Abstract
Background
Fulminant myocarditis is a catastrophic disease with high mortality and complications. A viral aetiology is frequent and the implication of SARS-CoV-2 is not yet known.
Case summary
A 38-year-old woman who recently arrived from Spain presented with palpitations that started suddenly 3 days prior to presentation and were associated with haemodynamic instability, without dyspnoea or chest pain. We found features of myopericarditis on the electrocardiogram and severe systolic dysfunction on the echocardiogram. The chest tomography showed findings which suggested COVID-19 infection, and PCR for SARS-CoV-2 was positive. The cardiac magnetic resonance image showed Lake Louise criteria for myocarditis. The patient was treated with immunomodulatory, steroid, and immunoglobulin therapy, with a favourable clinical response.
Discussion
The importance of this case lies in highlighting the severe cardiac involvement in a young patient, without previous risk factors, positive for COVID-19, and the favourable response to the medical treatment given.
Keywords: Myocarditis, Fulminant, Myopericarditis, Coronavirus, Case report, COVID-19
Learning points
Fulminant cardiac involvement may be present in the absence of respiratory symptoms from COVID-19.
COVID-19 fulminant myocarditis may benefit from steroid and human immunoglobulin therapy.
Introduction
Fulminant myocarditis is a catastrophic illness, with significant myocardial inflammatory compromise, which can lead to death. Its multiple aetiologies include autoimmunity and infections. A viral origin is one of the most common, with multiple microorganisms involved.1 The World Health Organization (WHO) recently confirmed the pandemic of SARS-CoV-2, which originated in Wuhan (China), as a serious public health problem.
SARS-CoV-2 is a single-stranded RNA virus with fast mutation and recombination, and high similarity to other coronaviruses which have appeared in previous years (SARS and MERS).2 The infectious disease which manifests mainly with symptoms such as cough and fever and that commonly causes pneumonia is called COVID-19 and, in patients with underlying cardiovascular diseases, it is more likely to have a severe manifestation or variability in clinical manifestations. We present a case of probable fulminant myocarditis secondary to COVID-19.
Timeline
Case presentation
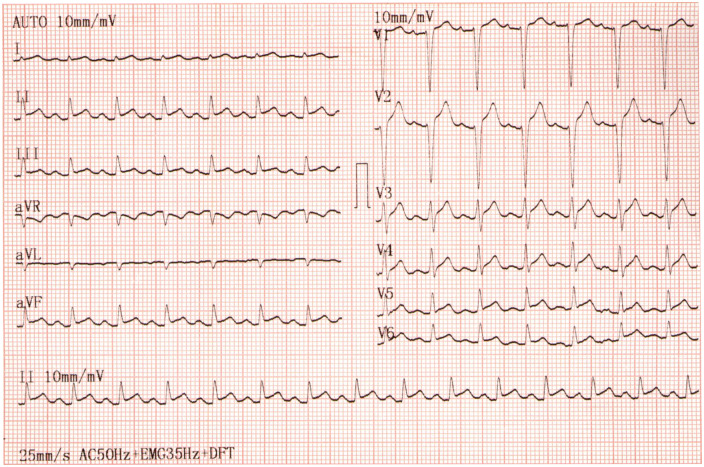
A 38-year-old mestizo Colombian woman without previous history of cardiovascular disease or any other medical history presented to the emergency room. The patient was not taking any medicine prior to her arrival. She had been having palpitations for 3 days that started suddenly, associated with general malaise, without dyspnoea, chest pain, or respiratory symptoms. The patient reported having recently arrived from Spain, 5 days before presenting herself to the hospital. Upon arrival, the patient’s cardiovascular examination revealed her to be tachycardic without audible heart murmurs or an elevated jugular venous pressure. The respiratory examination revealed soft inspiratory crackles bibasally. Her vital signs included a heart rate of 137 b.p.m., blood pressure of 98/54 mmHg (cardiogenic index >1.1 stage B cardiogenic shock), pulse oximetry of 95% SaO2, and body temperature of 36.5°C. The electrocardiogram showed a diffuse and concave elevation of the ST-segment, with PR segment depression and Spodick’s sign (Figure 1).
