Abstract
The ongoing pandemic of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses a serious threat to global public health and there is currently no effective antiviral therapy. It has been suggested that chloroquine (CQ) and hydroxychloroquine (HCQ), which were primarily employed as prophylaxis and treatment for malaria, could be used to treat COVID-19. CQ and HCQ may be potential inhibitors of SARS-CoV-2 entry into host cells, which are mediated via the angiotensin-converting enzyme 2 (ACE2), and may also inhibit subsequent intracellular processes which lead to COVID-19, including damage to the cardiovascular (CV) system. However, paradoxically, CQ and HCQ have also been reported to cause damage to the CV system. In this review, we provide a critical examination of the published evidence. CQ and HCQ could potentially be useful drugs in the treatment of COVID-19 and other ACE2 involved virus infections, but the antiviral effects of CQ and HCQ need to be tested in more well-designed clinical randomized studies and their actions on the CV system need to be further elucidated. However, even if it were to turn out that CQ and HCQ are not useful drugs in practice, further studies of their mechanism of action could be helpful in improving our understanding of COVID-19 pathology.
Keywords: coronavirus, angiotensin-converting enzyme 2, chloroquine, hydroxychloroquine, cardiovascular system
Introduction
The coronavirus disease 2019 (COVID-19) is due to infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1–3 The most common symptoms of COVID-19 are fever and cough.4,5 Both human-to-human and asymptomatic transmission have been reported.6 The COVID-19 pandemic has rapidly evolved into a global health crisis as there is currently no proven drug for treating coronavirus patients. However, the strategy of drug repurposing may offer hope for a new approach to COVID-19 treatment.
Among the myriad existing drugs that are potential repurposing candidates for treating COVID-19, the immunomodulatory agents, chloroquine (CQ) and hydroxychloroquine (HCQ), have captured great attention. CQ and its more soluble and less toxic metabolite HCQ are primarily used for prophylaxis and treatment of malaria, but they have also been reported to effectively inhibit the effects of certain viruses, such as SARS-CoV and influenza A H5N.7–10 Recently, the possible use of CQ/HCQ as a repurposed therapeutic agent against COVID-19 has been explored.11
Angiotensin-converting enzyme 2 (ACE2), a new homolog of ACE, can convert angiotensin II (Ang II) to Ang(1–7).12,13 Ang(1–7) binds and activates the G protein-coupled receptor Mas (MasR)14 and acts as a natural damping mechanism for the activation of the classical renin–angiotensin system (RAS),12,13,15,16 which plays a critical role in maintaining normal cardiovascular (CV) functions. Apart from its crucial role in CV disease, ACE2 has also been shown to be a functional host cellular entry receptor for coronavirus that directly binds the viral spike (S) protein, which is primed by the transmembrane serine protease 2 (TMPRSS2).17–20
The ongoing COVID-19 pandemic, caused by SARS-CoV-2, poses a serious threat to global public health, and cross-sectional data suggest that SARS-CoV-2 infected patients have a high prevalence of CV disease.21,22 Recent data indicated that CQ and HCQ (CQ/HCQ) may have a promising ability to inhibit SARS-CoV-2 and other ACE2-related viral diseases,7–9 but the effects of CQ/HCQ on the CV system seem paradoxical. CQ/HCQ shows CV benefits, including a reduction in the risk of developing hyperlipidemia and diabetes mellitus, but CV disorder has also been reported as one of the rare but severe side effects of CQ/HCQ.23
In this review, we summarize and evaluate the published evidence concerning the actions and mechanisms of action of CQ/HCQ in treating SARS-CoV-2 and other ACE2-related viral infections. We conclude that further mechanistic studies as well as well-designed clinical randomized trials are needed to investigate the molecular pathogenesis of SARS-CoV-2 infection and to examine the antiviral efficacy of CQ/HCQ against COVID-19. Furthermore, the effects and mechanisms of action of CQ/HCQ on the CV system should be further investigated.
