Abstract
Background
South Korea experienced the novel coronavirus disease (COVID-19) outbreak in the early period; thus data from this country could provide significant implications for global mitigation strategies. This study reports how COVID-19 has spread in South Korea and examines the effects of rapid widespread diagnostic testing on the spread of the disease in the early epidemic phase.
Methods
We collected daily data on the number of confirmed cases, tests and deaths due to COVID-19 from 20 January to 13 April 2020. We estimated the spread pattern with a logistic growth model, calculated the daily reproduction number (Rt) and examined the fatality pattern of COVID-19.
Results
From the start date of the epidemic in Korea (18 February 2020), the time to peak and plateau were 15.2 and 25 days, respectively. The initial Rt was 3.9 [95% credible interval (CI) 3.7 to 4.2] and declined to <1 after 2 weeks. The initial epidemic doubling time was 3.8 days (3.4 to 4.2 days). The aggressive testing in the early days of the epidemic was associated with reduction in transmission speed of COVID-19. In addition, as of 13 April, the case fatality rate of COVID-19 in Korea was 2.1%, suggesting a positive effect of the targeted treatment policy for severe patients and medical resources.
Conclusions
Our findings provide important information for establishing and revising action plans based on testing strategies and severe patient care systems, needed to address the unprecedented pandemic.
Keywords: COVID-19 epidemic, aggressive testing, epidemiological pattern, severe-patients-targeted treatment, South Korea
Key Messages
We examined epidemiological and spatiotemporal patterns of COVID-19 spread in South Korea.
We demonstrated associations suggesting the effectiveness of the early implementation of an ‘aggressive’ testing strategy to address transmission speed and reproduction number reduction.
Our results suggest the need for differentiated intervention policies: rapid and intensive interventions in the early period in the epicentre and surrounding areas, and long-term and continuous interventions in areas further from the epicentre.
A substantial reduction of the proportion of daily deaths was detected after the implementation of the updated COVID-19 response strategy for the separate care of mild and severe patients.
Case fatality rates were associated with available regional medical resources, such as number of negative pressure rooms, intensive care units and extracorporeal membrane oxygenation (ECMO) machines.
Introduction
A rapid increase in atypical pneumonia cases caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first observed in Wuhan, China, in Dec 2019.1 Since then, the novel coronavirus disease, named COVID-19, has rapidly spread to over 200 countries, areas and territories.2 The World Health Organization (WHO) declared the COVID-19 pandemic on March 11.3,4
South Korea (hereafter, Korea) has been affected by the COVID-19 epidemic, with more than 10 000 confirmed cases reported through April 2020.5 The first case in Korea was identified on 20 January 2020, and 27 cases were observed sporadically through 17 February.6 However, the spread exploded with the mass infection on 18 February, stemming from a ‘Shinchunji’ church service in the city of Daegu (with a population of about 2.5 million and the epicentre of COVID-19 in Korea), which marked the start of the nationwide epidemic in Korea.
Because Korea experienced the COVID-19 outbreak earlier than other countries, sharing information regarding epidemiological features and intervention strategies can aid the development and implementation of policies to prevent further devastation from the pandemic. Several international media outlets and governments have highlighted the ‘aggressive testing’ strategy that Korea implemented in the early stage of the epidemic and its consequences, leading to a drastic reduction in the spread and the lower fatality rate of COVID-19.7,8
However, despite growing evidence, studies that investigated the Korean case are sparse. Most have addressed the epidemic in a specific region, such as Wuhan,9 or at the country level,10 with limited information on differences in epidemiological patterns depending on regional or demographic characteristics.1 Further, a lack of quantified evidence of the effect of diagnostic tests in the early phase has been reported, although early testing, diagnosis and subsequent quarantine are acknowledged as the basic principles to reduce transmission rates.11
This study investigates the epidemiological and spatiotemporal patterns of the COVID-19 spread in Korea. We examine whether ‘aggressive testing’ in the early days of the epidemic contributed to a reduction in the spread of disease. We also explore whether improved patient care guidelines and medical resource mobilization influenced reducing the fatality rate.
Materials and Methods
Data
Study area and population
We analysed nationwide data in Korea, collected until 13 April 2020, including all eight metropolitan/special cities and nine provinces (hereafter, regions). The study area was classified into three sub-areas: Daegu (the epicentre), neighbouring areas around Daegu (Gyeongsang provinces, Busan and Ulsan) and other areas (not classified as Daegu and neighbouring areas).
COVID-19 case data
We collected daily time-series counts of the cumulative confirmed cases and deaths in Korea from 20 January to 13 April 2020, by extracting from press releases of the Korea Centers for Disease Control and Prevention (KCDC). The 382 cases confirmed at the airports and immediately quarantined were excluded in this study.5 The definition of COVID-19 cases in Korea is displayed in Table 1. The data were stratified based on age [0–19 years (y), 20–39 y, 40–59 y, 60–79 y, 80+ y) and sex. In addition, the data included the number of local and overseas imported cases; however, this classification was only provided from 25 March onwards and was not provided by age group and sex.
Table 1.
