Abstract
The use of telehealth to support, enhance or substitute traditional methods of delivering healthcare is becoming increasingly common in many specialties, such as stroke care, radiology and oncology. There is reason to believe that this approach remains underutilized within nephrology, which is somewhat surprising given the fact that nephrologists have always driven technological change in developing dialysis technology. Despite the obvious benefits that telehealth may provide, robust evidence remains lacking and many of the studies are anecdotal, limited to small numbers or without conclusive proof of benefit. More worryingly, quite a few studies report unexpected obstacles, pitfalls or patient dissatisfaction. However, with increasing global threats such as climate change and infectious disease, a change in approach to delivery of healthcare is needed. The current pandemic with coronavirus disease 2019 (COVID-19) has prompted the renal community to embrace telehealth to an unprecedented extent and at speed. In that sense the pandemic has already served as a disruptor, changed clinical practice and shown immense transformative potential. Here, we provide an update on current evidence and use of telehealth within various areas of nephrology globally, including the fields of dialysis, inpatient care, virtual consultation and patient empowerment. We also provide a brief primer on the use of artificial intelligence in this context and speculate about future implications. We also highlight legal aspects and pitfalls and discuss the ‘digital divide’ as a key concept that healthcare providers need to be mindful of when providing telemedicine-based approaches. Finally, we briefly discuss the immediate use of telenephrology at the onset of the COVID-19 pandemic. We hope to provide clinical nephrologists with an overview of what is currently available, as well as a glimpse into what may be expected in the future.
Keywords: CKD, dialysis, ESRD, quality of life, systematic review, technology, telemedicine, virtual consultation
INTRODUCTION
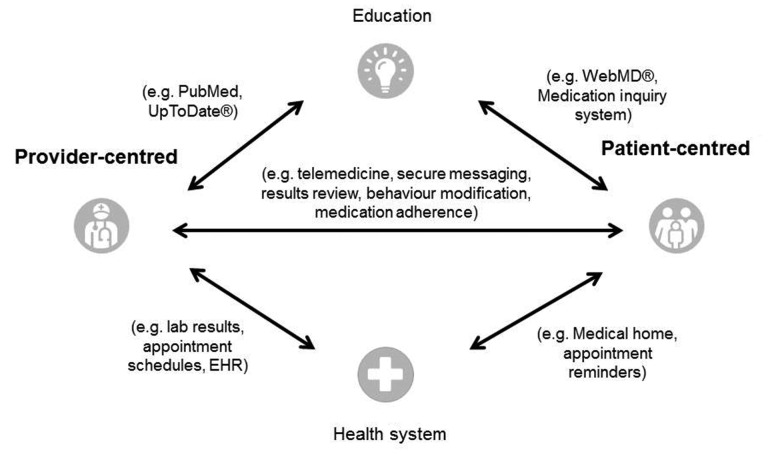
Telemedicine, a term coined in the 1970s, literally means ‘healing at a distance’, and describes the use of information technology to improve outcomes through care and patient information. Other similar terms currently in use include telehealth, which emphasizes the delivery of care outside traditional healthcare facilities, whereas eHealth focuses on information and communication. Telemedicine in a broader sense may differentiate between provider and patient-centred approaches, direct communication, education and data processing within the healthcare system (Figure 1) [1]. There is reason to believe that telemedicine is currently underutilized in our specialty [2]. Patients with significant kidney disease generally have a high burden of healthcare interactions but many of them share a desire to remain in employment and stay out of hospital. The discussion around climate change [3] and, more recently, the coronavirus disease 2019 (COVID-19) pandemic have prompted to rethink of traditional models of medicine. The latter has forced the renal community to embrace change and consider telemedicine in order to maintain patient care. Here, we provide a narrative review and an update on telemedicine in nephrology. Due to the limited number of large-scale studies, we have decided against a meta-analysis of published research on this topic, instead focusing on adult renal medicine with particular attention to applications for clinicians such as dialysis, inpatient care, virtual consultation and patient empowerment. We also review pitfalls and discuss potential future avenues of research. Our aim is to provide practicing nephrologists and allied healthcare professionals with an overview of what is currently possible and where the field may be heading in the next decade.
FIGURE 1.
Taxonomy of telemedicine [1].
DIALYSIS
Home dialysis has clear advantages over in-centre treatment in terms of flexibility, quality of life and cost [4], while a survival benefit remains difficult to prove [5]. Furthermore, ‘low-tech’ home therapies such as peritoneal dialysis (PD) are promising approaches to overcome the increasing discrepancy between patients requiring dialysis (14.5 million) and those receiving dialysis (5.4 million) worldwide in the next 10 years [6]. Barriers that may prevent a more widespread uptake of home therapy include patient-related factors such as lack of confidence and the perception of isolation, as well as socio-economic factors [7]. Geographical isolation is also a concern regarding therapy. As an example, Tonelli et al. described increased complications with PD if patients lived >50 km away from the renal centre [8]. This observation fits in with more recent data that describe distance to healthcare as a risk factor in patients with renal failure [9], although the precise nature of the association remains unclear. In theory, telemedicine should have clear advantages in dialysis (Table 1). Telemedicine is also seen as a tool to overcome distance: a collaborative project run by the Implementing Transnational Telemedicine Solutions team aims to increase access for geographically remote populations in Northern Europe by implementing telemedicine in a sustainable way [18].
Table 1.
Advantages of telemedicine use in dialysis [ 10–17]
Patient-related
|
For the health economy
|
For climate/environment
|
Peritoneal dialysis
Current evidence concerning the use of telemedicine in PD relates to either the use of remote biometric monitoring (RBM), with or without the addition of videoconferencing, or other bidirectional communication [19, 20]. The data collected by RBM remain variable, with most centres using a combination of blood pressure, weight, ultrafiltration volumes or dialysis exchanges. Breaches or predetermined trends can be flagged up and acted on accordingly, either by a telephone call, videoconference or an in-person visit. Recently, the introduction of a system allowing remote access to an automated PD (APD) system with little involvement on the side of the patient has fostered the use of telemedicine in PD [21, 22]. While the advantages seem obvious, studies with objective outcomes are few and often hampered by small sample size, heterogeneous populations or other study limitations. Some authors reported reduced unplanned hospital and emergency room visits with an associated reduction in utilization and cost of health resources, but no change in overall hospitalization rates [23, 24]. Wallace et al. describe a system for automatic collection of PD parameters and the benefits that this incurs, namely monitoring for and early detection of problems such as technique failure, non-adherence and presentation of factitious data at in-person review [10]. They also suggest that automatic monitoring of PD can help avoid logistical problems such as those related to last-minute provision of low supplies. In addition, it may enable ‘marginal’ patients to continue PD at home via either increased monitoring or early detection of problems preventing technique failure and modality switch [10]. These benefits however, were not quantified, and the authors emphasize that their service was not available at all times and did not replace the need to seek help in acute illness [10].
Overall, evidence suggests a positive patient experience when telemedicine is used within PD [25, 26]. Common themes identified are increased autonomy [26], reduced hospital visits (saving on travel, cost and time) [11, 26, 27], increased patient satisfaction compared with phone contact [26], increased confidence [26] and feeling of increased safety [11], decreased perception of ‘being a burden’ [26] and enabled more time for life [26]. Objectively, changes to quality of life scores have been inconsistent, with mainly similar [25, 27–29], but occasionally improved [20], scores reported.
Agarwal and Wilkie [30] noted that the use of telemedicine in PD has challenges as well: patients may perceive the technology as intrusive, worry about data security or miss the direct contact with healthcare providers. Some have reported low voluntary uptake of telehealth [26, 29]. The assumption that all patients on home dialysis are automatically ideal candidates for telemedicine due to their use of technology to deliver their treatment may also be premature: a Norwegian group assessed the perceived potential of telemedicine support for a small number of PD and home haemodialysis (HHD) patients [11] and found that those patients who used machinery to delivery their dialysis, i.e. HHD and APD, were receptive to the idea of using telemedicine, whereas those patients performing continuous ambulatory PD (CAPD) were not [11]. Preference for continuing with traditional rather than tablet-based recording of exchange information has also been reported in CAPD patients [31].
HHD
Studies concerning telehealth outcomes in HHD are even fewer, with most publications exploring perceptions and acceptability rather than objective outcomes [11, 12, 32–37]. These studies describe positive patient experience, with improved adherence and confidence [12, 32, 36], time and financial savings [11, 37] and reassurance to both patients and carers mainly to address acute problems on HHD [11, 32, 35, 36]. Positive staff experience has also been reported [12, 34]. Benefits of telemonitoring and RBM have been reported for home training, in terms of both reduced time and improved confidence transitioning home [32, 35, 38, 39] and reducing technique failure [39] (Figure 2). Regarding other objective measures, only one study has reported an increased frequency of HHD prescription change through use of an app [37]. However, negative experience has also been reported. Of note, since its conception in the mid-1990s, one of the initial pioneers of real-time telemonitoring on nocturnal HHD [40–42] discontinued its use in 2012 [38]. The authors argue that, while telemonitoring was initially perceived as useful and safe, patient reassurance and compliance monitoring can be better met through other means [38]. Therefore as with PD, much more evaluation and evidence is required.
