Several paediatric cases with a multisystem inflammatory syndrome and acute heart failure associated with SARS-CoV-2 infection have been reported recently. The mechanism for heart failure in this setting remains unclear. We performed endomyocardial biopsy in a young COVID-19 patient presenting with a delayed multisystem inflammatory syndrome with acute heart failure.
A 19-year-old patient was admitted for acute respiratory distress and a mixed distributive and cardiogenic shock. One month previously he was diagnosed with COVID-19 and was advised to recover at home because of mild illness that lasted 1 week. After 3 weeks without symptoms, he presented recurrent fever, cough, cervical adenopathy, and subsequently chest pain and dyspnoea. Upon arrival, he was febrile at 39°C, hypotensive (81/43 mmHg), and tachycardic (140 b.p.m.), with a respiratory rate of 45 breaths/min. Nasopharyngeal swab confirmed COVID-19. Laboratory test results revealed a profound elevation in inflammatory biomarkers: ferritin 4560 μg/L (normal <300), triglyceride 4 mM (normal <1.7), procalcitonin 155 μg/L (normal <0.5), type 1 interferon 6.1 (normal <2.3), neutrophil count 24 × 109/L (normal <8), lymphopenia 0.5 × 109/L (normal >1), and decreased complement activity CH50 <14 (normal >40). High-sensitivity troponin I and N-terminal probrain natriuretic peptide (NT-proBNP) were both elevated at 4200 ng/L (normal <79) and 17 377 pg/mL (normal <500), respectively. Left ventricular ejection fraction was impaired (20%) with a low cardiac output. The patient required protective mechanical ventilation. Emergent coronary angiography revealed normal coronary arteries. Endomyocardial biopsy was performed (four samples for pathological study and three for viral PCR). Histopathological study demonstrated a myocarditis with inflammatory infiltrates consisting of a majority of lymphocytes and neutrophils (≥14 leucocytes/mm2), oedema, but no typical myocyte necrosis. Cell-specific immunoperoxidase stains revealed CD4-positive T lymphocytes. CD20-positive B-lymphocytes were absent (Figure ). There was no granuloma, abscess, giant cell, or features of vasculitis. RT–PCR for SARS-CoV-2 and PCR for other cardiotropic viruses were negative on the myocardium. PCR was performed on a peripheral blood sample, to evaluate for co-existing viraemia, and was negative. Blood cultures were sterile. Bronchoalveolar lavage revealed a purulent fluid with high neutrophil count, without evidence of SARS-COV-2 or other infectious agent. Our management consisted of high-dose diuretics, broad-spectrum antibiotics, and supportive therapy with inotropic and vasopressive drugs. No immunosuppressive therapy was used. The patient improved promptly. Ejection fraction and inflammatory markers normalized spontaneously and the patient was discharged on day 12.
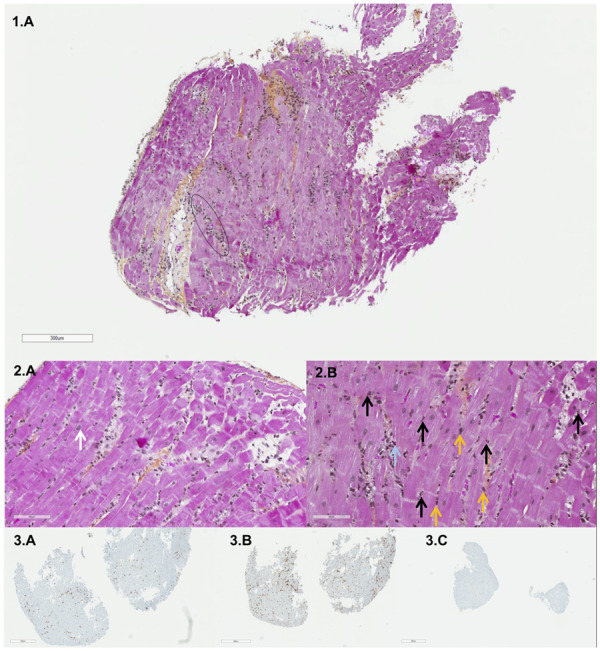
In young patients, COVID-19 can present as a delayed multisystem inflammatory syndrome (also reported as Kawasaki-like disease) associated with acute heart failure. Similarly to previous paediatric cases, prominent features are: delayed presentation, multisystem inflammatory response with multiple organ failure and in particular heart failure, and rapid recovery. The endomyocardial biopsy suggests a fulminant lymphocytic myocarditis secondary to an imbalanced proinflammatory response without evidence of direct viral cytopathic effect as a potential mechanism for heart failure in this setting.
Endomyocardial biopsy. Light microscopy, histopathological study (scale: 1, 300 μm; 2A, 90 μm; 2B, 80 μm). Inflammatory cell infiltration (black circle) with a majority of neutrophils (black arrows) and lymphocytes (yellow arrows) and rare eosinophils (blue arrow). The white arrow indicates a myocyte. Immunohistochemistry (staining: 3A, CD3; 3B, CD4; 3C, CD8) revealed T-lymphocyte infiltrates (CD3 positive) consisting of CD4-positive T lymphocytes.
Conflict of interest: none declared.


