The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has recently reached the pandemic level.1 COVID-19 is associated with higher case fatality in patients with comorbidities, including those with arterial hypertension or other cardiovascular diseases.2 Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) are extensively used for different clinical indications.3 It has been hypothesized that the use of ACEIs/ARBs may negatively impact on the clinical outcomes of COVID-19 patients by affecting the expression of angiotensin-converting enzyme 2 aminopeptidase (ACE-2), the human receptor for SARS-CoV-2.4 Accruing evidence is available that does not show a higher risk with ACEIs/ARBs in patients with COVID-19.5 However, available studies have limited statistical power to detect signals of increased mortality.6 In this context, a meta-analysis provides more precise estimates of the direction and strength of association between the use of ACEIs/ARBs and prognosis. Few meta-analyses have been published that do not show an increase in death with ACEIs/ARBs. However, new studies are now available that suggest the opportunity for an update.7,8
This meta-analysis complies with the MOOSE (Meta-analyses Of Observational Studies in Epidemiology) and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, and its protocol is registered in the PROSPERO database (CRD42020185053). We searched PubMed, Scopus, and web sources up to 7 June 2020 for studies reporting data on the association between ACEI/ARB use and clinical outcomes in patients with COVID-19. No language restrictions were used. The primary outcome of interest was all-cause death (in-hospital or within 28 days from symptom onset). The secondary outcome of interest was severe disease due to SARS-CoV-2. Where available, we extracted both the raw number of events and the adjusted risk estimates (e.g. multivariable or propensity score adjusted). When needed, the corresponding authors of the included studies were contacted, and one of them provided additional data that were not available in the original publication. Data quality of the included studies was evaluated using the Newcastle Ottawa scale (NOS). Data were pooled using inverse-variance weighted random-effects models. The between-study heterogeneity was calculated according to the DerSimonian and Laird method. The Hartung–Knapp method was used for adjustment of the estimates and confidence intervals (CIs). Publication bias was assessed using the Begg and Mazumdar test. Pooled estimates are presented as odds ratios (ORs) with the 95% CI.
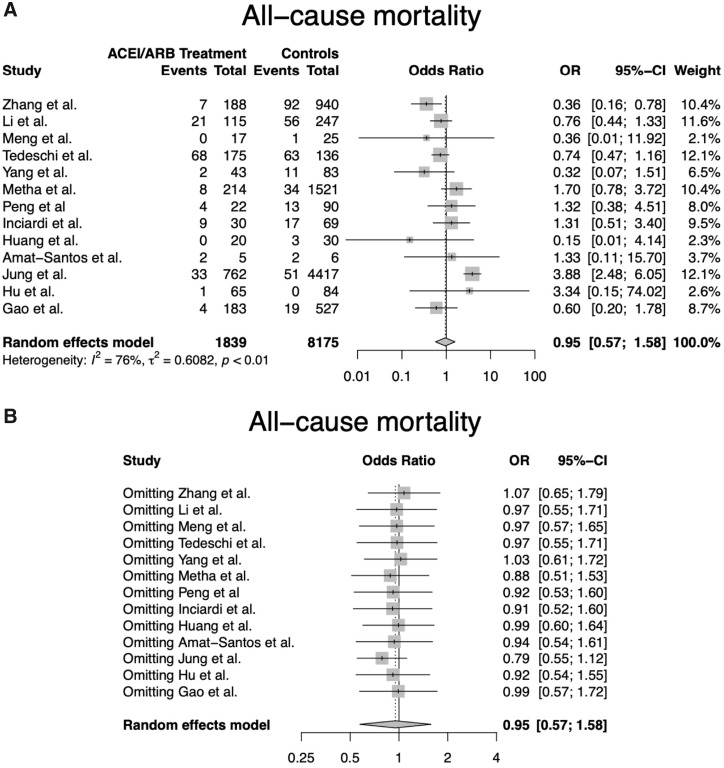
A total of 175 studies were screened at the title and abstract level, and 14 were finally included (13 with observational design and one interim analysis of a randomized clinical trial), encompassing 10 127 patients. Most patients were in their sixth decade, with age ranging across studies between 45 and 86 years. Hypertension was the only indication for ACEIs/ARBs in 8 studies out of 14. All-cause mortality was reported in 13 studies (n = 10 014) while severe disease was reported in 9 studies (n = 1675). The quality of the included studies ranged from low to high (average NOS score 5.5, range 3–8). In the unadjusted analysis, the use of ACEIs/ARBs was not associated with an increased risk of all-cause death (OR 0.95, 95% CI 0.57–1.58; Figure 1A) or severe disease (OR 0.88, 95% CI 0.60–1.31). The results were consistent in a sensitivity analysis restricted to studies with NOS score ≥5. A leave-one-out sensitivity analysis showed that none of the studies overly influenced the magnitude and significance of the summary estimate (Figure 1B). There were no concerns for publication bias (P-value >0.05).
Figure 1.
Forest plot (A) and leave-one-out sensitivity analysis (B) for all-cause mortality according to the use of ACEIs/ARBs. Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; OR, odds ratio.
We did not identify an increased risk of early death or severe disease associated with the use of ACEIs/ARBs. These findings support the recommendation of major international cardiovascular societies on the importance of continuing ACEIs/ARBs during the COVID-19 outbreak.9,10 Although no conclusions can be drawn on the benefit of ACEIs/ARBs in COVID-19, our results support the safety of the uninterrupted use of ACEIs/ARBs. In addition, since these drugs provide clear benefits in patients with cardiovascular diseases, their preventive discontinuation during COVID-19 should be discouraged.
Conflict of interest: none declared.
References
- 1. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL.. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O.. Potential effects of coronaviruses on the cardiovascular system. JAMA Cardiol 2020;doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016;37:2129–2200.27206819 [Google Scholar]
- 4. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N,, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S.. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Danser AHJ, Epstein M, Batlle D.. Renin–angiotensin system blockers and the COVID-19 pandemic. Hypertension 2020;75:1382–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jarcho JA, Ingelfinger JR, Hamel MB, D’Agostino RB, Harrington DP.. Inhibitors of the renin–angiotensin–aldosterone system and Covid-19. N Engl J Med 2020;382:2462–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pirola CJ, Sookoian S.. Estimation of renin–angiotensin–aldosterone–system (RAAS)-inhibitor effect on COVID-19 outcome: a meta-analysis. J Infect 2020;doi: 10.1016/j.jinf.2020.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Usman MS,, Siddiqi TJ, Khan MS, Ahmed A, Ali SS, Michos ED, Hall ME, Krasuski RA, Greene SJ, Butler J, Alkhouli M.. A meta-analysis of the relationship between renin–angiotensin–aldosterone system inhibitors and COVID-19. Am J Cardiol 2020;doi: 10.1016/j.amjcard.2020.05.038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ESC Council on hypertension. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang.
- 10.HFSA, ACC, AHA. HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. 2020. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19. [DOI] [PMC free article] [PubMed]