COVID-19 case definition and diagnostic testing information in Korea. KCDC: Korea Centers for Disease Control and Prevention. All contents are reported in the Korea Centers for Disease Control and Prevention (KCDC) website [http://ncov.mohw.go.kr/]
| Description | |
|---|---|
| Case definition |
|
| Diagnostic test |
|
| Testing eligibility criteria |
|
|
COVID-19 testing data
We collected daily reports of the number of diagnostic tests from the KCDC from 30 January to 13 April 2020, which were available only at a national level. During this period a total of 518 743 tests were performed, of which 2.0% were positive. Detailed information about the diagnostic test is reported in Table 1.
Medical indicators
We collected regional-level data for five medical indicators that could relate to the capacity for treatment of severe COVID-19 patients and thus are potentially linked to COVID-19 mortality. These indicators were the numbers of: available hospital beds; beds in the intensive care unit (ICU); negative pressure beds plus extracorporeal membrane oxygenation (ECMO); and numbers of hospitals equipped with the ECMO. More detailed information on data collection and variable selection can be found in the Supplementary materials (Supplementary Methods 1, available as Supplementary data at IJE online).
Statistical modelling
Growth curve estimation
We applied a logistic growth model to estimate cumulative confirmed cases on day t (Cmod, t). The logistic growth model can detect both starting and plateau points of an epidemic and assumes a ‘saturation effect’ indicating that the size of the at-risk population decreases due to public health interventions, changes in behaviour and quarantine.12 Parameters from the logistic model were used to derive the empirical distribution of the Cmod, t and daily new confirmed cases (Pmod, t = Cmod, t – Cmod, t-1) using Monte Carlo simulation, which assumes a multivariate normal distribution.13
Durations to peak and plateau points
We defined two time points related to transmission patterns over time: peaks and plateaus. We defined 18 February 2020 as the start date of the epidemic (the 31st case; the first confirmed case in Daegu, although this might not represent when the virus or disease was first present in Daegu). The peak was then defined as the day t when Pmod was the highest after 18 February, and the plateau point as the first day when Pmod was smaller than average Pmod from the start date of the epidemic until 13 April, the end date of the study, i.e. the first date of t for which Pmod, t <[Cmod, Apr. 13 – Cmod, Feb. 18] / [number of dates between 18 February and 13 April]. This point can be interpreted as an indication of maximum growth and therefore a plateau.14 Finally, we calculated the number of days from the start of the epidemic to each peak and plateau point. The empirical means and confidence intervals (eCI) for each duration were estimated by Monte Carlo simulation.
Daily reproduction numbers
We estimated the daily reproduction number (Rt; interpreted as the daily R0) following Cori et al.15 The reproduction number changes as intervention policies are implemented, thus the real-time reproduction numbers can guide control plans and provide insight into the effectiveness of intervention policies.15,16 Rt was calculated based on a combination of the observed daily confirmed cases and the distribution of the serial interval (the time interval between infection and subsequent transmission), then smoothed using a 7-day time window.17 The serial interval was assumed to follow a gamma distribution with a mean of 4.98 days and a standard deviation of 3.22 days1; sensitivity analysis was conducted based on other parameters suggested from earlier studies.18,19 Imported cases were included in the Rt calculation but not considered in the age- and sex-specific calculations, due to data limitations. All analyses were conducted using the EpiEstim R software package.15
Doubling time
The epidemic doubling time was calculated to assess the dynamic features of the COVID-19 transmission. Cmod was used to calculate the 7-day rolling doubling time from 18 February 2020 to the end date of the study (13 April 2020). The eCIs of the doubling time were estimated by Monte Carlo simulation.
Effects of aggressive testing
In this study, we defined ‘aggressive testing’ as a combination of rapid and widespread testing. Since KCDC has conducted full case investigations for any space with confirmed infections and testing on all potentially infected people with overlapping moving patterns and routines within a few days of the outbreak, we determined that testing policies in Korea were implemented quickly and showed degrees of fluidity and scaleability. Therefore we describe the Korean testing policies as ‘aggressive’. More detailed information on interventions in Korea indicating the rapid and widespread testing are provided in Supplementary Method 2, available as Supplementary data at IJE online.
We hypothesized that an increase in testing was associated with reduced transmission of COVID-19. To examine this hypothesis, we estimated the association between the daily number of tests and changes in the number of daily confirmed cases (Pmod, t – Pmod, t-1; i.e. the speed of transmission) using a distributed lag model. In this analysis, we restricted data to the early period of the epidemic defined as the first 4 weeks from the start date of the epidemic, because the daily number of tests was maintained at around 10 000 per day during the first 4 weeks and decreased afterwards, along with the stabilization in new confirmed cases. We used a natural cubic spline with two equally spaced knots on the log scale to consider the lagged effect of testing up to 14 lag days. For sensitivity analysis, we changed modelling specifications to examine the consistency of the results. Also, we assessed the association between the number of tests and daily Rt with a 7-day rolling regression model using a 7-day moving average as a predictor variable.