FIGURE 2.

Telemedicine in HHD: nurse providing instructions and observing patient setting up dialysis at home (patient consent provided).
Recently, in Canada, the development of a virtual ward to address gaps in care that are often present after a dialysis patient is discharged from hospital, or has another such change in care, has been reported [43]. Through the use of the virtual ward, 67% of HHD patients were found to have a gap in care; however, the presence of care gaps was not found to be related to other secondary adverse outcomes such as readmission to hospital. The study did not look at whether the use of a virtual ward resulted in a reduction of these secondary outcomes, but was proven to be a feasible and practical intervention [43]. A large prospective follow-up trial assessing the impact of the virtual ward for both PD and HHD patients after discharge has been designed and is currently underway [44].
In-centre haemodialysis
Many of the perceived benefits of telemedicine for dialysis patients have been for those undertaking home therapies. However, few studies have examined the role this can play for patients undergoing in-centre haemodialysis (ICHD). These have looked at either telemedical interventions on non-dialysis days, such as RBM, questionnaires and reminders [45–47], or during dialysis itself, such as videoconferencing, virtual rounds or real-time measurement of dialysis variables remotely [48–51]. As with PD, results have been variable. Sicotte et al. reported the use of two different telehealth strategies in the Canadian First Nations, and found no difference between the two modes, supporting the suggestion that telehealth can be tailored to the needs and preferences of the individual or population [48]. However, tensions among staff have been reported [49].
Due to the open nature of most ICHD units, the issue of privacy and confidentiality during videoconferencing has been raised. Whitten et al. found that patients again had overall positive perceptions of telehealth, and moreover did not feel it limited their privacy, however they were uncertain whether they would rather be seen in person or perhaps utilize telehealth only when this was not possible. These sentiments were echoed not only by staff, who had a mixed perception of when it was suitable to use [13], but also in a further study where only 45.5% of patients were satisfied with self-monitoring compared with 100% with nurse involvement [14]. As with PD and HHD, however, improved self-awareness, self-management and self-efficacy have been reported, despite technical factors, memory and lethargy being highlighted as barriers to use [14].
The advantages of telemedicine in dialysis patients seem obvious and patient experience is generally positive. These sentiments are echoed in other specialities where telehealth has been used more widely, for example, telehealth-supported thrombolysis in acute stroke having comparable clinical outcomes or supervising provision of chemotherapy to remote oncology patients [52]. There is also very little evidence of real risk or harm and in a meta-analysis of telemedicine in chronic conditions, Hanlon et al. concluded that this approach is generally safe [53]. However, the authors also emphasized that telehealth-mediated self-management was not consistently superior to usual care [53]. As far as the use of telemedicine in dialysis is concerned, evidence of true benefit with regards to clinical outcomes, cost or resource utilization is equally difficult to find. What one would like to see is more studies demonstrating not just patient satisfaction but also evidence of real benefit. There are good examples of such studies in other specialties, for example cardiology. A 2017 meta-analysis concluded that telemedicine reduced admission, shortened length of stay and reduced mortality in patients with congestive heart failure [54]. Further research is required in the field of telenephrology and dialysis, with upcoming trials such as Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision (CONNECT) [55] hopefully gleaning more evidence on objective outcomes to support this ever-growing field.
INPATIENT CARE AND IN-REACH CONSULTATION
In most countries, inpatient nephrology will only be provided in larger centres with some degree of in-reach into smaller surrounding hospitals and provision of dialysis care in satellite units. Northern Canada is often used to illustrate a challenging geography where the most remote dialysis satellite unit can be 750 km away from the regional centre [56]. Our own department in the northwest of the UK is slightly less challenged, but the distance to our most remote satellite hospital is still 100 km. Apart from the geography, our ability to in-reach is also hampered by the fact that the hospitals operate different IT systems, the lack of synchronization between our availability and that of our counterparts locally, and finally the challenge to assess from a distance whether a patient is well enough to be transferred.
The advantages of telenephrology in this scenario are quite obvious, and it is surprising that not much evidence exists in our specialty when compared with, for example, stroke medicine [57, 58]. Virtual inpatient consultations can also provide the patient with an opportunity to speak with the specialist, which may enhance the discussion and also allow the local teams to seek advice. On occasion, such a discussion may even avoid the need for the patient to transfer to the tertiary centre. Intuitive as the concept may be, the evidence to support the concept is currently mostly lacking, not only in terms of efficacy but also with regard to cost-effectiveness and governance.
Barriers also exist, in particular relating to organizational, technical and economic/regulatory forces [55], and developing a fully fledged virtual in-reach service with video dialogue will be a challenge for many. However, virtual in-reach consultations do not necessarily have to involve visual contact or voice; in our practice, we have gained read and write access to a neighbouring hospital’s electronic health record (EHR) so that we can review results, observations and medication, and read ward round entries. We document our advice in a ‘virtual ward round’, which our colleagues locally will see with immediate effect (Figure 3). Others have described a similar approach not to overcome distance but to address workload when providing in-reach advice on site but without the need to see the patient [59].
FIGURE 3.
‘Virtual ward round’ for a fictitious inpatient at Furness General Hospital, Barrow-in-Furness, UK. The clinician at the renal centre reviews all patient data and writes an entry directly into the EHR. Panel A: Fluid balance and ward round documentation by parent team locally. Panel B: Virtual nephrology consultation documented remotely. Not shown are medication, vital signs, and laboratory/imaging results, which are also accessible during the remote consultation. The distance between the renal centre and the satellite hospital is 64 miles (103 km) or 90 min by car; the satellite hospital has face-to-face inpatient care once a week in conjunction with an outpatient clinic there. With kind permission from Melanie Waszkiel and Dr Colin Brown, University Hospitals of Morecambe Bay NHS Foundation Trust, Kendal, UK.
VIRTUAL CONSULTATION FOR NON-DIALYSIS PATIENTS
One reason for the success of virtual clinics for home dialysis patients is that these patients are per se younger, proactive and usually IT literate. However, rolling this approach out to a population of patients attending general nephrology outpatient clinics can be more difficult. Virtual clinics for the triage and management of patients with chronic kidney disease (CKD) and for providing remote advice have been well described [60–67]. Two UK-based studies have implemented virtual clinics for the follow-up of patients with moderate CKD. Overall survival rates were higher in patients managed in the virtual nephrology clinic compared with those discharged to primary care follow-up [68]. Interestingly, of those patients requiring initiation of renal replacement, none was started in an emergency setting, and rates of definitive dialysis access were higher than regional and national figures [68]. A London group reported reduced waiting times from 64 to 5–10 days, with <15% of referrals requiring a face-to-face review [69]. Promising data have also emerged from Australia, where the use of a virtual clinic has been described as safe and efficient [60].
Others have described the use of virtual clinics for transplant assessment with improved waiting times and significant time and financial savings [70, 71], as well as for transplant aftercare [72–75]. The latter may be another very suitable use of such technology, again because the transplant population is on average younger and IT literate. The authors also emphasized the importance of involving patients in such service redesign and reported substantial cost savings [72] as has been described elsewhere [76]. Transitional care following renal transplantation is another very attractive use for telemedicine that is currently under investigation by a large German study [77]. It will be very interesting to see whether the intervention, which includes two smartphone apps, truly improves adherence and outcomes in this vulnerable population. Acceptability and patient perspectives on virtual clinics are overall positive for both CKD [60, 64, 65, 69, 78, 79] and transplant patients [75, 80], with the notable exceptions of what is lost from lack of face-to-face contact in terms of non-verbal communication or when faced with an acutely ill patient [65, 81].
It is worthwhile noting that even in the setting of a dedicated study as few as 12% of the referrals may be suitable for virtual clinics [67]. More worryingly, studies conducted in a real-life setting often fail to demonstrate a clear benefit of telemedicine over traditional CKD management and referral systems. A study in the Netherlands looked at the use of telenephrology in the management of CKD across 47 general practices [82]. Primary care providers reported a positive experience, but evidence of a clear-cut benefit was lacking. Any such approach also requires access to primary care records [83], which can be difficult to obtain. The cost aspect of this approach also deserves consideration. Some authors describe considerable savings as high as £111.56 per patient attendance [67]. Our own experience trying to argue the case of savings has been less than straightforward, mainly because in the UK a national tariff for a virtual clinic encounter does not exist. The general issues around reimbursement for telemedicine in Europe [84] and the USA [85] have been discussed elsewhere. Recent political developments in USA are also noteworthy: The Bipartisan Budget Act of 2018 removed restrictions based on geographical location, enabling telemedicine to be available to far greater numbers of patients than before [19]. Protagonists and supporters of this approach hope that it will reduce costs and also provide better care [86]. This trend could well have an effect on legislation in the rest of the world as well.
