chance of who became symptomatic first [21, 22]). Key workers in household quarantine who tested negative are assumed to self-isolate for 7 days until the infectivity of the case at home is assumed to have ended [14]. We explored different values for the durations in isolation and quarantine in sensitivity analyses (for more details, see Figure 1 and Supplementary Table 1).
RESULTS
With the baseline strategy of no testing and self-isolating based on COVID-19–like symptoms alone (S0), we expect 987–2267 days lost in isolation and 0.562–104.1 infected workers with the potential to spread per 1000 key workers. Testing strategies S1 and S2 are expected to reduce the total number of days of the absence durations, while S3 may increase it (Table 2). Conversely, strategies S1 and S2 may increase the total number of infected workers remaining at work, while S3 may reduce it (Table 2). The total number of tests for the 1000 key workers varies per testing strategy and re-testing scenario as is shown in Table 2.
Table 2.
Model Results for SARS-CoV-2 Testing in 1000 Key Workers, Including the Ranges of the Uncertainty of Infected Workers, Proportion Asymptomatic, Specificity, and Sensitivity
| Strategy | Number of Tests (per 1000 Workers) | Days in Isolation (per 1000 Workers) |
Workers Spreading (per 1000 Workers) |
Efficiency Compared With No Testing, S0 | Testing Accuracy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change of Days in Isolation (per Test) | Change of Workers Spreading (per 1000 Tests) | |||||||||||
| Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | |
| S0 | 0.0 | 0.0 | 987 | 2267 | 0.562 | 104.1 | n/a | n/a | n/a | n/a | n/a | n/a |
| S1A | 92.8 | 219.6 | 804 | 2049 | 0.653 | 122.4 | −1.97 | −1.00 | 0.983 | 83.048 | 0.831 | 0.988 |
| S1B | 51.3 | 168.2 | 802 | 1993 | 0.722 | 136.0 | −3.60 | −1.63 | 3.113 | 189.750 | 0.709 | 0.996 |
| S1C | 87.6 | 161.2 | 823 | 2118 | 0.585 | 108.7 | −1.87 | −0.93 | 0.195 | 28.283 | 0.884 | 0.991 |
| S1D | 46.4 | 109.8 | 821 | 2062 | 0.653 | 122.4 | −3.58 | −1.87 | 1.966 | 166.096 | 0.800 | 0.965 |
| S1E | 46.4 | 109.8 | 821 | 2062 | 0.653 | 122.4 | −3.58 | −1.87 | 1.966 | 166.096 | 0.800 | 0.965 |
| S2A | 93.9 | 210.4 | 661 | 1612 | 0.569 | 107.7 | −3.47 | −3.12 | 0.078 | 16.801 | 0.958 | 0.997 |
| S2B | 51.7 | 124.9 | 658 | 1587 | 0.575 | 110.3 | −6.89 | −5.44 | 0.247 | 49.525 | 0.940 | 0.999 |
| S2C | 89.2 | 190.7 | 694 | 1693 | 0.564 | 105.0 | −3.48 | −3.01 | 0.017 | 4.635 | 0.890 | 0.999 |
| S2D | 47.0 | 105.2 | 691 | 1669 | 0.569 | 107.7 | −6.93 | −5.69 | 0.155 | 33.603 | 0.880 | 0.997 |
| S2E | 47.0 | 105.2 | 754 | 1945 | 0.569 | 107.7 | −6.80 | −3.07 | 0.155 | 33.603 | 0.880 | 0.997 |
| S3A | 1979.2 | 1995.9 | 611 | 2580 | 0.239 | 47.8 | −0.22 | 0.16 | −28.455 | −0.162 | 0.944 | 0.995 |
| S3B | 1011.5 | 1212.8 | 491 | 2142 | 0.418 | 83.7 | −0.47 | −0.10 | −16.876 | 0.971 | 0.915 | 0.999 |
| S3C | 1756.0 | 1980.0 | 712 | 3934 | 0.048 | 11.9 | −0.20 | 0.95 | −52.488 | −0.265 | 0.890 | 0.999 |
| S3D | 989.6 | 998.0 | 676 | 3495 | 0.239 | 47.8 | −0.44 | 1.24 | −56.909 | −0.324 | 0.871 | 0.995 |
| S3E | 989.6 | 998.0 | 801 | 5803 | 0.239 | 47.8 | −0.31 | 3.57 | −56.909 | −0.324 | 0.871 | 0.995 |
Highlighting per testing strategy in bold: Lowest number of tests, days in isolation, and workers spreading (per 1000 tests); highest reduction/lowest increase of days in isolation, highest reduction/lowest increase of workers spreading (per 1000 tests), highest accuracy.