Figure 1.
Electrocardiogram. Diffuse and concave ST elevation with PR-segement depression and Spodick’s sign.
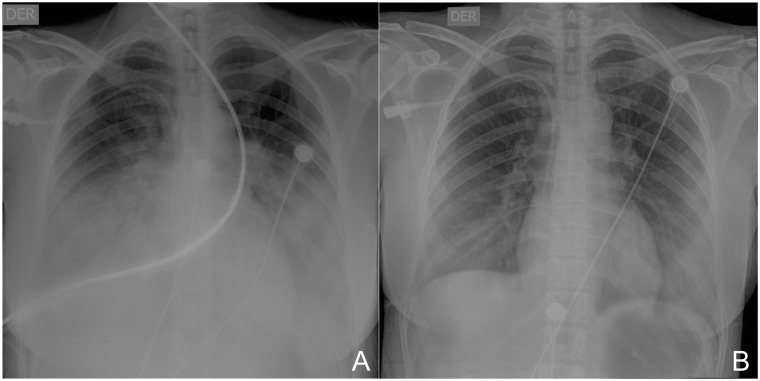
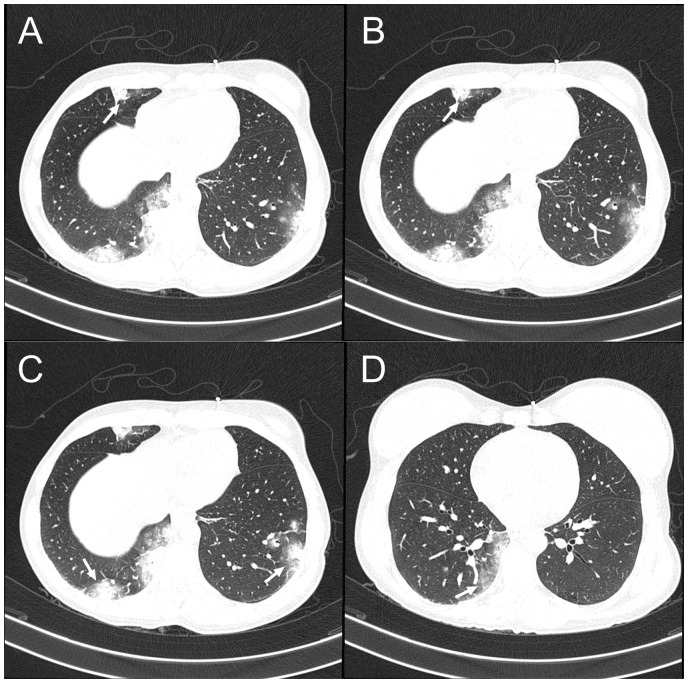
The initial chest X-ray showed reticular interstitial opacities (Figure 2A), and computed tomography (CT) of the chest showed compromise of the lung parenchyma, and mixed opacities, mainly alveolar and ground-glass of peripheral and basal predominance, a classic pattern of pulmonary involvement in COVID-19 (Figure 3). The biomarkers of myocardial injury were significantly elevated (Table 1). The transthoracic echocardiogram revealed a left ventricle with global hypokinesia, with severely reduced systolic function, an ejection fraction of 30%, without valvular heart disease, and mild pericardial effusion (2 mm). Nasopharyngeal and oropharyngeal swabs were taken in order to identify microorganisms causing the infection in the respiratory tract (14 viral and 4 bacterial); the swabs were negative, and nucleic acid amplification by PCR was positive for SARS-CoV-2.
Figure 2.
Chest X-ray. (A) Bilateral diffuse opacity. (B) After 10 days of treatment.
Figure 3.
Chest computed tomography. (A and B, arrows) A medial alveolar opacity of the middle lobe. (C, arrow) A posterior basal alveolar opacity of the right lower lobe and lateral basal opacity of the left lower lobe (left arrow). (D) A medial basal alveolar opacity of the right lower lobe. Ground-glass opacity is seen on all panels.