ACE2 and Its Role in Viral Infection
The RAS is a humoral regulation cascade that elegantly orchestrates key vascular physiology in humans. SARS-CoV-2 infection has been proposed to interfere with RAS through the ACE2 receptor for host cell entry (Figure 1).19,26 Severe COVID-19 infection has many clinical characteristics that are strikingly similar to the effects of overactivation of the RAS. It has been reported that coagulation is activated and accelerated in patients with SARS-CoV-2.27 The complex entry process of coronavirus into susceptible cells requires multistep actions of receptor-binding and proteolytic processing of the S protein to promote virus–cell fusion. S protein cleavage occurs at the boundary between the S1 and S2 subunits, and S is further cleaved at the S2′ site by host proteases to facilitate the fusion of viral and cellular membranes via extensive irreversible conformational changes.26,28–30 A recent study provided fresh evidence that SARS-CoV-2 exploits ACE2 and TMPRSS2 for host cell entry.26 Like SARS-CoV entry into host cells,17 the S glycoprotein domain B (SB) of SARS-CoV-2 binds to the human ACE2 (hACE2) receptor and is subsequently primed by TMPRSS2.31,32 Moreover, SARS-CoV-2 S has a similar or even higher (∼10- to 20-fold) affinity for binding to hACE2 as compared to SARS-CoV S.31,32 However, a novel and very important feature of SARS-CoV-2 S is that it harbors a furin cleavage site at the S1/S2 boundary, which is processed during biosynthesis.32 Therefore, the presence of the polybasic cleavage site in SARS-CoV-2 S, processed by furin-like proteases, may modulate tropism, transmissibility, and pathogenicity of SARS-CoV-2, making it a highly pathogenic virus, like avian influenza viruses.33 The relationship between the expression level of ACE2 and susceptibly to SARS-CoV-2 infection still remains elusive. It will thus be interesting to determine whether SARS-CoV-2 interferes with ACE2 expression and activity as well as to evaluate the functional consequence of the potential cleavage site used in SARS-CoV-2 and its impact on transmissibility and pathogenesis in animal models.
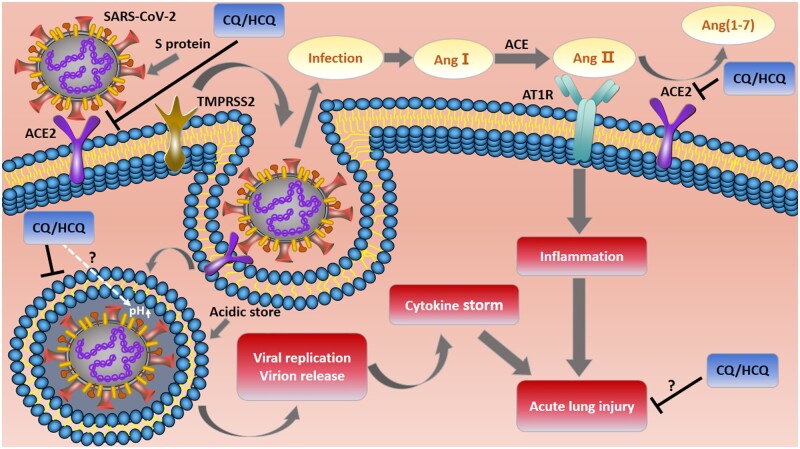
Figure 1.
The effects of chloroquine and hydroxychloroquine on ACE2-related viral infection. The initial entry of SARS-CoV-2 (an enveloped virus)24 into host cells depends on ACE2 and TMPRSS2. The S protein of SARS-CoV-2 binds to the functional receptor ACE2 and employs TMPRSS2 for its priming. S protein is cleaved by TMPRSS2 at S2′ site which results in virus/membrane fusion.25 Both ACE2 and TMPRSS2 facilitate the virus transport into the target cell through the early and late endosomes where eventually the viral genome will be released into the cell cytoplasm. SARS-CoV-2 infection could influence the balance of RAS, which leads to Ang II accumulation through the ACE/AngII/AT1R axis and eventually causes acute lung injury. CQ/HCQ may block SARS-CoV-2 fusion with the host cell and entry into the target cell through elevating the pH in the endolysosomal system and/or by interfering with the glycosylation of the ACE2 receptor and the S protein.8
In order to better understand the initial step of SARS-CoV-2 infection, elucidation of the interactional mechanism between the receptor-binding domain (RBD) of SARS-CoV-2 S and ACE2 appears to be particularly important. Two recent independent studies have reported the cryogenic electron microscopy (cryo-EM) structure of the SARS-CoV-2 spike trimer.31,32 Moreover, another study presented the cryo-EM structures of the full-length hACE2-B0AT1 (the neutral amino acid transporter) complex and a complex between the RBD of SARS-CoV-2 and the hACE2-B0AT1 complex as well as the hACE2–RBD interface.34 In addition, analytical modeling of structure predicted the potential residues of SARS-CoV-2 RBD that are recognized by ACE2.35 Furthermore, X-ray crystallography data at a higher resolution