Fatalities
We compared daily changes in the proportion of new deaths relative to the cumulative confirmed cases before and after revision of the COVID-19 response guidance announced by the KCDC on 1 March 2020 (COVID-19 Action Procedures, 7th edition),20 using two-sample t tests. Before the revision, all confirmed patients were hospitalized. However, the exponentially growing number of new cases created challenges in securing hospital beds, and therefore the KCDC modified its directive to hospitalize only severe cases. Patients with mild symptoms were transferred to a temporary care centre and treated under quarantine. We repeated the t test by areas, age groups and sex.
Second, we examined how the medical resources for each region were associated with the case fatality rates (CFRs) using weighted regression models. We considered population, sex ratio, the percentage of people aged ≥ 60 years, gross regional domestic product (GRDP) per capita and age-sex standardized smoking prevalence as confounders. Detailed information on data collection and frameworks of the regression model are described in the Supplementary data (Supplementary Method 3, available as Supplementary data at IJE online).
Results
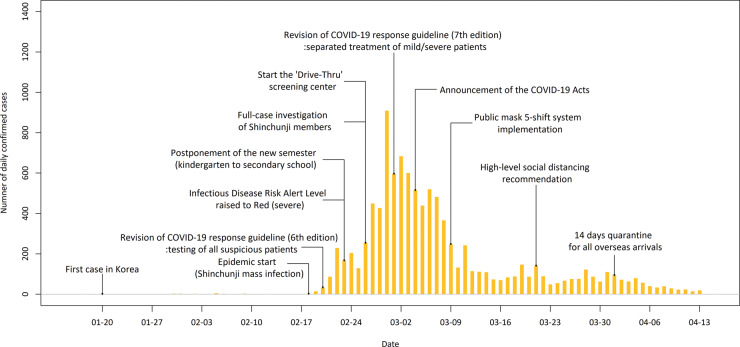
Figure 1 displays the time trend of daily confirmed cases in Korea and the timing of major interventions implemented by the KCDC and Korean government. Full lists of the interventions and major epidemic events with timelines are provided in Supplementary Tables S3 and S4, available as Supplementary data at IJE online. Through 13 April, a total of 10 155 confirmed COVID-19 cases were reported in Korea, with 6819 (67.1% of the total cases) in Daegu, 1619 (15.9%) in areas neighbouring Daegu and 1717 (16.9%) in other areas. Detailed descriptive information is provided in the Supplementary Table S5, available as Supplementary data at IJE online.
Figure 1.
Daily series of COVID-19 cases with major interventions in Korea. The period shown ranges from 20 January (date of the first confirmed case in Korea) to 13 April 2020 (last day of the study). The major interventions indicate those implemented by the Korea Centers for Disease Control and Prevention (KCDC) and the Korean government
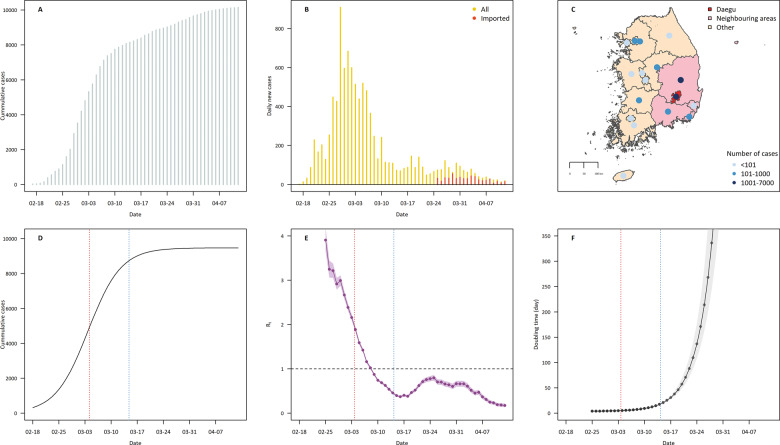
Figure 2A shows the time trend of cumulative cases from the date of the first diagnosed case in Korea. Figure 2B displays daily confirmed cases with imported cases from 25 March and shows that over 60% of confirmed cases were imported after 1 April. Figure 2C displays geographical distributions of the numbers of cumulative cases on 13 April . The fitted logistic growth model for cumulative cases is displayed in Figure 2D. The R2 of the model was 0.99. Figure 2E shows the daily reproduction number (Rt), which was initially 3.9 [95% credible interval (CI) 3.7 to 4.2] and declined to <1 after 14 days. The results of sensitivity analysis for Rt are reported in Supplementary Figure S1, available as Supplementary data at IJE online, and the results were influenced by the mean value of the serial interval. Figure 2F shows the epidemic doubling time; the initial doubling time was 3.8 days (95% eCI 3.4 to 4.2 days). The logistic growth curves and Rts for each region are displayed in Supplementary Figures S2 and S3, available as Supplementary data at IJE online.
Figure 2.