Table 1.
Laboratory data
| Variable | Initial presentation | 10 days after initial presentation | Reference range, adults |
|---|---|---|---|
| ScvO2 | 55.8% | 70% | >70% |
| PO2 | 69.3 | 75.3 | 72–104 mmHg |
| PCO2 | 28 | 35 | 35–45 mmHg |
| Lactic acid | 28.7 | 20 | 4.5–19–8 mg/dL |
| Troponin I | 1190 | 80 | <14 ng/L |
| BNP | 13 000 | 1000 | <100 pg/mL |
| C-reactive protein | 25.7 mg/mL | 7 mg/mL | 0–5 mg/mL |
| White cell count | 19 000 | 6.74 | 4.5–11.3 × 103/μL |
| Neutrophil count | 15700 | 9.10 | 2.25–8.48 ×103/μL |
| Lymphocyte count | 400 | 1.400 | 0.90–4.52 × 103/μL |
| Haemoglobin | 14 | 12 | 12.3–15–3 g/dL |
| Haematocrit | 42 | 34 | 35–47% |
| Platelet count | 258 000 | 239.000 | 150–450 × 103/μL |
| Creatinine | 1.2 mg/dL | 0.80 | 0.51–0.95 mg/dL |
| Ureic nitrogen | 36 mg/dL | 16 | 6–20 mg/dL |
PO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; ScvO2, central venous oxygen saturation; BNP, brain natriuretic peptide.
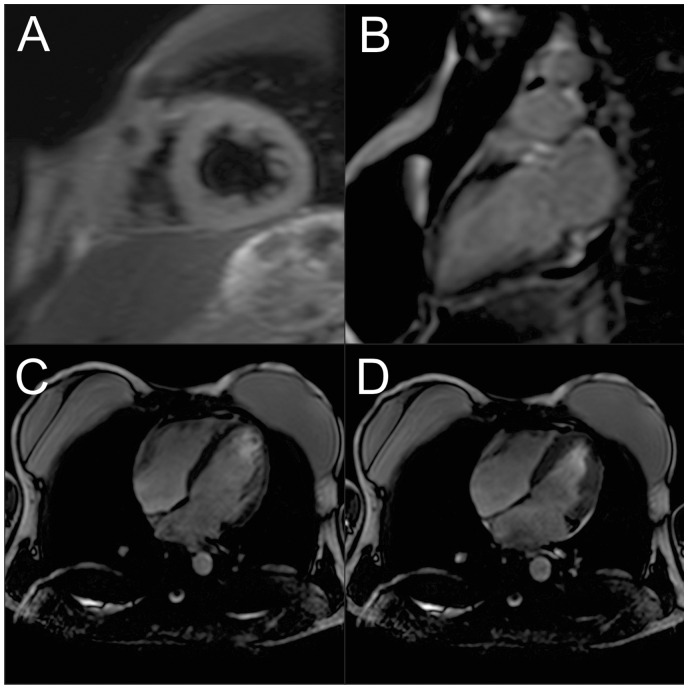
Finally, it was concluded that the patient should be diagnosed with fulminant myocarditis with stage B cardiogenic shock and COVID-19 pneumonia. Treatment in the intensive care unit (ICU) consisted of oxygen therapy without mechanical ventilation, methylprednisolone (200 mg/day), and intravenous human immunoglobulin (IVIG; 20 g/day) for 4 days, hydroxychloroquine (800 mg on day 1, then 400 mg/day) and azithromycin (500 mg/day), both during 5 days without a QTc greater than 450 ms, and lopinavir/ritonavir (800/200 mg/day, 10 days). The initial support was performed with norepinephrine and subsequently inotropic support with dobutamine and levosimendan, in addition to intravenous furosemide, without the need for circulatory assistance devices. The patient showed a sustained clinical, haemodynamic, and respiratory improvement (Table 1). After 16 days of hospital stay (10 days in the ICU), the patient was discharged with heart failure management which included bisoprolol 5 mg/day, spironolactone 25 mg/day, and enalapril 10 mg b.i.d. Before discharge, cardiac magnetic resonance imaging (MRI) was performed (Figure 4), which showed inflammatory manifestations, with the recovery of the ejection fraction. The performance of a myocardial biopsy after the clinical improvement was considered, but the patient did not consent to the procedure.