Providers should consider cost early on and agree a sustainable funding. Cost is also a key consideration for the use of telemedicine in developing countries [87], where this approach appears attractive to address the huge workforce shortage and inequity of service provision [88]. Whether telemedicine is actually beneficial and cost-effective in this scenario remain unclear [87].
PATIENT PORTALS AND mHEALTH
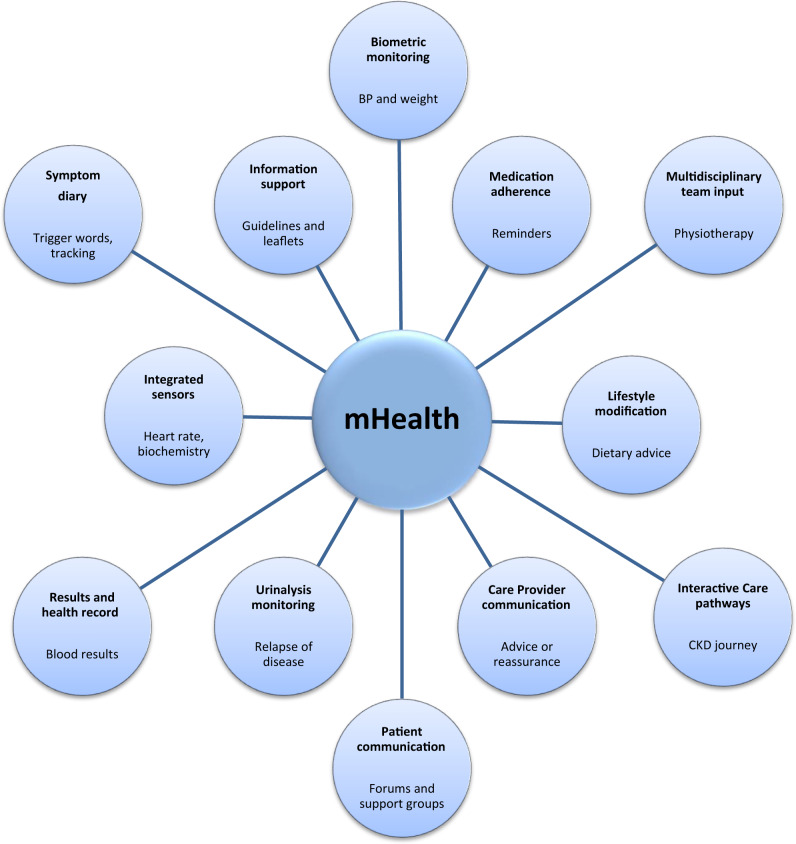
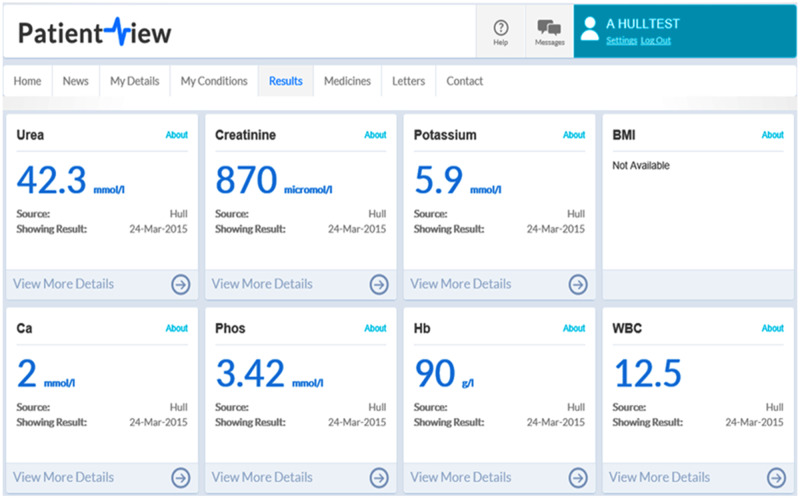
An increasing number of resources are designed around patient information, engagement and empowerment (see Figure 4). A good example from the UK is RenalPatientView™, a web-based system that grants patients access to their laboratory results (Figure 5). The aims of this particular portal are to encourage patients to engage with their health issues and give them a sense of ownership, as discussed in this journal recently [89]. Typically, such portals will also provide patient information, for example, around laboratory results and normal ranges. More recently, such portals have been equipped with added interactivity, that is contact with a physician for advice [89].
FIGURE 4.
Schematic illustrating range of patient portals and mobile applications available, with examples of potential uses underneath.
FIGURE 5.
RenalPatientView™—screenshot with laboratory results (fictitious patient) from [86] (open access licence).
In comparison with RenalPatientView™, which is a technology originally developed for desktop computers, mHealth describes the use of mobile devices for similar purposes. Ideally, mHealth addresses the patients and relatives’ need for communication and also enables chronically ill patients to have access to the information relevant to them at the right time (‘small data principle’) or on the go. Some studies suggest that interactive therapy plans can promote adherence [90] and improve safety [91]. A good example of mHealth in our specialty is the use of smartphone-based apps such as Transplant Hero™ to remind transplant patients of their immunosuppressive medication and thus improve adherence [92].
More advanced platforms can also promote healthy lifestyle [93], provide patients with tailored information about their care pathway and more. A commercial provider in Germany has developed a more sophisticated approach [94] that is based on the MyTherapy app [95]. The patient has the option to share certain information, such as medication or vital parameters, with their care team so that they can intervene early. A Japanese study looked at the use of a smart phone app as a way of facilitating patients’ engagement with issues such as dry weight targets and diet and demonstrated improved quality of life [96]. Patient satisfaction was particularly demonstrated in a retrospective paediatric study that looked at the parents of paediatric renal patients when having access to an online resource in which they could seek advice from a specialist. Over 90% of the responders would recommend the resource and felt they trusted the online consultation [96].
Importantly, the patient experience with apps is not always positive. A recent contribution by a patient emphasized that these apps are often clunky and poorly designed [97]. Moreover, ratings by physicians do not often correlate with patients’ experience [98] and anybody seeking to embark on a new mHealth project is probably well advised to seek patient involvement from the start [97]. It is also clear that apps are not universally trustworthy when it comes to the information provided [99], which raises the question of whether the renal community should perhaps consider ways to signpost reliable apps [100].
ARTIFICIAL INTELLIGENCE
Artificial intelligence (AI) is often defined as the use of intelligent, non-human agents that perceive their environment and take actions to maximize its chance of successfully achieving its goals. It is becoming one of the biggest areas of development engineering in healthcare and our specialty is no exception [101]. In particular, the continuing euphoria and success of Deep Learning are raising high expectations. Deep Learning is a special form of machine learning in which artificial deep neural networks inspired by the human brain are used and trained on huge amounts of data to identify patterns. Cardiologists have recently described Deep Learning for automated identification of atrial fibrillation based on patient videos [102]. Another good example is the use of Deep Learning in imaging. Sharma et al. show that such an approach can be used to calculate the kidney volume of autosomal dominant polycystic kidney disease (ADPKD) patients with a high degree of certainty [103]. The use of AI in nephropathology is also conceivable [104, 105]. Deep Learning through analysis of historical patient data also offers possibilities for decision support or the identification of high-risk patients. Esteban et al. for example, used Deep Learning with a set of data from the Charite Hospitals renal transplant database to develop an algorithm to predict clinical events [106].
Others have described the use of AI in haemodialysis patients mainly to determine target weight and dialysis prescription. The benefit of AI in this setting is that the machines can respond in real-time to the changes in a patient’s homoeostasis and aim to reduce the delay in response thus preventing episodes of symptomatic intradialytic hypotension or large variations in ultrafiltration [107]. These systems also allow for continuous changes in dialysis prescriptions and generate data that can be useful in aiding predictions on prognosis or cardiovascular risks. Further applications of AI include renal anaemia, and the ongoing ANEMEX trial (ClinicalTrials.gov Identifier: NCT03214627) uses AI to develop an algorithm that makes recommendations on erythropoietin dosing. The algorithm generates recommendations based on the information acquired when analysing previous medication lists and doses, demographics and recent investigations [108].
Natural language processing is another useful application of AI. Texts can describe a lot of information and relevant findings, be it in the form of biomedical publications, in doctor’s letters in hospitals or in medical forums. In order to access this data efficiently and automatically, approaches from natural language processing are often used. The technology can access text but also spoken language. Possible uses include extracting new histories from EHRs, trawling social media for possible side effects of new medication or finding warning signals in patient diaries. Especially in clinical everyday life, methods from speech processing can be used to access historical text data more easily, to generate cohorts, to summarize patient histories or to collect relevant information in order to incorporate it into prediction models with structured information (e.g. laboratory values, vital parameters). An interesting approach that combines big data analysis and natural speech has been tested on a cohort of patients with rare diseases [109]. In many cases, the system was able to detect and suggest the correct diagnosis early in the course of the disease on the basis of the symptoms [109]. Through machine learning and multiple closed-feedback loops, the system becomes more intelligent with each patient contact assess. A similar system could be useful to support decision making in rare glomerular diseases, inherited renal disease or vasculitis/multisystem disease.