Abbreviations: Max, maximum; Min, minimum; n/a, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Efficiency of Testing Strategies as Compared With No Testing (S0)
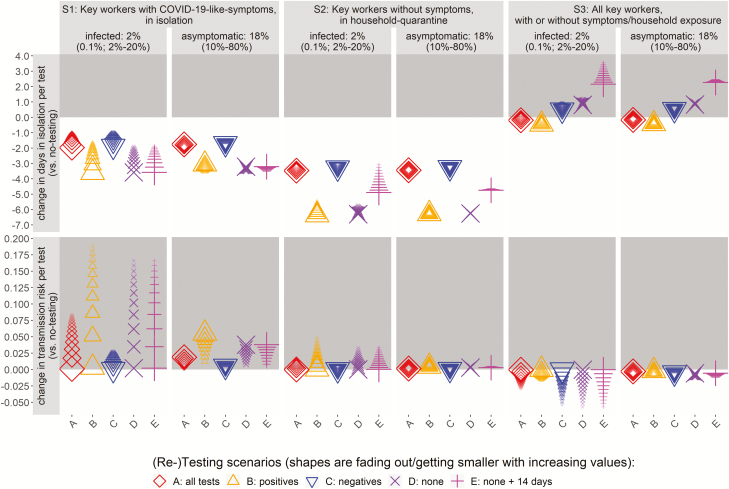
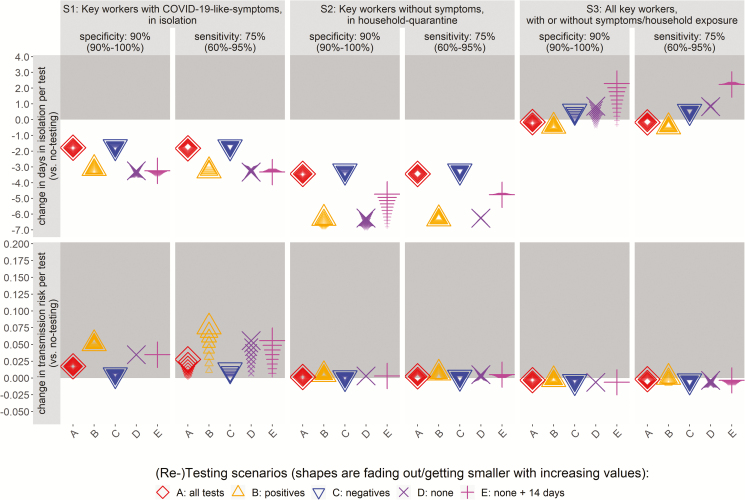
In order to optimize the testing strategies in reducing staff absences and workplace transmission risk it is important to consider the efficiency of the number of tests performed. Figures 2 and 3 present the main findings for the 3 different testing strategies (S1–S3) in terms of changes in absence duration and the transmission risk per test against the baseline of no testing (S0). Note that negative values of change thus represent desirable reductions (in transmission risk or days in isolation; indicated in white-shaded areas). Also, the impact of the variation in key epidemiological parameters (the proportion of infected workers and asymptomatic in Figure 2 and the RT-PCR specificity and sensitivity in Figure 3) is illustrated with the different sizes and fading of the shapes, with smaller shapes that are fading out representing increasing parameter values. All other parameters were kept at their base value, and their impact on results are presented in the Supplementary Material and discussed below (note that the variation of results for all other disease parameters is captured within the range of these 4 key parameters).
Figure 2.
Change in the days in self-isolation and the transmission risk per test (in rows), shown per strategy (in columns) for different proportions of SARS-CoV-2–infected workers and asymptomatic SARS-CoV-2 infection. Note that negative values of changes represent desirable reductions. Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Figure 3.
