This commentary refers to ‘Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy’, by R.M. Inciardi et al., doi: 10.1093/eurheartj/ehaa583.
We read with interest the paper by Dr Inciardi et al.1 showing higher mortality and thrombo-embolic events in cardiac patients with COVID-19. Higher prevalence of heart failure and hypertension in this cohort, conditions associated with reduced angiotensin-converting enzyme 2 (ACE2) levels, leads us to propose that ACE2 may mediate some of the prothrombotic effects of the SARS-CoV-2 virus.
Thrombotic complications are prevalent in COVID-19, contributing to mortality and morbidity.2 Following the recognition that SARS-CoV-2 enters the host cell by binding to ACE2, there has been controversy regarding use of angiotensin-converting enzyme inhibitors (ACEIs) in infected individuals, since ACEIs up-regulate ACE2 and theoretically may increase infectability of the host. The tissue distribution of ACE2 includes lung alveolar epithelial cells, enterocytes, arterial and venous endothelial cells, arterial smooth muscle, and nasal and oral epithelium, mirroring the clinical manifestations of COVID-19 in acute respiratory distress syndrome, diarrhoea, thrombosis, anosmia, and ageusia.
ACEI use has been associated with increased ACE2 expression and, in animals, ACEI infusion increased the number of ACE2 receptors in the cardiopulmonary circulation. An increase in pulmonary ACE2 receptors for SARS-CoV-2 to bind may increase the risk of severe disease manifestation.
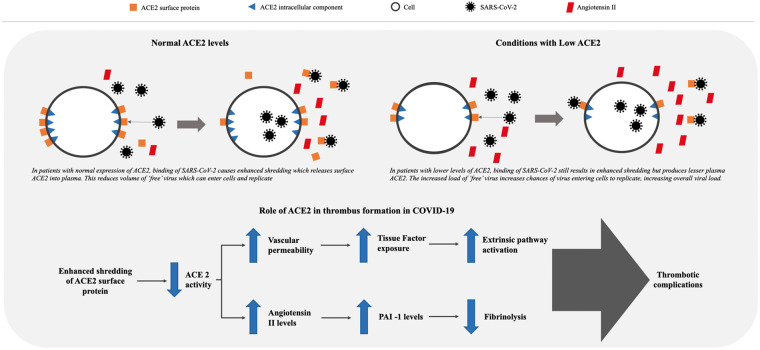
However, ACEI use is protective against COVID-19 mortality,3 which initially appears counter-intuitive. Following the binding of the virus, expression and enzymatic activity of ACE2 are significantly reduced, probably due to enhanced shedding, whereby the extracellular component of ACE2 is cleaved, with release of the resultant soluble protein, reducing surface expression and activity. This reduction in ACE2 expression may contribute to the virulence of SARS-CoV-2. Patient characteristics associated with severe disease, such as hypertension, diabetes, and obesity, have been linked to lower plasma ACE2 levels, and may explain why ACEI-treated patients appear protected. The location of the ACE2 gene on the X-chromosome, and oestrogen-dependent activation of ACE2, may account for the better prognosis in women with COVID-19.
Reduction in ACE2 activity increases vascular permeability, leading to tissue factor (TF) expression in subendothelial cells as well as in leucocytes and platelets, which can trigger coagulation, thrombosis, and disseminated intravascular coagulation (Take home figure).3 ACE2 may exert antithrombotic effects, through a number of mechanisms, the most important being the renin–angiotensin pathway, in which angiotensin I is converted by ACE to angiotensin II (Ang II), which is then broken down by ACE2 to Ang(1-7). Reduction in ACE2 leads to an increase in Ang II, which stimulates plasminogen activator inhibitor 1 (PAI-1) expression in a variety of cells, including smooth muscle cells, endothelial cells, and adipocytes.4 PAI-1 is the major inhibitor of the plasma fibrinolytic cascade, and ACEIs reduced PAI-1 levels in experimental and clinical studies.4 Ang II also appears to sensitize platelets to the effects of classical platelet agonists.4 Patients with COVID-19 appear to have elevated levels of plasma Ang II, which in turn correlate with viral load and degree of lung injury.5
Take home figure.
Thrombotic complications in patients with COVID-19 related to angiotensin-converting enzyme 2 (ACE2) activity. SARS-CoV-2 binding to ACE2 receptors induces enhanced shredding of ACE2, with a resultant (1) increase vascular permeability, subsequent tissue factor exposure, and activation of extrinsic coagulation pathway; and (2) increase in angiotensin II levels, subsequent increase in plasminogen activator inhibitor 1 (PAI-1) levels, and reduction of fibrinolytic activity.
In conclusion, reduction of ACE2 due to SARS-CoV-2 infection and the resultant endothelial dysfunction, release of TF, and impaired fibrinolysis could explain the thrombotic and thrombo-embolic phenomena observed in patients with severe COVID-19 disease.
Conflict of interest: none declared.
References
- 1. Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, Italia L, Zaccone G, Tedino C, Fabbricatore D, Curnis A, Faggiano P, Gorga E, Lombardi CM, Milesi G, Vizzardi E, Volpini M, Nodari S, Specchia C, Maroldi R, Bezzi M, Metra M.. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J 2020;41:1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020;75:2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Rohit L, Liu PP, Li H.. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;26:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dielis AW, Smid M, Spronk HM, Hamulyak K, Kroon AA, ten Cate H, de Leeuw PW.. The prothrombotic paradox of hypertension: role of the renin–angiotensin and kallikrein–kinin systems. Hypertension 2005;46:1236–1242. [DOI] [PubMed] [Google Scholar]
- 5. Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L.. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63:364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]