THE DIGITAL DIVIDE, LEGAL ASPECTS AND OTHER BARRIERS AND PITFALLS
Telemedicine has pitfalls beyond the lack of robust evidence [110], in particular around the ‘digital divide’ [111]. This term was originally used to describe differences in Internet ‘access’ between urban, educated and wealthy patients on one side and underserved populations on the other [112]. More recently, this concept has also included eHealth literacy [113, 114]. All attempts to empower patients by digital means will only ever reach a part of the population that is already quite well-activated and engaged, whereas another substantial part of the patient population (the elderly, non-users of the internet, the less well-off) is essentially ignored. Several approaches have been suggested to address and overcome this divide, such as community-based education, focusing on underserved populations or using primary care to support patients with low eHealth literacy [111].
Another concern is around legislation and confidentiality. There is reason to believe that our renal patients may worry less about this topic than those in other specialties [115], and perhaps also less than their physicians. However, legal requirements around data confidentiality have increased in recent years and clinicians need to be mindful of this risk. As an example, earlier this year, the UK information commissioner issued a £183 million fine against British Airways for a significant data breach [116].
Other barriers and pitfalls also need to be considered. Agarwal and Wilkie also note potential challenges for providers, such as the need to manage change, find additional resources and generate evidence of benefit that is required for funding [30]. Other concerns include the volume of data presented could be too large to analyse, and lead to ‘fatigue’ among providers [10]. One study reports that 47% of alerts generated required an intervention [25].
Others have emphasized that the balance between face-to-face and virtual visits is delicate [117]. Telemedicine also has potentially significant effects on the doctor–patient relationship [118]. Finally, there is the question of accountability: what if, say, a significant laboratory result has slipped through the safety net of a virtual approach or a patient has underestimated the significance of a new symptom during a long period of unsupervised or virtual follow-up? It is probably safe to say that robust evaluation will be crucial to the success of any such approach, coupled with a degree of vigilance for unexpected side effects. Table 2 summarizes potential barriers and pitfalls. Table 3 provides a glossary of terms.
Table 2.
Barriers to telemedicine [ 10–17]
Patient-related
|
Physician-related
|
Related to law, governance and infrastructure
|
Table 3.
Glossary
| AI: Computer systems that can perform tasks that would usually require human intelligence, and are able to ‘think’ and ‘learn’ in order to do so. |
| Bidirectional communication: Any means of communication in which two or more parties communicate together at the same time. For example, telephone call or videoconferencing. |
| Deep Learning: Specifically relates to the use of artificial neural networks, which are computerized networks that mimic the neural networks in the human brain. It enables AI to ‘learn’ as a human brain would, and is a subset of machine learning, see below. |
| Digital divide: Traditionally used to describe the gap between those who do and do not have adequate access to information and communication technology. Can now also be used to include those who may have access, but are less capable of using these technologies, that is, those who are less eHealth literate, see below. |
| e-Alert: An alert that is automatically generated by a machine, without the need for a person to review data. |
| eHealth: Healthcare that is supported by electronic systems and processes. For example, an EHR, or automatically generated reminders. |
| eHealth literacy: The ability to obtain, understand and use healthcare information through electronic means. |
| EHR: A digital database containing a breadth of information regarding a patient or a certain population. Typically includes patient demographics, medication and allergy lists, medical notes, laboratory or imaging results and physiological parameters, amongst others. It may be accessible to a variety of different healthcare providers, depending on local arrangements. |
| Machine learning: A process used in AI where computer programmes or algorithms automatically improve through experience and repeated exposure. Linked the deep learning, which mimics the neural structures of the human brain, see above. |
| mHealth: Healthcare that is provided through a mobile device, such as a mobile phone or tablet. |
| Natural language processing: How computers can process and analyse human (natural) language. For example, speech recognition. |
| Patient portal: A secure, online platform (for example, a website or an application) through which a patient can access their personal healthcare information. There may also be the option of communicating with their healthcare provider. |
| RBM: The measurement and electronic recording of various parameters, which is done with the patient away from the usual clinical setting where this would take place, that is, remotely. Biometrics can include a variety of measurements that the patient can take themselves, for example blood pressure and weight, or that are automatically collected by the machine, for example ultrafiltration volumes in PD. |
| Telehealth: Healthcare that is provided remotely through the use of information and communication technology, with the patient being located at a different place to the healthcare provider. It encompasses not only diagnosis, treatment, monitoring and prevention of disease, but also education, research and continued service development. |
| Telemedicine: Often used synonymously with telehealth, however can be used to describe the provision of care via only medical physicians, as opposed to other allied healthcare professions. |
| Telemonitoring: The process of using technology to monitor a patient remotely, using audio, video, sensors, electronic data or a combination of any of the above. |
| Telenephrology: The use of telehealth, or telemedicine, specifically within the field of nephrology. |
| Videoconferencing: A form of communication that uses both audio (via microphone, for the transmission of sound or voice) and video (via camera, for the transmission of real-time picture) at the same time, enabling both users to see and hear the other party. |
| Virtual: Something that can be done or simulated using a computer, without the need for a physical presence in that location. Examples include virtual consultations, virtual ward rounds, virtual in-reach and virtual clinics, which can all be done using various electronic means away from the usual place they occur. |
Telemedicine during the COVID-19 pandemic
Very recently, COVID-19 [119] has demonstrated another unique feature of telemedicine to the renal community, namely to enable ongoing care when traditional forms of healthcare are temporarily no longer available. In our own practice, we were positively surprised by how something we have always aspired to could be implemented within days, and with good patient feedback. In addition to transitioning almost all clinics to videoconferencing or telephone services, and expanding our home therapy capacity, we have also introduced smartphone technology to remotely assess urine dipstick results (Figure 6) [120]. Telemedicine has not only enabled us to keep patients out of a high-risk hospital setting but also allowed us to provide reassurance to anxious patients who are in self-isolation far away. However, we have also encountered limitations, particularly in older patients and others who are less familiar with technology. We have also learned that with current UK tariff structures, our approach is financially ruinous and unsustainable due to a difference between face-to-face clinics and a virtual consultation of around £250 per patient. Irrespective of such concerns, it is clear from our interaction with patients in the last couple of weeks that this development will be irreversible after the crisis: most patients will not return to previous rituals of commuting to clinic appointments. We can only hope that patient groups and other stakeholders will lobby the government to change tariffs and make this approach viable in the mid- to long-term.
FIGURE 6.

Smartphone technology to remotely monitor urine dipstick results [120] to diagnose urinary tract infection or monitor proteinuria. healthy.io, Tel Aviv, Israel, with permission. The kit comprises a beaker, a solitary urine dipstick and a colour chart. Patients also receive the link to an app via text message that takes them through the process and uses the smartphone camera to assess the dipstick result. The result is uploaded to a secure web platform and the requesting clinician is notified [120].
FUTURE USE OF TELEMEDICINE IN NEPHROLOGY
It is likely that the use of smartphone mobile apps to enhance patient autonomy and self-management (mHealth) will increase further within the specialty, for example in renal transplant recipients [121]. We speculate that within a decade, many or most renal patients in the developed world will have access to such technology, with access to their laboratory results and clinic letters, often with an option for communication with healthcare providers. It is also likely that commercial providers will develop an interest in such technology and at some stage healthcare providers will have to be reimbursed for providing advice to their patients through such technology. There are also implications for education in that we need to train up a workforce that has more advanced information technology skills than ever before [122].
The use of telemedicine is also likely to grow in the and at pace acute hospital setting, accelerated by the ongoing COVID-19 pandemic, concerns regarding climate change and workload pressures overall. In 2014, National Health Service (NHS) England outlined a requirement for all acute NHS hospitals in the UK to develop automated acute kidney injury (AKI) alerts [123]. In response to this a London group, in collaboration with DeepMind Technologies Ltd (London, UK), developed the Streams app: a ‘mobile AKI detection and management application’. Despite a significant reduction in the number of unrecognized AKI episodes and time to recognition within the emergency department, there were no differences in renal recovery [124]. Other e-alert systems have also shown variable results, with no improvement in clinical outcomes in AKI seen in the USA (alerts via text message) [125], improved diagnosis and nephrologist review but limited translated clinical effects in China (e-alerts to physician workstation) [126] and Korea (automated nephrologist consultation generated) [127], and numerous improved clinical outcomes in the UK [128]. We speculate that within the next decade most hospitals in the developed world will have some form of AKI alert system and that the nephrologists of the future and their teams will spend more time assessing patients highlighted in this way.