Change in the days in self-isolation and the transmission risk per test (in rows), shown per strategy (in columns) for different proportions of RT-PCR specificity and sensitivity. Note that negative values of changes represent desirable reductions. Abbreviations: COVID-19, coronavirus disease 2019; RT-PCR, reverse transcriptase–polymerase chain reaction.
Testing workers with COVID-19–like symptoms (S1) may reduce absences by 0.9–3.6 days per test for those uninfected with SARS-CoV-2 or with false-negative results; the latter may increase transmission risk to others when back at work by 0.2–189.8 infected workers per 1000 tests (depending on the rate of infection) (Figure 2). Re-testing negatives (S1C) increases the transmission risk the least (0.2–28.3 workers per 1000 tests) (Figure 2), while still reducing absence durations (0.9–1.9 days). The number of tests is the second highest (87.6–161.2 tests per 1000 workers) at an accuracy of 88.4–99.1% (Table 2).
Similarly, testing workers in household quarantine (S2) may reduce absences by 3.0–6.9 days per test but increase transmission risk to others by 0.02–49.5 workers per 1000 tests (Figure 2). Re-testing negatives (S2C) increases the transmission risk the least (0.02–4.6 workers per 1000 tests), while reducing absence durations (3.0–3.5 days per test), at 89.2–190.7 tests per 1000 workers and an accuracy of 89.0–99.9% (Table 2).
Testing all key workers (S3) may change absence durations between −0.5 and +3.6 days per test, largely due to finding asymptomatic infections and false-positive results. It changes the transmission risk between −56.9 and +0.97 workers per 1000 tests (Figure 2), with an increased risk of workplace transmission only at low sensitivity of 60% and when re-testing positives (S3B). The accuracy (87.1–99.9%) and testing needs (989.6–1995.9 tests per 1000 workers) are high.
Sensitivity Analyses
The reductions in absence were most sensitive to the test specificity (affecting false-positives) (Figure 3, the vertical dispersion of values), while transmission risk was most sensitive to the rate of infection (affecting numbers of infections) (Figure 2). The accuracy was most dependent on the positive-predictive value of testing strategies (Supplementary Table 2, Supplementary Figures 3 and 4). If the proportion of infections that are asymptomatic is higher and there are fewer symptomatic cases, then fewer individuals are expected to isolate at home even in the baseline scenario without testing, which, in turn, changes the number of staff who are being tested by the different testing strategies. This difference between testing strategies and the baseline of no-testing may lead to nonproportional changes in the efficiency of testing, and hence small variation in results even when testing all staff (Figure 2).
The sensitivity of the other parameters showed that none of the rates of illness changed results beyond the impact of the 4 key epidemiological parameters; more extreme numbers of days spent in isolation may be less plausible, but they would lead to largely different absence durations (Supplementary Figures 5–8). Our separate scenario analysis of testing the symptomatic index case in households in quarantine increases the number of tests, which decreases the magnitude of the outcomes but not their direction (Supplementary Figure 2).
DISCUSSION
Self-isolation depletes the supply of key workers essential during the COVID-19 pandemic and whose work is valued very highly [23, 24]. For example, with public services in healthcare under pressure from COVID-19 internationally, maximizing the availability of staff is critical. Our model results indicate that, as compared with no testing (S0), testing all workers (S3) may reduce the risk of workplace transmission the most by finding asymptomatic infections (particularly at an RT-PCR sensitivity of ≥65%), but with increased staff absences and greatly increased testing capacity needs. In contrast, testing workers in quarantine (S2) reduces absence durations the most by identifying those who are not SARS-CoV-2 infected, allowing some of these staff to return to work earlier than without testing but at an increased risk of workplace transmission. This risk can be mitigated but not eliminated through re-testing initially negative samples for staff in quarantine (S2C).
The optimal testing strategy will be context dependent and setting specific, and also depends on the risk of workplace exposure, the levels of infection that will differ by occupation and during the course of the epidemic, the availability of testing capacity and resources, and the relative weight given to reducing staff absences (which may be valued higher during staff shortages) and reducing transmission risk to others (which may be valued higher for frontline healthcare staff working with vulnerable individuals in hospitals and care homes). It will also differ for frontline workers in frequent contact with vulnerable individuals versus those in important supporting roles in the back office, which carry a lower risk of exposure. For instance, strategy S3 might be targeted at workers who contact vulnerable individuals such as immunocompromised patients or nursing-home residents. Weekly testing of asymptomatic workers could be considered for those who have never been tested before (where the chance of being positive equals the prevalence) or for workers who tested negative a week ago (where the chance of being positive equals the risk of being infected in the last 7 days).