showed the interaction between SARS-CoV-2 RBD and ACE2, demonstrating that SARS-CoV-2 and SARS-CoV RBD share high structural similarity.36 It remains to be investigated how SARS-CoV-2 alters the conformations of S glycoprotein trimers and the interactions between ACE2 and S proteins in receptor-mediated endocytosis. Interestingly, single-cell RNA-sequencing data from multiple healthy human tissues discovered that the SARS-CoV-2 entry receptor ACE2 and the viral entry-associated protease TMPRSS2 are highly expressed in nasal goblet and ciliated cells.37 These new insights indicate that the primary viral SARS-CoV-2 transmission occurs through infectious droplets. Although TMPRSS2 activity is essential for viral transmission, it still needs to be determined whether the endosomal cysteine proteases cathepsin B and L or other proteases, as reported in SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV),28,29,38–40 are involved in priming SARS-CoV-2 S. Hence, further mechanistic studies are needed to elucidate the underlying detailed mechanism of SARS-CoV-2 entry into host cell and to test the potential of SARS-CoV-2 neutralizing antibodies.41,42
Several studies have reported that 3%–29% of COVID-19 patients develop acute respiratory distress syndrome (ARDS) which is a common complication and cause of death as a result of SARS-CoV-2 infection.4,5,21,22 Although the pathophysiology of COVID-19 has not been completely unraveled, the potential main mechanism of COVID-19-associated ARDS would appear to be the immune–pathological event of the so-called cytokine storm. Laboratory tests showed that patients infected with SARS-CoV-2 express high amounts of pro-inflammatory cytokines and chemokines, including interleukin (IL)-1β, tumor necrosis factor α (TNFα), interferon-γ (IFN-γ), C-X-C motif chemokine ligand-10, and monocyte chemoattractant protein 1.4 The evidence obtained from the postmortem biopsy study of a 50-year-old male patient suggested that the severe immune injury in COVID-19-associated ARDS is related to over-activation of T cells, manifested by the elevation of T-helper-17 (Th17) and high cytotoxicity of CD8 T cells.43SARS-CoV-2, SARS-CoV, and MERS-CoV cause acute lethal disease characterized by dysregulated and excessive immune responses and lung damage during viral infection. It was reported that relative delayed Type I interferon (IFN-I) signaling promoted inflammatory monocyte–macrophage accumulation in BALB/c mice infected with SARS-CoV.44 Consequently, these accumulated mononuclear macrophages produce more monocyte chemoattractants through activating the IFN-α/β receptors and mononuclear macrophage-derived pro-inflammatory cytokines, such as TNFα, IL-1β, and IL-6, induce apoptosis of T cells. Robust virus replication and excessive inflammatory responses induced the release of IFN-α/β and IFN-γ, causing inflammatory cell infiltration via Fas–Fas ligand (FasL) signaling or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)–death receptor 5 (DR5) signaling. This eventually results in the apoptosis of airway and alveolar epithelial cells.45–47 The apoptosis of these endothelial and epithelial cells could potentially lead to vascular leakage and alveolar edema, which is regarded as playing a key role in the pathogenesis of virus infection-associated ARDS. It seems to be this deadly uncontrolled cytokine storm that triggers the frantic attack on the body by the immune system causing ARDS and finally unprecedented mortality in severe cases of SARS-CoV-2 infection. Future work needs to investigate the details of the IFN signaling involved in SARS-CoV-2 infection, how the inflammatory response is triggered as well as the type of cell death that occurs during COVID-19. Also, further autopsy or biopsy studies, including more patients of different ages and backgrounds, would be needed to examine the histopathological changes and ACE2 levels in different tissues.