Wearable technology in combination with remote monitoring is surely another field with potential growth [129]. Of note, wearable devices for haemodialysis [130] and, more recently, PD [131] have been described and reviewed elsewhere [132] (Figure 7). We speculate that within the next decade such devices will become safe and established options for some patients and that they will become smaller and potentially implantable [132]. They could be linked to wearable sensors to monitor renal function [133], calcium or pH [134] and other parameters. Assessment of oedema (SmartSock™) has been described [135], as well as other sensors such as wireless detection of blood leaks for HHD [136]. Outside of nephrology, recent research has demonstrated the ability of a smartwatch to detect atrial fibrillation, with no app-related adverse events reported [137]. Such technology could be extrapolated to nephrology, for example a detected decrease in oxygen saturations coupled with increasing weight or oedema triggering increased ultrafiltration volumes. The use of such wearables in dialysis patients has been reviewed recently [138]. The combination of wearable technology with mHealth apps is also tempting. As an example, others have speculated that an app could integrate data from heart rate and blood pressure measurement and the word ‘dizzy’ in its patient diary to suggest a change in antihypertensive medication [100]. We believe that the current COVID-19 pandemic will act as a proof of concept for some of these applications and that some of these systems will influence care in selected patients such as those with nephrotic syndrome within the next 5 years.
FIGURE 7.
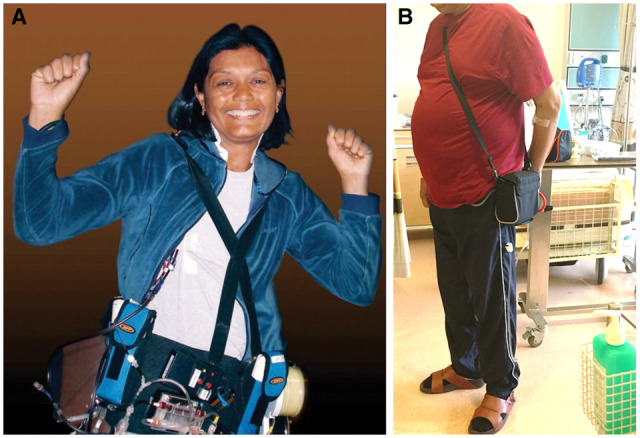
(A) Wearable haemodialysis device. Courtesy of Dr Victor Gura, Cedars Sinai Medical Center of Medicine at UCLA, Beverley Hills, USA. (B) Automated Wearable Artificial Kidney (AWAK) device for PD. Courtesy of Dr Marjorie Foo, Senior Consultant/Head. Director of SGH-Peritoneal Dialysis Program, Department of Renal Medicine, Singapore General Hospital.
AI will, we believe, be another field with significant growth. An AI approach to predicting AKI has been described recently [139]. This opens up the possibilities and implications of using AI within nephrology further. Examples include the above-mentioned changes in biometric measurements resulting in an automatic alteration to dialysis prescription coupled with machine learning regarding the patient’s physiological ‘norm’, alterations in laboratory or physiological parameters leading to an earlier (or later) than planned outpatient appointment or use of AI for the triage and management of AKI. We also view screening for CKD in the community as a potential use of AI and we speculate that within the next decade many renal centres in the developed world will use AI for this purpose.
CONCLUSION
A decade ago, one of us concluded an article on the Internet and Nephrology in this journal with Bob Dylan’s notion that ‘times they are a changing’ [140]. In hindsight, we underestimated just how much mobile technology would change our specialty. Ten years later, we have again underestimated the pace of change: in our initial version of this article in late January 2020, we stated that ‘the next decade will undoubtedly involve even more change to the way we go about our daily work’. We could not have been more wrong: Telemedicine has become a reality as a result of the pandemic, and at pace: During the week preceding this resubmission, we have already carried out numerous virtual consultations, assessed patients' general appearance and peripheral oedema via a smartphone camera and used an app to analyse urine dipsticks remotely. We speculate that the change brought about by the COVID-19 pandemic [141] as well as stark choices around climate change [142] will force rapid change away from the traditional model of care whereby most patients commute to renal centres by means of fossil-fuelled transport and return home often with no change of treatment and no real benefit other than ‘reassurance’. Our patients have made it abundantly clear already that they will not return to this model even after the pandemic has ended. We therefore believe that within a year most nephrologists in developed countries will have some form of telemedicine in their portfolio. Patients and their self-help groups will help overcome barriers, be they regulatory or financial. We acknowledge the scepticism within the renal community as evidenced in a recent EDTA survey [143], as well as the fact that the true potential of AI remains difficult to gauge [144]. A glaring oversight in our 2009 contribution was to ignore the digital divide as a potential issue. We applaud all enthusiasm for telemedicine within the specialty but we must also ensure that we cater for our population as a whole irrespective of access to the Internet or eHealth literacy. Finally, it is sobering that a worldwide pandemic was required to question regulatory red tape [145], overcome real and perceived obstacles, and enable substantial change. In that sense, the ‘cloud’ now has a silver lining, remains full of opportunities and is also a lot closer than anticipated.
CONFLICT OF INTEREST STATEMENT
A.W. is the clinical lead for a project with healthy.io™ around the use of mobile technology for the remote interpretation of urine dipsticks, which started during the COVID-19 pandemic in late March 2020. He has no contract with the company, received no speaker fees and has no shares in the company, nor any other link. The remaining authors declare no conflict of interest.
REFERENCES
- 1. Diamantidis CJ, Becker S. Health information technology (IT) to improve the care of patients with chronic kidney disease (CKD). BMC Nephrol 2014; 15: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hailey D. Telehealth in nephrology care 2014: Promises and challenges. Am J Kidney Dis 2016; 68: 5–7 [DOI] [PubMed] [Google Scholar]
- 3. Horton R. Offline: extinction or rebellion? Lancet 2019; 394: 1216. [DOI] [PubMed] [Google Scholar]
- 4. Vinson AJ, Perl J, Tennankore KK. Survival comparisons of home dialysis versus in-center hemodialysis: A narrative review. Can J Kidney Health Dis 2019; 6:205435811986194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Korevaar JC, Feith GW, Dekker FW et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int 2003; 64: 2222–2228 [DOI] [PubMed] [Google Scholar]
- 6.N.N. Global Kidney Health Atlas. 2020. https://www.theisn.org/all-articles/665-global-kidney-health-atlas (28 January 2020, date last accessed)
- 7. Chan CT, Wallace E, Golper TA et al. Exploring barriers and potential solutions in home dialysis: An NKF-KDOQI Conference Outcomes Report. Am J Kidney Dis 2019; 73: 363–371 [DOI] [PubMed] [Google Scholar]
- 8. Tonelli M, Hemmelgarn B, Culleton B . et al. Mortality of Canadians treated by peritoneal dialysis in remote locations. Kidney Int 2007; 72: 1023–1028 [DOI] [PubMed] [Google Scholar]
- 9. Kosnik MB, Reif DM, Lobdell DT et al. Associations between access to healthcare, environmental quality, and end-stage renal disease survival time: Proportional-hazards models of over 1,000,000 people over 14 years. PLoS One 2019; 14: e0214094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallace EL, Rosner MH, Alscher MD et al. Remote patient management for home dialysis patients. Kidney Int Rep 2017; 2: 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rygh E, Arild E, Johnsen E et al. Choosing to live with home dialysis-patients’ experiences and potential for telemedicine support: a qualitative study. BMC Nephrol 2012; 13: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ditchburn JL, Marshall A. Renal telemedicine through video-as-a-service delivered to patients on home dialysis: A qualitative study on the renal care team members’ experience. J Ren Care 2017; 43: 175–182 [DOI] [PubMed] [Google Scholar]
- 13. Whitten P, Buis L. Use of telemedicine for haemodialysis: perceptions of patients and health-care providers, and clinical effects. J Telemed Telecare 2008; 14: 75–78 [DOI] [PubMed] [Google Scholar]
- 14. Minatodani DE, Chao PJ, Berman SJ. Home telehealth: Facilitators, barriers, and impact of nurse support among high-risk dialysis patients. Telemed J e-Health 2013; 19: 573–578 [DOI] [PubMed] [Google Scholar]
- 15. Lew SQ, Sikka N, Thompson C et al. Adoption of telehealth: Remote biometric monitoring among peritoneal dialysis patients in the United States. Perit Dial Int 2017; 37: 576–578 [DOI] [PubMed] [Google Scholar]