Testing capacity is limited and is also required by patients. For instance, at 1 testing episode with a re-test per key worker per 3 months, testing the estimated 670 000 frontline healthcare workers in the United Kingdom [25] requires approximately 15 000 tests per day. With approximately 11.8 million healthcare workers in the European Union and the UK [26], capacity for more than 250 000 tests per day would be needed just to test healthcare staff (possibly increased 10-fold to capture all key workers essential to the pandemic response, as seen by the estimated 7.1 million key workers in total in the United Kingdom [9]). Similarly, testing the 3.8 million registered nurses and physicians in the United States alone requires capacity for more than 80 000 tests per day [27]. Given the possible shortages of laboratory supplies for extensive testing and contact tracing [11, 12], targeted testing thus remains important internationally. Testing healthcare workers will also remain a priority because, despite using personal protective equipment, (1) most frontline healthcare workers have a high rate of exposure to infection, due to patients with COVID-19 seeking care and due to staff being in close proximity to those patients and performing aerosol-generating procedures such as tracheal intubation/extubation [28, 29], and (2) infected workers are at risk of transmission to vulnerable patients (and other healthcare workers) not infected with SARS-CoV-2. However, it is important to be aware of the wide range of alternative uses for testing in the community with different resource implications—for example, screening symptomatic cases, screening their contacts, or more intensive screening in semi-enclosed settings such as care homes or prisons, etc. Quantifying these alternative uses of testing resources will be important but is outside the scope of this paper.
Strengths and Limitations
This rapid analysis was based on the known epidemiology of SARS-CoV-2 infection and international recommendations for self-isolation [1–4]. We considered a wide range of values for all input parameters and expressed the variation in results for 1000 key workers to increase generalizability of findings and to provide insights for different audiences, reflecting the uncertainty in various occupations, activities, and settings. Furthermore, test sensitivity using swabbing and RT-PCR has been reported to be as low as 71% in hospitalized patients [17]. However, SARS-CoV-2 causes both upper respiratory tract infection (URTI) and lower respiratory tract infection (LRTI); the latter causes pneumonia while the former will be more important for transmission and will be detected more reliably by swabbing. Therefore, swab sensitivity in hospitalized patients may be lower than in community cases of illness because hospitalized patients will be more likely to have pneumonia caused by LRTI, and some of them might have very little URTI. Also, hospitalization typically occurs after several days of illness, when viral shedding is declining (unless in the case of severe COVID-19) [30]. Hence, swabbing may be more sensitive for detection of community cases who are a transmission risk (ie, those with URTI) than for all cases of COVID-19. It is possible that asymptomatic infection is associated with lower viral shedding, which may reduce the sensitivity of testing but which may also reduce infectivity; further research is required.
Our analysis assumes compliance with testing and with isolation/quarantine rules. Noncompliance would reduce the beneficial effects of testing. However, noncompliance with testing of symptomatic cases would require hiding of symptoms from colleagues; noncompliance with screening of asymptomatic individuals would be even harder if all staff are required to be tested; and noncompliance with staying at home for the required isolation/quarantine period by those who test positive would be difficult if staff are not allowed to work. Noncompliance with household quarantine (ie, staying at home due to a household member’s illness) may be easier to achieve because it simply requires nondisclosure, but it may be less likely to occur with healthcare workers who are motivated to care for patients. Furthermore, people evading household quarantine and attending work would have done so in the absence of a testing strategy, and infection could be found by asymptomatic screening. Those required to stay at home may choose to leave their home for shopping, leisure, or socializing, and thus transmit infection in the community, but this is not the focus of our analysis.