Epidemiological aspects of COVID-19 in Korea after the beginning of the epidemic in Daegu (18 February 2020). (A) Daily series of cumulative confirmed COVID-19 cases. (B) Daily series of confirmed cases after 18 February 2020 (yellow) with imported cases counted from 25 March. (C) Geographical distribution of confirmed cases across all metropolitan/special cities and provinces in Korea. Metropolitan/special cities and provinces were divided into three areas: Daegu (the epicentre), neighbouring (regions adjacent to Daegu) and other areas. Coloured dots indicate the cumulative number of confirmed cases on 13 April. (D) Estimated logistic growth curve for the cumulative COVID-19 cases in Korea with peak (red) and plateau (blue) points. (E) Time-varying reproduction number (Rt) of COVID-19 in Korea with 95% credible intervals. The vertical lines indicate peak (red) and plateau (blue) points. (F) Time-varying epidemic doubling time; 7-day rolling estimates were applied. The vertical lines indicate peak (red) and plateau (blue) points.
Table 2 shows information on the national spread pattern of COVID-19. The number of days from the start date of the epidemic to peak and plateau was 15.2 (95% eCI 15 to 16 days) and 25.8 (95% eCI 25 to 26 days), respectively. Daily confirmed cases at peak and plateau were estimated as 537.8 and 167.0, respectively, and Rt was 2.2 at peak and 0.5 at plateau point.
Table 2.
Epidemiological aspects of the COVID-19 in Korea. Time: the number of days from the beginning of the COVID-19 outbreak in Korea (18 February 2020: the first diagnosed case in Daegu) to each time point. Daily cases: the modelled daily confirmed cases at each time point. Rt: estimated reproduction numbers at each time point, t. Doubling time: epidemic doubling time from 18 February 2020 to each time point. Neighbouring: neighboring areas around Daegu
| Time points (t) | Time from initial outbreak (days) | Daily cases | Rt | Doubling time (days) | |
|---|---|---|---|---|---|
| National | After a week | – | 244.9 (226.7 to 263.3) | 3.9 (3.7 to 4.2) | 3.8 (3.4 to 4.2) |
| Peak | 15.2 (15 to 16) | 537.8 (495.9 to 583.6) | 2.2 (2.1 to 2.2) | 4.8 (4.3 to 5.3) | |
| Plateau | 25.8 (25 to 26) | 167.0 (152.5 to 184.0) | 0.5 (0.4 to 0.5) | 17.4 (16.2 to 18.9) | |
| Areas | |||||
| Daegu | After a week | – | 174.1 (161.4 to 185.5) | 4.2 (3.8 to 4.5) | 2.9 (2.6 to 3.1) |
| Peak | 14.3 (14 to 15) | 483.1 (455.2 to 512.7) | 2.7 (2.6 to 2.8) | 3.4 (3.1 to 3.7) | |
| Plateau | 23.4 (23 to 24) | 120.3 (103.9 to 133.6) | 0.6 (0.6 to 0.7) | 11.3 (10.7 to 11.9) | |
| Neighbouring | After a week | – | 52.8 (50.3 to 55.4) | 3.5 (3.2 to 4.0) | 3.6 (3.3 to 3.9) |
| Peak | 13.5 (13 to 14) | 93.1 (87.4 to 99.3) | 1.7 (1.5 to 1.8) | 4.6 (4.2 to 5.0) | |
| Plateau | 23.7 (23 to 24) | 26.6 (24.2 to 29.7) | 0.6 (0.5 to 0.6) | 16.9 (15.9 to 18.0) | |
| Others | After a week | – | 14.0 (13.4 to 14.5) | 3.8 (3.0 to 4.7) | 9.2 (8.7 to 9.8) |
| Peak | 32.3 (31 to 33) | 44.7 (43.3 to 46.2) | 0.9 (0.8 to 1.0) | 14.1 (13.5 to 14.7) | |
| Plateau | 46.3 (45 to 48) | 29.9 (28.8 to 30.8) | 0.5 (0.4 to 0.6) | 29.0 (27.5 to 30.8) | |
| Age groups | |||||
| 0–19 y | After a week | – | 11.5 (10.3 to 12.7) | 6.8 (4.5 to 9.7) | 4.2 (3.7 to 4.7) |
| Peak | 17.9 (17 to 18) | 32.6 (29.9 to 35.7) | 1.6 (1.4 to 1.8) | 5.6 (4.9 to 6.4) | |
| Plateau | 28.8 (28 to 30) | 11.3 (10.4 to 12.3) | 0.4 (0.3 to 0.5) | 19.1 (17.5 to 21.0) | |
| 20–39 y | After a week | – | 101.7 (91.0 to 111.4) | 4.6 (4.1 to 5.2) | 3.4 (3.0 to 3.9) |