- 16. Lew SQ, Sikka N, Thompson C et al. Impact of remote biometric monitoring on cost and hospitalization outcomes in peritoneal dialysis. J Telemed Telecare 2019; 25: 581–586 [DOI] [PubMed] [Google Scholar]
- 17. Rosner MH, Ronco C. Remote monitoring for continuous peritoneal dialysis. Contrib Nephrol 2012; 178: 68–73 [DOI] [PubMed] [Google Scholar]
- 18. Casey M, Hayes PS, Heaney D et al. Implementing transnational telemedicine solutions: a connected health project in rural and remote areas of six Northern Periphery countries series on European collaborative projects. Eur J Gen Pract 2013; 19: 52–58 [DOI] [PubMed] [Google Scholar]
- 19. Lew SQ. Telehealth in peritoneal dialysis: Review of patient management. Adv Perit Dial 2018; 34: 32–37 [PubMed] [Google Scholar]
- 20. Gallar P, Vigil A, Rodriguez I et al. Two-year experience with telemedicine in the follow-up of patients in home peritoneal dialysis. J Telemed Telecare 2007; 13: 288–292 [DOI] [PubMed] [Google Scholar]
- 21. Milan Manani S, Rosner MH, Virzi GM et al. Longitudinal experience with remote monitoring for automated peritoneal dialysis patients. Nephron 2019; 142: 1–9 [DOI] [PubMed] [Google Scholar]
- 22. Bunch A, Vesga JI, Camargo DO et al. Remote automated peritoneal dialysis management in Colombia. Kidney Int Rep 2019; 4: 873–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uchiyama K, Washida N, Yube N et al. The impact of a remote monitoring system of healthcare resource consumption in patients on automated peritoneal dialysis (APD): A simulation study. Clin Nephrol 2018; 90: 334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Makhija D, Alscher MD, Becker S et al. Remote monitoring of automated peritoneal dialysis patients: assessing clinical and economic value. Telemed J e-Health 2018; 24: 315–323 [DOI] [PubMed] [Google Scholar]
- 25. Dey V, Jones A, Spalding EM. Telehealth: Acceptability, clinical interventions and quality of life in peritoneal dialysis. SAGE Open Med 2016; 4:205031211667018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magnus M, Sikka N, Cherian T et al. Satisfaction and improvements in peritoneal dialysis outcomes associated with telehealth. Appl Clin Inform 2017; 26: 214–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nayak Karopadi A, Antony S, Subhramanyam SV et al. Monitoring of peritoneal dialysis: Why? Where? How? Hong Kong J Nephrol 2013; 15: 6–13 [Google Scholar]
- 28. Nayak A, Karopadi A, Antony S et al. Use of a peritoneal dialysis remote monitoring system in India. Perit Dial Int 2012; 32: 200–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kiberd J, Khan U, Stockman C et al. Effectiveness of a web-based eHealth portal for delivery of care to home dialysis patients: A single-arm pilot study. Can J Kidney Health Dis 2018; 5:205435811879441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Agarwal S, Wilkie M. Remote patient management in peritoneal dialysis: opportunities and challenges. Contrib Nephrol 2019; 197: 54–64 [DOI] [PubMed] [Google Scholar]
- 31. Harrington DM, Myers L, Eisenman K et al. The use of a tablet computer platform to optimize the care of patients receiving peritoneal dialysis: a pilot study. Blood Purif 2014; 37: 311–315 [DOI] [PubMed] [Google Scholar]
- 32. Cafazzo JA, Leonard K, Easty AC et al. Patient perceptions of remote monitoring for nocturnal home hemodialysis. Hemodial Int 2010; 14: 471–477 [DOI] [PubMed] [Google Scholar]
- 33. Liu N, Kim J, Jung Y . et al. Remote monitoring systems for chronic patients on home hemodialysis: Field test of a copresence-enhanced design. JMIR Hum Factors 2017; 4: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chow JD, Fortnum D, Frasca S et al. Beyond dialysis—telehealth initiatives Renal Society of. Australasia J 2016; 12: 18–25 [Google Scholar]
- 35.Lindsay RM, Leitch R, Heidenheim AP, et al. The London daily/nocturnal hemodialysis study—study design, morbidity, and mortality results. Am J Kidney Dis 2003; 42 (Suppl 1): 5–12 [DOI] [PubMed] [Google Scholar]
- 36. Mitchell JG, Disney AP, Roberts M. Renal telemedicine to the home. J Telemed Telecare 2000; 6: 59–62 [DOI] [PubMed] [Google Scholar]
- 37. Nicdao MK, Baldacchino T, Youn HJ et al. ‘My Home Hemo’ app—a new telehealth tool for remote monitoring of patients on home haemodialysis. Ren Soc Australas J 2016; 12: 41–47 [Google Scholar]
- 38. Marshall MR, Pierratos A, Pauly RP. Delivering home hemodialysis: is there still a role for real-time treatment monitoring? Semin Dial 2015; 28: 176–179 [DOI] [PubMed] [Google Scholar]
- 39. Weinhandl ED, Collins AJ. Relative risk of home hemodialysis attrition in patients using a telehealth platform. Hemodial Int 2018; 22: 318–327 [DOI] [PubMed] [Google Scholar]
- 40. Pierratos A, Ouwendyk M, Francoeur R et al. Nocturnal hemodialysis: Three-year experience. J Am Soc Nephrol 1998; 9: 859–868 [DOI] [PubMed] [Google Scholar]
- 41. Skiadas M, Agroyiannis B, Carson E et al. Design, implementation and preliminary evaluation of a telemedicine system for home haemodialysis. J Telemed Telecare 2002; 8: 157–164 [DOI] [PubMed] [Google Scholar]
- 42. Agroyannis B, Fourtounas C, Romagnoli G et al. Telemedicine technology and applications for home hemodialysis. Int J Artif Organs 1999; 22: 679–683 [PubMed] [Google Scholar]
- 43. Raphael MJ, Nadeau-Fredette AC, Tennankore KK et al. A virtual ward for home hemodialysis patients—a pilot trial. Can J Kidney Health Dis 2015; 2: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schachter ME, Bargman JM, Copland M et al. Rationale for a home dialysis virtual ward: Design and implementation. BMC Nephrol 2014; 15: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Minatodani DE, Berman SJ. Home telehealth in high-risk dialysis patients: A 3-year study. Telemed J e-Health 2013; 19: 520–522 [DOI] [PubMed] [Google Scholar]
- 46. Som A, Groenendyk J, An T et al. Improving dialysis adherence for high risk patients using automated messaging: Proof of concept. Sci Rep 2017; 7: 4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neumann CL, Wagner F, Menne J et al. Body weight telemetry is useful to reduce interdialytic weight gain in patients with end-stage renal failure on hemodialysis. Telemed J e-Health 2013; 19: 480–486 [DOI] [PubMed] [Google Scholar]
- 48. Sicotte C, Moqadem K, Vasilevsky M et al. Use of telemedicine for haemodialysis in very remote areas: The Canadian First Nations. J Telemed Telecare 2011; 17: 146–149 [DOI] [PubMed] [Google Scholar]
- 49. Lehoux P, Daudelin G, Poland B et al. Designing a better place for patients: Professional struggles surrounding satellite and mobile dialysis units. Soc Sci Med 2007; 65: 1536–1548 [DOI] [PubMed] [Google Scholar]
- 50. Rumpsfeld M, Arild E, Norum J et al. Telemedicine in haemodialysis: A university department and two remote satellites linked together as one common workplace. J Telemed Telecare 2005; 11: 251–255 [DOI] [PubMed] [Google Scholar]
- 51. Bellazzi R, Magni P, Bellazzi R. Improving dialysis services through information technology: from telemedicine to data mining. Studies Health Technol Inform 2001; 84: 795–799 [PubMed] [Google Scholar]
- 52. Jhaveri D, Larkins S, Sabesan S. Telestroke, tele-oncology and teledialysis: A systematic review to analyse the outcomes of active therapies delivered with telemedicine support. J Telemed Telecare 2015; 21: 181–188 [DOI] [PubMed] [Google Scholar]
- 53. Hanlon P, Daines L, Campbell C et al. Telehealth interventions to support self-management of long-term conditions: A systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. J Med Internet Res 2017; 19: e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin M-H, Yuan W-L, Huang T-C et al. Clinical effectiveness of telemedicine for chronic heart failure: A systematic review and meta-analysis. J Invest Med 2017; 65: 899–911 [DOI] [PubMed] [Google Scholar]
- 55. Jeffs L, Jain AK, Man RH et al. Exploring the utility and scalability of a telehomecare intervention for patients with chronic kidney disease undergoing peritoneal dialysis-a study protocol. BMC Nephrol 2017; 18: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ferguson TW, Zacharias J, Walker SR et al. An economic assessment model of rural and remote satellite hemodialysis units. PLoS One 2015; 10: e0135587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wechsler LR, Demaerschalk BM, Schwamm LH et al. Telemedicine quality and outcomes in stroke: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48: e3–e25 [DOI] [PubMed] [Google Scholar]