We did not model transmission dynamics to calculate numbers of transmission events because this is highly context dependent and requires knowledge of transmission probabilities per contact (by type of contact), rates of contact (by type of contact), mixing patterns (ie, characteristics of individuals with whom contact is made), etc. Hence, we report on the change in transmission risk from infected workers returning to work, which we acknowledge may underestimate transmission reductions from finding asymptomatic infection and transmission increases from releasing false-negatives from isolation or household quarantine. Findings for strategy S3 could thus lead to shorter absence durations but at increased transmission risks, and additional labor shortages as a result from secondary infections of infected workers returning to work. Other benefits of testing, such as reduced anxiety [7, 13] and improved surveillance, were not explicitly included in our analysis. Additional occupational measures will also be needed to protect, maintain, and restore the physical and mental health of key workers [31, 32].
Conclusions
Our model results suggest the largest reduction of days in isolation at minimally increased transmission risk when testing key workers in household quarantine for SARS-CoV-2 infection as compared with no testing and self-isolating based on COVID-19–like symptoms alone, or if capacity allowed, testing all staff if preventing workplace transmission is paramount.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. F. G. S. and M. R. conceived the study. F. G. S. performed the analysis and wrote the first draft of the manuscript with feedback from all other authors. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication. All authors contributed to the interpretation of the data and findings, contributed to the manuscript, and approved the work for publication.
Disclaimer. The study sponsors had no role in the study design, data collection, data analysis, data interpretation, report writing, or decision to submit for publication. The views expressed are those of the authors and not necessarily those of the UK Department of Health and Social Care (DHSC), the Department for International Development (DFID), the European Union (EU), the UK Medical Research Council (MRC), the National Health Service (NHS), the National Institute for Health Research (NIHR), or Public Health England (PHE).
Financial support. This work was supported by Public Health England (PHE), which is an executive agency of the Department of Health and Social Care (DHSC). F. G. S., P. J. W., and M. J. were supported by the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Modelling and Health Economics, a partnership between PHE, Imperial College London, and the London School of Hygiene and Tropical Medicine (LSHTM) (grant number NIHR200908). P. J. W. was supported by the MRC Centre for Global Infectious Disease Analysis (grant number MR/R015600/1); this award is jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 programme supported by the European Union (EU). P. J. W. was supported by the NIHR HPRU in Modelling Methodology at Imperial College London in partnership with PHE (HPRU-2012–10080). M. J. was supported by the NIHR HPRU in Immunisation at LSHTM in partnership with PHE (grant number HPRU-2012–10096) and the European Commission (EC) Horizon 2020 Research and Innovation Programme—project EpiPose (grant number 101003688).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. Home care for patients with COVID-19 presenting with mild symptoms and management of their contacts: interim guidance, 17 March 2020.2020. Available at: https://www.who.int/publications/i/item/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts. Accessed 19 June 2020.
- 2. European Centre for Disease Prevention and Control. Technical report: contact tracing: public health management of persons, including healthcare workers, having had contact with COVID-19 cases in the European Union—second update. 8 April 2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/Contact-tracing-Public-health-management-persons-including-healthcare-workers-having-had-contact-with-COVID-19-cases-in-the-European-Union%E2%80%93second-update_0.pdf. Accessed 19 June 2020.
- 3. Public Health England. Stay at home: guidance for households with possible or confirmed coronavirus (COVID-19) infection. Updated 18 June 2020 Available at: https://www.gov.uk/government/publications/covid-19-stay-at-home-guidance/stay-at-home-guidance-for-households-with-possible-coronavirus-covid-19-infection. Accessed 19 June 2020.
- 4. Centers for Disease Control and Prevention. Social distancing, quarantine, and isolation—keep your distance to slow the spread.2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/social-distancing.html. Accessed 10 April 2020.
- 5. World Health Organization. Laboratory testing strategy recommendations for COVID-19: interim guidance, 22 March 2020 Available at: https://www.who.int/publications/i/item/laboratory-testing-strategy-recommendations-for-covid-19-interim-guidance. Accessed 19 June 2020.
- 6. Iacobucci G. Covid-19: “illogical” lack of testing is causing healthy staff to self-isolate, BMA chief warns. BMJ 2020; 368:m1277. [DOI] [PubMed] [Google Scholar]
- 7. Hunter E, Price DA, Murphy E, et al. First experience of COVID-19 screening of health-care workers in England. Lancet 2020; 395:e77–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Implementing safety practices for critical infrastructure workers who may have had exposure to a person with suspected or confirmed COVID-19.2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/critical-workers/implementing-safety-practices.html. Accessed 19 June 2020.