Figure 4.
Cardiac magnetic resonance imaging (MRI). (A) Left ventricular short-axis view using a short inversion time inversion recovery (STIR) T2-weighted showing the transmural extent of myocardial oedema to both ventricles. (B) Left ventricular long-axis view with inferobasal late gadolinium enhancement (LGE). (C and D) Long-axis view of the left ventricular end-diastolic and end-systolic using MRI with an ejection fraction of 60%.
The medical control performed after 2 weeks showed that the patient had no new symptoms and transthoracic echocardiography was normal.
Discussion
We describe a patient presenting sudden palpitations, with an electrocardiogram showing ST-segment diffuse and concave elevation, PR-segment depression compatible with acute myopericarditis, plus the increase of biomarkers, and systolic dysfunction by echocardiogram. Cardiac MRI shows an inflammatory process of a clinical picture compatible with a diagnosis of fulminant myocarditis. The presence of fulminant myocarditis is rare; few cases have been described since the beginning of the pandemic. The increase of cardiac biomarkers could be related to acute myocardial compromise in 8–12% of the cases, correlated with the prognosis and need for UCI admission by 80% of cases.3
In our case, we observe an increase in the markers of myocardial injury with severe systolic dysfunction in the echocardiograph; this implies that the possible myocardial injury mechanisms due to COVID-19 could be, in the first place, by direct injury to the myocytes as a result of the migration of infected macrophages from the lung, as observed in the infiltration of myocardial macrophages in the autopsy samples of patients with SARS4 and in a current SARS-CoV-2 case through a endomyocardial biopsy.5 A second probable mechanism is the indirect injury of the myocardium due to the cytokine storm that the infection triggers, demonstrated by the increase of interleukin (IL) IL-6, IL-10, IL-2 receptor (IL-2R), and tumour necrosis factor (TNF)-α in severe cases.6,7 A third probable mechanism is the presence of a significant amount of angiotensin-converting enzyme 2 (ACE2) receptors in the myocardium with down-regulation which alters the cardioprotective effects of angiotensin 1-7, with an increase of TNF-α production associated with the myocardial dysfunction.8 These mechanisms suggest that the severe inflammatory response is a probable mediator of myocardial compromise in SARS-COV2 cases, although there is a lack of studies that confirm this theory.
There are few fulminant myocarditis cases described in the SARS-CoV-2 pandemic. The inflammatory process in this case takes place in the lower respiratory tract, as observed in the chest CT at the time of diagnosis, which could explain a cardiac injury triggered by the inflammatory response. Currently, fulminant myocarditis is not known as a secondary common complication of COVID-19, and therefore a high clinical suspicion is required for an early diagnosis.
In our case, after treatment, the patient presented clinical and haemodynamic improvement, achieving recovery of systolic function and decreasing pulmonary compromise (Table 1; Figure 2B). The decision to use dual inotropic therapy was substantiated by the persistence of the ScvO2 at <60% despite the use of dobutamine and norepinephrine, with subsequent clinical improvement, without circulatory support. This is supported by scientific evidence from a study that noted improvement in refractory acute heart failure.9
Perhaps the cytokine release syndrome, a hypothesis related to the severity of inflammation in the infection by COVID-19 that generates cardiac involvement, encourages us to use systemic steroids and IVIG to counteract it. Currently, there are no clinical trials to support this therapeutic approach; the decision was based on the clinical improvement described in case reports and the decrease of mortality observed in a comprehensive support treatment for patients with fulminant myocarditis.10–12 In light of the hypothesis that the combined use hydroxychloroquine and azithromycin results in viral clearance, our team administered these dugs on the seventh day of treatment.13 We also administered lopinavir/ritonavir based on their use leading to a shorter stay in the ICU as a secondary outcome.14 We controlled the QTc daily given the likely myocardial damage and possible adverse events not observed. Nevertheless, emphasis is placed on the lack of clinical trials that provide solid scientific evidence with regard to treatment in cases of fulminant myocarditis associated with COVID-19.