- 58. Silva GS, Farrell S, Shandra E et al. The status of telestroke in the United States: a survey of currently active stroke telemedicine programs. Stroke 2012; 43: 2078–2085 [DOI] [PubMed] [Google Scholar]
- 59. Rushakoff RJ, Rushakoff JA, Kornberg Z et al. Remote monitoring and consultation of inpatient populations with diabetes. Curr Diabetes Rep 2017; 17: 70. [DOI] [PubMed] [Google Scholar]
- 60. Katz IJ, Pirabhahar S, Williamson P et al. iConnect CKD—virtual medical consulting: a web-based chronic kidney disease, hypertension and diabetes integrated care program. Nephrology (Carlton, Vic) 2018; 23: 646–652 [DOI] [PubMed] [Google Scholar]
- 61. Tan J, Mehrotra A, Nadkarni GN et al. Telenephrology: providing healthcare to remotely located patients with chronic kidney disease. Am J Nephrol 2018; 47: 200–207 [DOI] [PubMed] [Google Scholar]
- 62. Ladino MA, Wiley J, Schulman IH et al. Tele-nephrology: a feasible way to improve access to care for patients with kidney disease who reside in underserved areas. Telemed J e-Health 2016; 22: 650–654 [DOI] [PubMed] [Google Scholar]
- 63. Ishani A, Christopher J, Palmer D et al. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis 2016; 68: 41–49 [DOI] [PubMed] [Google Scholar]
- 64. AlAzab R, Khader Y. Telenephrology application in rural and remote areas of Jordan: benefits and impact on quality of life. Rural Remote Health 2016; 16: 3646. [PubMed] [Google Scholar]
- 65. Narva AS, Romancito G, Faber T et al. Managing CKD by telemedicine: The Zuni Telenephrology Clinic. Adv Chronic Kidney Dis 2017; 24: 6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fernandes NM, Bastos MG, Oliveira NA et al. Telemedicine: development of a distance care system for pre-dialysis chronic kidney disease patients. J Bras Nefrol 2015; 37: 349–358 [DOI] [PubMed] [Google Scholar]
- 67. Mark DA, Fitzmaurice GJ, Haughey KA et al. Assessment of the quality of care and financial impact of a virtual renal clinic compared with the traditional outpatient service model. Int J Clin Pract 2011; 65: 1100–1107 [DOI] [PubMed] [Google Scholar]
- 68. Harnett P, Jones M, Almond M et al. A virtual clinic to improve long-term outcomes in chronic kidney disease. Clin Med 2018; 18: 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hull SA, Thomas N, Rainey H et al. Development and evaluation of a renal learning health system across inner east London. The Health Foundation Innovating for Improvement Report London: The Health Foundation, 2018
- 70. Forbes RC, Broman KK, Johnson TB et al. Implementation of telehealth is associated with improved timeliness to kidney transplant waitlist evaluation. J Telemed Telecare 2018; 24: 485–491 [DOI] [PubMed] [Google Scholar]
- 71. Forbes RC, Rybacki DB, Johnson TB et al. A cost comparison for telehealth utilization in the kidney transplant waitlist evaluation process. Transplantation 2018; 102: 279–283 [DOI] [PubMed] [Google Scholar]
- 72. Udayaraj UP, Watson O, Ben-Shlomo Y et al. Establishing a tele-clinic service for kidney transplant recipients through a patient-codesigned quality improvement project. BMJ Open Qual 2019; 8: e000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kalil RT, Winetroub C, Abel S. Improving Access to Specialized Care: The Telehealth Kidney Transplant Clinic at the Iowa City VAMC (Issue Brief) Washington, DC: VHA Office of Rural Health Veterans Rural Health Resource Center—Central Region, 2013
- 74. Schmid A, Hils S, Kramer-Zucker A et al. Telemedically supported case management of living-donor renal transplant recipients to optimize routine evidence-based aftercare: a single-center randomized controlled trial. Am J Transplant 2017; 17: 1594–1605 [DOI] [PubMed] [Google Scholar]
- 75. Connor A, Mortimer F, Higgins R. The follow-up of renal transplant recipients by telephone consultation: three years experience from a single UK renal unit. Clin Med 2011; 11: 242–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kaier K, Hils S, Fetzer S et al. Results of a randomized controlled trial analyzing telemedically supported case management in the first year after living donor kidney transplantation - a budget impact analysis from the healthcare perspective. Health Econ Rev 2017; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kreuzer M, Prufe J, Bethe D et al. ; Study group of the German Society for Pediatric Nephrology (Gesellschaft für Pädiatrische Nephrologie, GPN). The TRANSNephro-study examining a new transition model for post-kidney transplant adolescents and an analysis of the present health care: study protocol for a randomized controlled trial. Trials 2014; 15: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Campbell M, Akbari A, Amos S et al. Feasibility of providing nephrology services to remote communities with videoconferencing. J Telemed Telecare 2012; 18: 13–16 [DOI] [PubMed] [Google Scholar]
- 79. Rifkin DE, Abdelmalek JA, Miracle CM et al. Linking clinic and home: a randomized, controlled clinical effectiveness trial of real-time, wireless blood pressure monitoring for older patients with kidney disease and hypertension. Blood Press Monit 2013; 18: 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Thompson DA, Leimig R, Gower G et al. Assessment of depressive symptoms during post-transplant follow-up care performed via telehealth. Telemed J e-Health 2009; 15: 700–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Udayaraj UP, Watson O, Ben-Shlomo Y . et al. Establishing a tele-clinic service for kidney transplant recipients through a patient-codesigned quality improvement project. BMJ Open Qual 2019; 8: e000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. van Gelder VA, Scherpbier-de Haan ND, van Berkel S et al. Web-based consultation between general practitioners and nephrologists: a cluster randomized controlled trial. Fam Pract 2017; 34: 430–436 [DOI] [PubMed] [Google Scholar]
- 83. Stoves J, Connolly J, Cheung CK et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual Saf Health Care 2010; 19: e54. [DOI] [PubMed] [Google Scholar]
- 84. Rosano GMC, Spoletini I, Vitale C. Who approves/pays for additional monitoring? Eur Heart J Suppl 2019; 21: M64–M67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lin JC, Kavousi Y, Sullivan B et al. Analysis of outpatient telemedicine reimbursement in an integrated healthcare system. Ann Vasc Surg 2019; 65: 100–106 [DOI] [PubMed] [Google Scholar]
- 86. Bieber SD, Weiner DE. Telehealth and home dialysis: a new option for patients in the United States. Clin J Am Soc Nephrol 2018; 13: 1288–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Osman MA, Okel J, Okpechi IG et al. Potential applications of telenephrology to enhance global kidney care. BMJ Glob Health 2017; 2: e000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Osman MA, Alrukhaimi M, Ashuntantang GE et al. Global nephrology workforce: gaps and opportunities toward a sustainable kidney care system. Kidney Int Suppl 2018; 8: 52–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hazara AM, Durrans K, Bhandari S. The role of patient portals in enhancing self-care in patients with renal conditions. Clin Kidney J 2020; 13: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mertens A, Brandl C, Miron-Shatz T et al. A mobile application improves therapy-adherence rates in elderly patients undergoing rehabilitation: a crossover design study comparing documentation via iPad with paper-based control. Medicine (Baltimore) 2016; 95: e4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Diamantidis CJ, Ginsberg JS, Yoffe M et al. Remote usability testing and satisfaction with a mobile health medication inquiry system in CKD. Clin J Am Soc Nephrol 2015; 10: 1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Levine D, Torabi J, Choinski K et al. Transplant surgery enters a new era: increasing immunosuppressive medication adherence through mobile apps and smart watches. Am J Surg 2019; 218: 18–20 [DOI] [PubMed] [Google Scholar]
- 93. Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. J Cardiovasc Nurs 2013; 28: 320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Smartpatient Website. Smartpatient Gmbh https://www.smartpatient.eu/ (3 December 2019, date last accessed)
- 95.Schmidt D, Graf V, Roller R et al. Integrierte Versorgung chronisch kranker Patienten am Beispiel von MACSS. In: Burchardt A, Uszkoreit H (eds.): IT für soziale Inklusion: Digitalisierung – Künstliche Intelligenz – Zukunft für alle. De Gruyter Oldenbourg, 2018, , et al.