| Peak | 14.7 (14 to 15) | 227.1 (203.7 to 254.0) | 2.2 (2.1 to 2.3) | 4.4 (3.8 to 5.1) | |
| Plateau | 24.5 (24 to 25) | 64.5 (57.7 to 71.2) | 0.4 (0.4 to 0.5) | 16.7 (15.0 to 18.6) | |
| 40–59 y | After a week | – | 85.5 (80.1 to 90.9) | 3.8 (3.4 to 4.2) | 4.0 (3.6 to 4.4) |
| Peak | 15.0 (15 to 15) | 171.5 (159.3 to 185.1) | 2.1 (1.9 to 2.2) | 5.1 (4.6 to 5.6) | |
| Plateau | 25.7 (25 to 26) | 54.6 (50.1 to 60.0) | 0.5 (0.5 to 0.6) | 18.3 (16.9 to 19.9) | |
| 60–79 y | After a week | – | 41.6 (39.0 to 44.2) | 3.1 (2.6 to 3.6) | 4.7 (4.2 to 5.1) |
| Peak | 16.9 (16 to 17) | 89.3 (83.1 to 96.7) | 1.6 (1.5 to 1.7) | 6.2 (5.6 to 6.9) | |
| Plateau | 28.3 (28 to 29) | 33.3 (30.9 to 35.8) | 0.4 (0.4 to 0.5) | 18.9 (17.6 to 20.3) | |
| 80+ y | After a week | – | 4.9 (4.5 to 5.3) | 4.0 (1.8 to 7.0) | 5.8 (5.3 to 6.3) |
| Peak | 24.0 (23 to 25) | 17.5 (16.5 to 18.7) | 1.0 (0.8 to 1.2) | 8.4 (7.8 to 9.1) | |
| Plateau | 36.4 (36 to 37) | 8.4 (7.9 to 8.9) | 1.4 (1.2 to 1.6) | 21.9 (20.6 to 23.5) | |
| Sex | |||||
| Male | After a week | – | 87.8 (80.9 to 94.5) | 3.4 (3.1 to 3.8) | 4.8 (4.3 to 5.4) |
| Peak | 16.8 (16 to 17) | 177.7 (162.9 to 196.9) | 1.6 (1.5 to 1.7) | 6.5 (5.7 to 7.3) | |
| Plateau | 28.4 (27 to 29) | 68.1 (63.2 to 73.1) | 0.4 (0.4 to 0.5) | 19.2 (17.6 to 21.0) | |
| Female | After a week | – | 153.2 (141.3 to 164.1) | 4.3 (3.9 to 4.7) | 3.8 (3.4 to 4.2) |
| Peak | 15.1 (15 to 16) | 337.9 (310.5 to 366.7) | 2.1 (2.1 to 2.2) | 4.7 (4.2 to 5.3) | |
| Plateau | 25.6 (25 to 26) | 103.2 (94.0 to 113.7) | 0.4 (0.4 to 0.4) | 17.9 (16.5 to 19.4) |
COVID-19 spread patterns in Korea differed by area as well as by age and sex. Figure 3 displays the logistic growth curves and Rts by area, age and sex. In Daegu and neighbouring areas, rapid increases in the logistic growth curves were observed in the first 3 to 4 weeks. Other areas showed distinctively slower growth patterns, reaching plateau about 3 weeks later. The initial Rts of the three areas were similar (Daegu: 4.2, neighbouring areas: 3.5, others: 3.8); however, the duration over which Rt stably decreased to <1 was longer in other areas than in Daegu and neighbouring areas. The spread was greater and faster in the younger age groups, with older groups showing flatter and slower growth patterns. The number of cumulative cases and initial Rt were greater in females than in males.
Figure 3.
Logistic growth curve and time-varying reproduction number (Rt) of COVID-19 by area, age-group and sex in Korea. (A) and( B) by area for Daegu, neighbouring area and other areas; (C) and (D) are by age groups; and (E) and (F) by sex. Panels A, C and E show logistic growth curves; B, D and F show time-varying Rt. The dots on the curves indicate the estimates at peak (red) and plateau (blue) points.
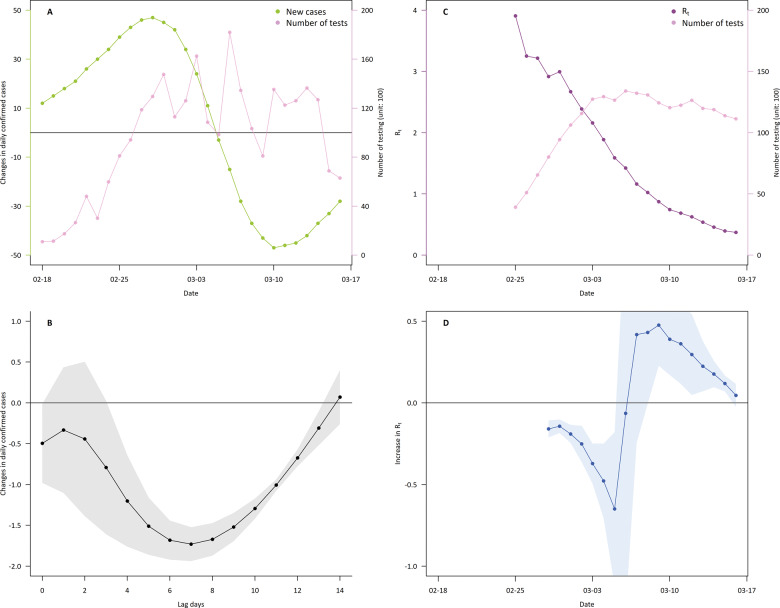
Figure 4 presents the associations of number of tests with changes in daily confirmed cases and with Rt during the first 4 weeks of the epidemic. We found that a higher number of tests was associated with a reduced number of new daily cases. Specifically, an increase of 1000 tests was associated with an overall decrease by 14.5 new cases (95% CI 10.4 to 18.8) over 14 lag days. The estimate was generally consistent with the results with changes in modelling specifications (Supplementary Table S6, available as Supplementary data at IJE online). We also found that an increase in testing was associated with a decrease in Rt within 2–3 weeks, as Rt was reduced by up to 0.65 (95% CI 0.25 to 0.71) per 1000 tests in about 2 weeks.