CQ/HCQ Actions on pH
The acid milieu in endosomes and lysosomes (pH between 5 and 6) is due to a bafilomycin-sensitive pump that concentrates H+ in the lumen of endosomes/lysosomes.48 This low pH is essential for virus/cell membrane fusion.8,49–52 CQ was reported to cause an increase in the intra-lysosomal pH of macrophages.53 However, there is still no evidence showing the effect of HCQ on the pH dynamics of endosomes/lysosomes. Nevertheless, both CQ and HCQ are weak bases so they should both be able to elevate endosomal pH and could thereby inhibit virus/cell membrane fusion. It has recently been reported that CQ is highly effective in the control of SARS-CoV-2 infection in vitro.11 Compared to remdesivir (GS-5734), the time-of-addition assay showed that CQ functioned at entry as well as at post-entry stages of the SARS-CoV-2 infection in Vero E6 cells.11 Similarly, another in vitro study also found that HCQ can efficiently inhibit SARS-CoV-2 infection via the same routes.54 The therapeutic effect of both CQ and HCQ may be due to blockade of the transport of SARS-CoV-2 from early endosomes (EEs) to endolysosomes (ELs), which seems to be the same viral genome releasing mechanism that operates in the case of SARS-CoV. However, the mode of actions of CQ and HCQ showed discrepancy in certain aspects such as in the morphology and pH values of endosomes/lysosomes.54 Another recent study found that HCQ exhibited a smaller EC50 than CQ in in vitro anti-SARS-CoV-2 activity and physiologically-based pharmacokinetic models indicated that HCQ is likely to be more effective than CQ in the treatment of SARS-CoV-2 infection.55 However, there are controversies about the effect of CQ/HCQ in altering endosomal/lysosomal pH and treating viral infection. It was reported that CQ/HCQ could directly bind to nucleic acids and inhibit the activation of the endosomal Toll-like receptor (TLR) by masking TLR ligand-binding epitopes rather than increase the endosomal pH.56 In addition, CQ was shown to inhibit autophagy mainly through impairing autophagosome fusion with lysosomes rather than by increasing pH in this organelle.49 Furthermore, there was a study showing that CQ could enhance porcine circovirus 2 infection of porcine epithelial cells via inhibition of endosome–lysosome system acidification.57 The effect of CQ/HCQ may differ from cell type to cell type and between virus species. Nevertheless, whether CQ/HCQ are able to affect the acidity of EEs and ELs in SARS-CoV-2 infection should be examined carefully in the future.
The progressive acidification that normally occurs from EEs to ELs depends on a high Ca2+ concentration in the EEs. The pH in the lumen of these organelles decreases in line with a decrease in the Ca2+ concentration.57 Ca2+ signaling has been demonstrated to be involved in viral fusion into host cells of many viruses such as Ebola virus (EBOV), MERS-CoV, and SARS-CoV.58 Ca2+ release from intracellular stores within the endolysosomal system, via two-pore channels (TPC1, TPC2) and channels belonging to the mucolipin family (eg TRPML1) can be evoked by nicotinic acid adenine dinucleotide phosphate (NAADP) and phosphatidylinositol 3,5-bisphosphate (PIP2).58,59 In pancreatic acinar cells, antibodies against TPC2 are very effective, and much more effective than antibodies against TPC1, in reducing NAADP-elicited Ca2+ release from acidic stores.60 In this context, it is of particular interest that it has very recently been shown that blocking TPC2 activity by tetrandrine decreases entry of SARS-COV-2 S pseudovirions.61 In contrast, a TRPML1 inhibitor had no effect.61 The endolysosomal Ca2+ level and pH, altered by TPC activity,62–64 regulate the activity of furin required for proteolytic activation of the S protein and viral fusion.65,66 It was reported that inhibition of TPCs rather than TRPML1 could block MERS-CoV infectivity.67 Moreover, it has been demonstrated that the endosomal calcium channels TPC1 and TPC2 are necessary for EBOV infection.58 Interestingly, tetrandrine has been identified as a highly potent and low cytotoxic TPCs inhibitor.58 Because of its ability to disrupt TPCs function, tetrandrine can prevent EBOV from escaping the endosomal network into the cell cytoplasm and thus block EBOV infection. Some other Ca2+ channel blockers such as amiodarone, verapamil, nimodipine, diltiazem, bepridil, and lomerizine could effectively protect against filoviral entry into target cells.68,69 Since these calcium channels are responsible for controlling trafficking and translocation of endosomes containing virus particles, it would be potentially intriguing to explore the effect of TPCs in SARS-CoV-2 infectivity and to screen Ca2+ channel blockers for their effects in halting SARS-CoV-2 infection.