- 96. Hayashi A, Yamaguchi S, Waki K et al. Testing the feasibility and usability of a novel smartphone-based self-management support system for dialysis patients: a Pilot Study. JMIR Res Protoc 2017; 6: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rogers D. Patient perspective of smartphone-based apps for CKD self-care. Clin J the Am Soc Nephrol 2019; 14: 483–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Singh K, Diamantidis CJ, Ramani S et al. Patients’ and nephrologists’ evaluation of patient-facing smartphone apps for CKD. Clin J Am Soc Nephrol 2019; 14: 523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lambert K, Mullan J, Mansfield K et al. Should we recommend renal diet-related apps to our patients? An evaluation of the quality and health literacy demand of renal diet-related mobile applications. J Ren Nutr 2017; 27: 430–438 [DOI] [PubMed] [Google Scholar]
- 100. Topf JM, Hiremath S. Got CKD? There’s an app for that! Clin J Am Soc of Nephrol 2019; 14: 491–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Niel O, Bastard P. Artificial intelligence in nephrology: core concepts, clinical applications, and perspectives. Am J Kidney Dis 2019; 74: 803–810 [DOI] [PubMed] [Google Scholar]
- 102. Yan BP, Lai WHS, Chan CKY et al. High-throughput, contact-free detection of atrial fibrillation from video with deep learning. JAMA Cardiol 2020; 5: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sharma K, Rupprecht C, Caroli A et al. Automatic segmentation of kidneys using deep learning for total kidney volume quantification in autosomal dominant polycystic kidney disease. Sci Rep 2017; 7: 2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Boor P. Artificial intelligence in nephropathology. Nat Rev Nephrol 2020; 16: 4–6 [DOI] [PubMed] [Google Scholar]
- 105. Hermsen M, de Bel T, den Boer M et al. Deep learning-based histopathologic assessment of kidney tissue. J Am Soc Nephrol 2019; 30: 1968–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Esteban C, Staeck O, Baier S et al. Predicting clinical events by combining static and dynamic information using recurrent neural networks. ARXIV 2016; 93–101 [Google Scholar]
- 107. Barbieri C, Cattinelli I, Neri L et al. Development of an artificial intelligence model to guide the management of blood pressure, fluid volume, and dialysis dose in end-stage kidney disease patients: proof of concept and first clinical assessment. Kidney Dis 2019; 5: 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Barbieri C, Mari F, Stopper A et al. A new machine learning approach for predicting the response to anemia treatment in a large cohort of End Stage Renal Disease patients undergoing dialysis. Comput Biol Med 2015; 61: 56–61 [DOI] [PubMed] [Google Scholar]
- 109. Ronicke S, Hirsch MC, Türk E et al. Can a decision support system accelerate rare disease diagnosis? Evaluating the potential impact of Ada DX in a retrospective study. Orphanet J Rare Dis 2019; 14: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Stevenson JK, Campbell ZC, Webster AC et al. eHealth interventions for people with chronic kidney disease. Cochrane Database Syst Rev 2019; 8: CD012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chesser A, Burke A, Reyes J et al. Navigating the digital divide: A systematic review of eHealth literacy in underserved populations in the United States. Inform Health Social Care 2016; 41: 1–19 [DOI] [PubMed] [Google Scholar]
- 112. Wilhelm A. The digital divide: facing a crisis or creating a myth. Inform Soc 2002; 18: 415–416 [Google Scholar]
- 113. Blau A. Access isn’t enough: merely connecting people and computers won’t close the digital divide. Am Libraries 2002; 33: 50–52 [Google Scholar]
- 114. Norman CD, Skinner HA. eHealth literacy: Essential skills for consumer health in a networked world. J Med Internet Res 2006; 8: e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Goldberg DJ. Digital photography, confidentiality, and teledermatology. Arch Dermatol 2004; 140: 477–478 [DOI] [PubMed] [Google Scholar]
- 116.BBC 2019. British Airways Faces Record £183m Fine for Data Breach https://www.bbc.co.uk/news/business-48905907 (27 November 2019, date last accessed)
- 117. Haveman ME, Kleiss SF, Ma KF et al. Telemedicine in patients with peripheral arterial disease: is it worth the effort? Expert Rev Med Devices 2019; 16: 777–710 [DOI] [PubMed] [Google Scholar]
- 118. Onor ML, Misan S. The clinical interview and the doctor–patient relationship in telemedicine. Telemed J e-Health 2005; 11: 102–105 [DOI] [PubMed] [Google Scholar]
- 119.World Health Organisation 2020. Pneumonia of Unknown Cause—China https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ (27 March 2020, date last accessed)
- 120. Burke AE, Thaler KM, Geva M et al. Feasibility and acceptability of home use of a smartphone-based urine testing application among women in prenatal care. Am J Obstet Gynaecol 2019; 221: 527–528 [DOI] [PubMed] [Google Scholar]
- 121. McGillicuddy JW, Gregoski MJ, Weiland AK et al. Mobile health medication adherence and blood pressure control in renal transplant recipients: A proof-of-concept randomized controlled trial. JMIR Res Protoc 2013; 2: e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Waseh S, Dicker AP. Telemedicine training in undergraduate medical education: Mixed-methods review. JMIR Med Educ 2019; 5: e12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Selby NM, Hill R, Fluck RJ et al. ; on behalf of the NHS England ‘Think Kidneys’ AKI Programme. Standardizing the early identification of acute kidney injury: The NHS England national patient safety alert. Nephron 2015; 131: 113–117 [DOI] [PubMed] [Google Scholar]
- 124. Connell A, Montgomery H, Martin P et al. Evaluation of a digitally-enabled care pathway for acute kidney injury management in hospital emergency admissions. NPJ Digit Med 2019; 2: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wilson FP, Shashaty M, Testani J et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet 2015; 385: 1966–197425726515 [Google Scholar]
- 126. Wu Y, Chen Y, Li S et al. Value of electronic alerts for acute kidney injury in high-risk wards: A pilot randomized controlled trial. Int Urol Nephrol 2018; 50: 1483–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Park S, Baek SH, Ahn S et al. Impact of electronic acute kidney injury (AKI) alerts with automated nephrologist consultation on detection and severity of AKI: a quality improvement study. Am J Kidney Dis 2018; 71: 9–19 [DOI] [PubMed] [Google Scholar]
- 128. Ebah L, Hanumapura P, Waring D et al. A multifaceted quality improvement programme to improve acute kidney injury care and outcomes in a large teaching hospital. BMJ Qual Improv Rep 2017; 6: u219176.w7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wieringa FP, Broers NJH, Kooman JP et al. Wearable sensors: can they benefit patients with chronic kidney disease? Expert Rev Med Devices 2017; 14: 505–519 [DOI] [PubMed] [Google Scholar]
- 130. Castro AC, Neri M, Nayak Karopadi A et al. Wearable artificial kidney and wearable ultrafiltration device vascular access-future directions. Clin Kidney J 2019; 12: 300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Foo MWY, Htay H, Gow S et al. Effect of Automated Wearable Artificial Kidney (AWAK) Device on Toxin Clearance and Safety in Peritoneal Dialysis Patients. https://www.asn-online.org/education/kidneyweek/2019/program-abstract.aspx?controlId=3234367, , et al. [DOI] [PubMed]
- 132. Salani M, Roy S, Fissell W. Innovations in wearable and implantable artificial kidneys. Am J Kidney Dis 2018; 72: 745–751 [DOI] [PubMed] [Google Scholar]
- 133. 2013. Romper Suit To Protect Against Sudden Infant Death https://www.fraunhofer.de/en/press/research-news/2013/january/romper-suit-to-protect-against-sudden-infant-death.html (15 October 2019, date last accessed)
- 134. Nyein HY, Gao W, Shahpar Z et al. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca(2+) and pH. ACS Nano 2016; 10: 7216–7224 [DOI] [PubMed] [Google Scholar]
- 135. Fallahzadeh R, Pedram M, Ghasemzadeh H. SmartSock: A wearable platform for context-aware assessment of ankle edema. In: Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, FL, 2016, 6302–6306 [DOI] [PubMed] [Google Scholar]
- 136. Lin CH, Chen WL, Li CM et al. Assistive technology using integrated flexible sensor and virtual alarm unit for blood leakage detection during dialysis therapy. Healthc Technol Lett 2016; 3: 290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Perez MV, Mahaffey KW, Hedlin H et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019; 381: 1909–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kooman JP, Wieringa FP, Han M et al. Wearable health devices and personal area networks: can they improve outcomes in haemodialysis patients? Nephrol Dial Transplant 2020; 35: ii43–ii50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tomasev N, Glorot X, Rae JW et al. A clinically applicable approach to continuous prediction of future acute kidney injury. Nature 2019; 572: 116–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ande P, Chiu D, Rayner S et al. What’s on the web for nephrology? NDT Plus 2009; 2: 119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med 2020; 382: 1679–1681 [DOI] [PubMed] [Google Scholar]
- 142. Howard C, Tcholakov Y, Holz C. The Paris agreement: charting a low-emissions path for a child born today. Lancet Planet Health 2020; 4: e4–e6 [DOI] [PubMed] [Google Scholar]
- 143. Zoccali C, Blankestijn PJ, Bruchfeld A et al. The nephrology crystal ball: the medium-term future. Nephrol Dial Transplant 2020; 35: 222–226 [DOI] [PubMed] [Google Scholar]
- 144. Muse ED, Topol EJ. A brighter future for kidney disease? Lancet 2020; 395: 179. [DOI] [PubMed] [Google Scholar]
- 145.White D, Kribs Z. 2020. CARES Act to Improve Options for People on Home Dialysis. https://www.kidneynews.org/policy-advocacy/leading-edge/cares-act-to-improve-options-for-people-on-home-dialysis (31 March 2020, date last accessed)