Figure 4.
Effects of testing on reduction in COVID-19 spread: during 4 weeks from the beginning of epidemic (18 February 2020) in Korea. (A) Changes in the number of daily confirmed cases (i.e. the speed of transmission; green), and the number of tests (pink). (B) Changes in newly confirmed cases per 1000 tests for 14 lag-days. (C) Seven-day moving average number of tests (pink) and time-varying reproduction number (Rt; purple). (D) Seven-day rolling associations between the 7-day moving average number of tests and time-varying Rt.
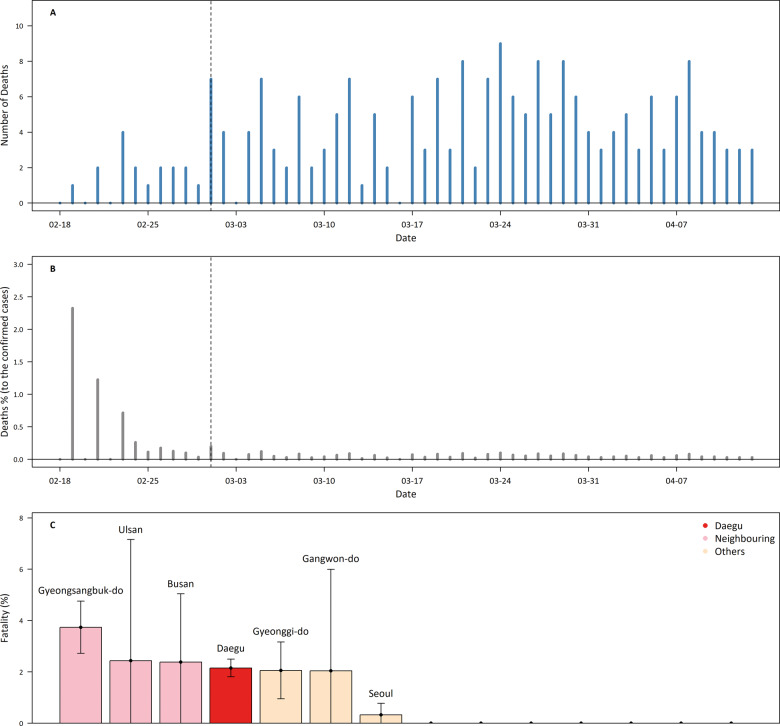
The temporal and regional trends of fatalities are displayed in Figure 5. Figure 5A shows the daily death counts. By 13 April 2020 a total of 217 deaths had occurred in Korea, 147 of which occurred in Daegu, in areas neighbouring Daegu 54 and only 16 in other areas. Among the total deaths, 199 deaths occurred in people aged 60+ y. As of 13 April, the CFR in the total population was 2.1% and was higher in Daegu and neighbouring areas than elsewhere (Supplementary Table S5). Figure 5B presents daily percentages of deaths relative to the numbers of cumulative confirmed cases during the study period. After the first day of the epidemic, the average death percentage was 0.44% before the revision of the COVID-19 response guidance (1 March), after which it decreased to 0.05% (P-value = 0.08). Furthermore, the reduction was observed in all areas (from 0.04% in other areas to 3.41% in neighbouring areas), age groups (0.01% in 80+ y to 1.3% in 60–79 y) and for men (0.67%) and women (0.1%). Figure 5C shows fatality rates by regions. Higher availability of medical resources was generally associated with lower CFR at the regional level (Table 3).
Figure 5.
Fatality aspects of COVID-19 in Korea. (A) Daily death counts of COVID-19. (B) Daily percentage of new deaths compared with the number of cumulative confirmed cases. Dashed lines in Panels A and B indicate the date of the announcement of the seventh revised guidance for the COVID-19 response system. (C) Case fatality rates on 13 April 2020 for each region in Korea. The study region was divided into three areas: Daegu (the epicentre), neighbouring (areas neighbouring Daegu) and other areas. Regions are arranged in order of case fatality rate.
Table 3.