- 9. Institute for Fiscal Studies. Key workers: key facts and questions 2020. Available at: https://www.ifs.org.uk/publications/14763. Accessed 19 June 2020.
- 10. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 2020; 25:2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. European Centre for Disease Prevention and Control. Rapid risk assessment: coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK—seventh update: 25 March 2020. Stockholm, Sweden: European Centre for Disease Prevention and Control, 2020. [Google Scholar]
- 12. European Centre for Disease Prevention and Control. Rapid risk assessment: coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK—tenth update: 11 June 2020. Stockholm, Sweden: European Centre for Disease Prevention and Control, 2020. [Google Scholar]
- 13. Keeley AJ, Evans C, Colton H, et al. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020; 25:2000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferguson NM, Laydon D, Nedjati-Gilani G, et al. Report 9: impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand. London: Imperial College, 2020. [Google Scholar]
- 15. Cromer D, van Hoek AJ, Jit M, Edmunds WJ, Fleming D, Miller E. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect 2014; 68:363–71. [DOI] [PubMed] [Google Scholar]
- 16. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020; 25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology 2020; 296:E115–17. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Public Health England. Guidance COVID-19: laboratory investigations and sample requirements for diagnosis. Updated 28 March 2020. Available at: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-guidance-for-clinical-diagnostic-laboratories/laboratory-investigations-and-sample-requirements-for-diagnosing-and-monitoring-wn-cov-infection. Accessed 10 April 2020.
- 19. European Centre for Disease Prevention and Control. Laboratory support for COVID-19 in the EU/EEA.2020. Available at: https://www.ecdc.europa.eu/en/novel-coronavirus/laboratory-support. Accessed 31 March 2020.
- 20. Cori A, Valleron AJ, Carrat F, Scalia Tomba G, Thomas G, Boëlle PY. Estimating influenza latency and infectious period durations using viral excretion data. Epidemics 2012; 4:132–8. [DOI] [PubMed] [Google Scholar]
- 21. Zhanwei D, Xu X, Wu Y, et al. Serial interval of COVID-19 among publicly reported confirmed cases. Emerg Infect Dis J 2020; 26:1341–3. doi: 10.3201/eid2606.200357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cowling BJ, Fang VJ, Riley S, Malik Peiris JS, Leung GM. Estimation of the serial interval of influenza. Epidemiology 2009; 20:344–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sandmann FG, Robotham JV, Deeny SR, Edmunds WJ, Jit M. Estimating the opportunity costs of bed-days. Health Econ 2018; 27:592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mark K, Steel K, Stevenson J, et al. Coronavirus disease (COVID-19) community testing team in Scotland: a 14-day review, 6 to 20 February 2020. 2020; 25:2000217. doi: 10.2807/1560-7917.ES.2020.25.12.2000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. NHS Digital. NHS workforce statistics, December 2019: England and organisation 2019. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/nhs-workforce-statistics/december-2019. Accessed 19 June 2020.
- 26. European Statistical Office (Eurostat). Healthcare personnel statistics—nursing and caring professionals 2019. Available at: https://ec.europa.eu/eurostat/statistics-explained/index.php/Healthcare_personnel_statistics_-_nursing_and_caring_professionals. Accessed 19 June 2020.
- 27. US Bureau of Labor Statistics. Healthcare occupations.2020. Available at: https://www.bls.gov/ooh/healthcare/home.htm. Accessed 19 June 2020.
- 28. Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7:e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weissman DN, de Perio MA, Radonovich LJ Jr. COVID-19 and risks posed to personnel during endotracheal intubation. JAMA 2020; 323: 2027–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020; 20:656–7. doi: 10.1016/S1473-3099(20)30232-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization (WHO). Coronavirus disease (COVID-19) outbreak: Rights, roles and responsibilities of health workers, including key considerations for occupational safety and health.2020. Available at: https://www.who.int/publications/i/item/coronavirus-disease-(covid-19)-outbreak-rights-roles-and-responsibilities-of-health-workers-including-key-considerations-for-occupational-safety-and-health. Accessed 19 June 2020.
- 32. Greenberg N, Docherty M, Gnanapragasam S, Wessely S. Managing mental health challenges faced by healthcare workers during covid-19 pandemic. BMJ 2020; 368:m1211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.