Unlike the cases described,10,11 our current case occured at a younger age, there were no respiratory symptoms or intestinal problems, nor was there a history of cardiovascular disease, and from the beginning the clinical picture was manifested with cardiogenic shock. The findings in the cardiac MRI showed biventricular oedema and, despite not being a specific pattern of an inflammatory process triggered by COVID-19, it has been described in cases so far. Performance of an endomyocardial biopsy was considered for histological confirmation, but it could not be done since the patient did not give consent. Regarding the background, in a study of 138 patients with COVID-19,15 it was noted that 11.8% of the patients who died had substantial heart damage without an underlying cardiovascular disease, which implies that the incidence of cardiovascular complications is relevant. Also, in our case, clinical improvement was observed with the use of steroids and IVIG, in addition to the treatments described so far for COVID-19. It seems that the use of dual inotropics plus an intravenous vasopressor helps to improve the haemodynamic condition of the patient. It also decreases the need for left ventricular assist devices in centres where there is no availability. At present, the evidence is not sufficient and requires further study.
Conclusion
The infection by SARS-CoV-2 can affect multiple systems, with mainly lung involvement. However, myocardial injury may be frequently evidenced in patients with COVID-19 with variable clinical manifestation. Fulminant myocarditis is a rare complication that requires clinical suspicion for early diagnosis and treatment. The importance of this case lies in the use of steroids, immunoglobulins, and immunomodulating agents as part of the successful therapy, which to date requires further study.
Lead author biography

Wikler Bernal Torres is currently an Internal Medicine Physician from the Universidad Libre de Cali. He is practising in the Department of Cardiology of the DIME Clinic. He has a special interest in Cardiology.
Supplementary Material
Acknowledgements
We thank Jhon Blanco, of Universidad Libre, Cali, and ESC Translate.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, Shah RV, Sims DB, Thiene G, Vardeny O; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Recognition and initial management of fulminant myocarditis. A Scientific Statement from the American Heart Association. Circulation 2020;141:e69–e92. [DOI] [PubMed] [Google Scholar]
- 2. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W.. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis 2020;doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babapoor-Farrokhran S, Gill D, Walker J, Rasekhi RT, Bozorgnia B, Amanullah A.. Myocardial injury and COVID-19: possible mechanisms. Life Sciences 2020;253:1177230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E.. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 2020;22:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, Yan J, Ping H, Zhou Q.. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol 2020;311:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q.. Clinical and immunologic features in severe and moderate forms of coronavirus disease 2019. J Clin Invest 2020;20:2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oudit GY, Kassiri Z, Jiang C, Liu PP,, Poutanen SM, Penninger JM, Butany J.. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 2009;39:618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juguet W, Faivre L, Deguillard C, Fard D, Pelletier V, Oliver L, Damy T, Mongardon M, Mekonso-Dessap A, Randé Dubois, Gallet L, Huguet R, Lim R. P. Levosimendan added to dobutamine in acute decompensated heart failure refractory to dobutamine. Arch Cardiovasc Dis Suppl 2020;12:1878–6480. [Google Scholar]
- 10. Hu H, Ma F, Wei X, Fang Y.. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Euro Heart J 2020;doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M.. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li S, Xu S, Li C, Ran X, Cui G, He M, Miao K, Zhao C, Yan J, Hui R, Zhou N, Wang Y, Jiang J, Zhang J, Wang D.. A life support-based comprehensive treatment regimen dramatically lowers the in-hospital mortality of patients with fulminant myocarditis: a multiple center study. Sci China Life Sci 2019;62:369–380. [DOI] [PubMed] [Google Scholar]
- 13. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D.. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020;doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C.. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med 2020;382:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z.. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.