Associations between medical indicators and fatality rate with the total cases at region level. Associations are expressed as changes in case fatality rate (CFR) with 95% confidence interval per unit increase in each indicator. Regional population, sex ratio, the percentage of people aged ≥ 60 years, gross regional domestic product (GRDP) per capita and age-sex standardized smoking prevalence were considered as confounders
| Indicator | Reduction in CFR(95% CI) | P-value |
|---|---|---|
| Number of beds in the hospital (per 1000 beds) | 0.51 (-0.03 to 1.04) | 0.106 |
| Number of beds in intensive care unit (ICU; per bed) | 0.01 (0.00 to 0.02) | 0.065 |
| Number of negative pressure beds (per bed) | 0.25 (0.06 to 0.45) | 0.038 |
| Number of hospitals equipped with the ECMOa (per hospital) | 0.47 (-0.03 to 0.98) | 0.107 |
| Number of ECMO (per machine) | 0.13 (0.03 to 0.23) | 0.042 |
ECMO machine: extracorporeal membrane oxygenation machine.
Discussion
This study investigated the epidemiological features of the COVID-19 outbreak in Korea. Increased testing was related to reductions in transmission and reproduction numbers, and the availability of medical interventions and resources for severe patients was related to a reduction in CFR.
The initial Rts we estimated at 3.9 nationally and 4.2 for Daegu. These values are generally similar to those in European countries, for which initial Rt were reported with range of 3.0 to 5.0,10 and generally lower than those reported for Wuhan (2.2 to 2.7)9,21,22 and other provinces in China (1.1 to 1.7).1 Our data showed that the initial Rt in Korea differed by age and sex: higher initial Rts were observed in younger age groups (<60 y) than in older groups (60+ y) and females compared with males. These results were due to the high fraction of women and younger people in Shinchunji; such patterns may be highly linked to the characteristics of specific mass infection events in the early epidemic phase.
Unlike reports from other countries in which that the initial Rt lasted for more than 1–2 weeks,1,9,10 the Korean data show a steady and rapid decline over time (with an Rt <1 after 2 weeks from the start date of the epidemic) and a relatively short duration between peak and plateau (about 10 days). Our results indicate that the aggressive testing approach that was implemented early in the epidemic partly contributed to the rapid reduction of the spread of COVID-19. In addition, we postulate that the higher number of tests may be related to mitigation of the spread on a multi-country scale. As of 13 April, European countries and the USA had relatively lower positive rates (≥15% in Italy, the UK, Spain and the USA) and generally had higher incidences of COVID-19 (all incidence cases per 10 000: ≥12 cases), compared with Korea (positive rate: 2%, and incidence cases per 10 000: 2 cases). Other countries reported lower positive rates (Taiwan, Australia and New Zealand; positive rates: <2%, and incidence cases per 10 000: <3 cases).23 Since multiple interventions, such as mitigation measures that encourage social/physical distancing and work-at-home policies, influence the spread of infectious disease, analysis of the isolated effectiveness of active testing is limited. However, given that aggressive testing is a leading intervention policy, this international comparison can support the hypothesis that Korea’s testing strategy contributed to the decline in COVID-19 spread.
However, we acknowledge that the decline in Rt and short durations from the start date of the epidemic to peak and plateau are unlikely to be solely attributable to aggressive testing; rather, our findings should be interpreted as a comprehensive result of a number of social and policy efforts. In particular, the following events and interventions should be considered (see Supplementary Table S3, available as Supplementary data at IJE online): first, the KCDC conducted a full case investigation of all Shinchunji members (about 200 000 persons). Moreover, the KCDC and the Korean government have strongly and repeatedly recommended a high-level social distancing strategy and the wearing of masks. On 4 March 2020, Korea announced the COVID-19 Acts, allowing prosecution of uncooperative suspected cases and suspension of entry to confirmed or suspected cases. Further, the KCDC and local governments have operated ‘Drive-Thru’ screening centres to reduce testing time and protect medical staff from cross-infection.24
Our results show that the spread and fatality patterns of COVID-19 in Korea differed between areas and age groups, which implies the benefit of differentiated prevention and mitigation strategies. Areas further away from Daegu and people aged 80+ y showed longer epidemic periods than other areas and age groups, despite having fewer cases. These findings might be linked to differences in transmission patterns centred on community and nursing hospitals for the elderly (see Supplementary Table S4). Furthermore, we found that most deaths occurred in an epicentre and neighbouring areas and older age groups. These result may contribute to prioritization of resource allocations by sub-population. We also found that treatment targeting severe patients might have contributed to the flattening of the fatality curve. As of 13 April 2020, the fatality rate in Korea (2.1%) was considerably lower than in most Western countries.10 In the early stage, the rapid increase in confirmed cases resulted in a lack of resources, and thus a fast increase in deaths. The KCDC thereafter revised its treatment guidelines20 and our results suggest the effectiveness of this approach, particularly in the epicentre and neighbouring areas. In addition, we found that fewer medical resources were related to higher fatality rates. However, since these results were not based on an individual trial and were limited in sample size, these findings should be interpreted with caution.
This study has several limitations. First, we based our serial interval distributions, used to calculate Rt, in previous studies,1,18 not on the Korean cases; infectivity profiles may differ depending on interventions, cultures and living conditions.1,25 In addition, there are potential confounding variables that we were unable to account for when assessing the association between numbers of tests and spread patterns, such as the various interventions implemented during the epidemic period. Although we conjecture that many interventions were related to diagnostic testing, further studies are needed to identify potential confounders in the effects of testing on transmission.
Despite these limitations, this study has several strengths. Firstl using statistical modelling with data from the initial period to the stabilization period of the epidemic, we reported on the overall transmission pattern of COVID-19 in Korea. Second, the study shows that an increase in testing is associated with a decline in the transmission speed in the early phase. Third, we show differences in the spread and fatality patterns depending on areas, age and sex, which suggests the benefit of differentiated intervention policies and prioritization of medical resources.
In conclusion, our study describes the epidemiological spread patterns of COVID-19 in Korea and suggests the effectiveness of intervention policies based on aggressive testing and policies for severe patient care. Findings in this study can offer important implications for the establishment and modification of action plans against this unprecedented worldwide pandemic.
Funding
This study was funded by the Korea Ministry of Environment via the ‘Climate Change Correspondence Program’ (project number: 2014001310007).
Supplementary Material
Acknowledgements
We extend appreciation to Ki Ho Hong, MD, (Department of Laboratory Medicine, Seoul Medical Center, Seoul, Korea) who provided comments regarding laboratory diagnostic testing of COVID-19 in Korea. We appreciate the funding opportunity from the Korea Ministry of Environment. We also thank the KCDC, medical staff and volunteers working to end COVID-19.
Conflict of Interest
The authors declare they have no competing financial interests.
References
- 1. Zhang J, Litvinova M, Wang W. et al. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outside Hubei province, China: a descriptive and modelling study. Lancet Infect Dis 2020, Apr 2. doi: 10.1016/S1473-3099(20)30230-9. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report – 90 Geneva: World Health Organization (WHO), 2020.
- 3.World Health Organization. WHO Virtual Press Conference on COVID-19. Geneva: WHO, 2020.
- 4.World Health Organization. Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV). Geneva: World Health Organization (WHO), 2005.
- 5.Korea Centers for Disease Control and Prevention. Updates on COVID-19 in Republic of Korea (as of 13 April) Seoul: Korea Centers for Disease Control and Prevention (KCDC), 2020.
- 6.Korea Centers for Disease Control and Prevention. Updates on COVID-19 in Republic of Korea (as of 19 February) Seoul:Korea Centers for Disease Control and Prevention (KCDC),2020.
- 7.TKealey T. South Korea listened to the experts. Cable News Network (CNN) 7 April 2020.
- 8.Sean Fleming. South Korea's Foreign Minister Explains how the Country Contained COVID-19 Cologny, Switzerland: World Economic Forum, 2020.
- 9. Kucharski AJ, Russell TW, Diamond C. et al. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis2020. PMID: 32171059. [DOI] [PMC free article] [PubMed]
- 10. Flaxman S, Mishra S, Gandy A. et al. Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries. Nature 2020, Jun 8. doi: 10.1038/s41586-020-2405-7.Onlineaheadofprint. [DOI] [Google Scholar]
- 11. Dhillon RS, Srikrishna D, Garry RF, Chowell G.. Ebola control: rapid diagnostic testing. Lancet Infect Dis 2015;15:147–48. [DOI] [PubMed] [Google Scholar]
- 12. Chowell G, Simonsen L, Viboud C, Kuang Y.. Is West Africa approaching a catastrophic phase or is the 2014 Ebola epidemic slowing down? Different models yield different answers for Liberia. PLoS Curr 2014;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee W, Kim H, Hwang S, Zanobetti A, Schwartz JD, Chung Y.. Monte Carlo simulation-based estimation for the minimum mortality temperature in temperature-mortality association study. BMC Med Res Methodol 2017;17:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Passos J, Pinho SZd, Carvalho LRd, Mischan MM.. Critical points in logistic growth curves and treatment comparisons. Sci Agric (Piracicaba, Braz) 2012;69:308–12. [Google Scholar]
- 15. Cori A, Ferguson NM, Fraser C, Cauchemez S.. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol 2013;178:1505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson R, Stockwin J, van Gaalen R. et al. Improved inference of time-varying reproduction numbers during infectious disease outbreaks. Epidemics 2019;29:100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cowling BJ, Ali ST, Ng TW. et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health 2020;5:e279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shim E, Tariq A, Choi W, Lee Y, Chowell G.. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis 2020;93:339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishiura H, Linton NM, Akhmetzhanov AR.. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis 2020;93:284–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Central Disaster Management Headquarters. COVID-19 Action Procedures. 7th edn. Seoul: Korea Centers for Disease Control and Prevention (KCDC), 2020.
- 21. Li Q, Guan X, Wu P. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med 2020;382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu JT, Leung K, Leung GM.. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet 2020;395:689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Our World in Data. Coronavirus (COVID-19) Testing 2020. https://ourworldindata.org/coronavirus-testing (28 May 2020, date last accessed).
- 24.Central Disaster Management Headquarters. COVID-19 Central Disaster Management Headquaters Regular Briefing Seoul: Korea Centers for Disease Control and Prevention (KCDC), 2020.
- 25. Litvinova M, Liu Q-H, Kulikov ES, Ajelli M.. Reactive school closure weakens the network of social interactions and reduces the spread of influenza. Proc Natl Acad Sci U S A 2019;116:13174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.