Although an open-label nonrandomized clinical trial with a small sample size showed that HCQ treatment is significantly associated with SARS-CoV-2 load reduction/disappearance in COVID-19 patients,70 there are several serious limitations in that study. Furthermore, a multicenter prospective observational study reported that CQ has the potential to shorten the time to SARS-CoV-2 viral suppression and duration of fever in patients with moderate symptoms at earlier stages of the disease.71 Another open-label, randomized controlled trial (RCT) did not show additional benefits of virus elimination from adding HCQ to the current standard of care in patients with mainly persistent mild to moderate COVID-19.72 Meanwhile, a retrospective analysis of 368 cases with confirmed SARS-CoV-2 infection indicated that using HCQ, either with or without azithromycin, could not reduce the risk of needing mechanical ventilation in patients hospitalized with COVID-19.73 Fortunately, at least 14 clinical trials are already registered in the clinicaltrials.gov74 to evaluate the effects of CQ/HCQ to SARS-CoV-2.
CQ/HCQ Relationships to ACE2
There is much evidence indicating that SARS-CoV and SARS-CoV-2 infect host cells through ACE2.17,18,61,75,76 Furthermore, CQ could block SARS-CoV fusion with and entry into the host cell through interfering with the glycosylation of the ACE2 receptor and the S protein.8 CQ/HCQ have promising ability to inhibit SARS-CoV-2 and ACE2-related viral infection. It has been shown that CQ is an effective inhibitor of the replication of the SARS-CoV in Vero E6 cell culture.77 Another study further confirmed that CQ is effective against SARS-CoV in Frankfurt and Urbani strains.8 In addition, this study found that CQ impaired the terminal glycosylation of ACE2, suggesting that the variations in its glycosylation status might result in the ACE2–SARS-CoV interaction being less efficient and therefore inhibit virus entry when the cells are treated with CQ. Also, it was shown that the recombinant SARS-CoV S protein downregulates ACE2 expression.78
In experimental mouse models, infection with avian influenza A H5N1 virus resulted in downregulation of ACE2 expression in the lung.79 Genetic inactivation of ACE2 caused severe lung injury in H5N1-challenged mice, suggesting a role for ACE2 in H5N1-induced lung pathologies.79 CQ was found to effectively inhibit autophagy in the lungs of avian influenza H5N1 mice and to ameliorate the acute lung injury and further, significantly improve the survival rate in mice infected with live avian influenza A H5N1 virus.9 There is also evidence that CQ had an inhibitory effect against the replication of human influenza A virus H1N1 and H3N2 in vitro.7 ACE2 could mediate the severe acute lung injury induced by influenza A (H7N9) virus infection in an experimental mouse model. Moreover, ACE2 deficiency worsened the disease pathogenesis markedly, mainly by targeting the angiotensin II receptor type 1 (AT1 receptor, AT1R).80 Therefore, the potential effects and mechanism of CQ/HCQ against ACE2-related viruses appear worth further investigation.
Paradoxical Effect of CQ/HCQ in the CV System
CQ/HCQ show CV benefits, including reductions in the risks of developing hyperlipidemia, diabetes mellitus, and thrombosis, as well as improving insulin sensitivity, glucose profiles, and HbA1c, and decreasing cholesterol, triglycerides, and low-density lipoprotein-cholesterol (LDL-c).81,82 CQ/HCQ is extensively used in the treatment of rheumatic diseases, the patients of which are at higher risk of CV disease.83,84 A retrospective study of a cohort of 1266 patients with rheumatoid arthritis (RA) found that HCQ was associated with an approximately 72% reduction in the risk of CV disease.85 Another longitudinal registry showed that, compared to nonusers, RA patients with HCQ treatments had significantly lower levels of total and low-density cholesterol.86 A longitudinal cohort study of 264 systemic lupus erythematosus patients found lowered serum cholesterol levels associated with HCQ treatment.87 A prospective, multicenter observational study of 4905 adults with RA also reported that use of HCQ is associated with a 38% reduction of diabetes risk.88 HCQ is also found to reduce blood pressure variability among 899 systemic lupus erythematosus patients.89
There are a few small clinical trials studying the effects of CQ/HCQ on CV risks in humans. A RCT carried out among 116 patients with metabolic syndrome found that a 1-year CQ treatment decreased blood pressure, lipids, and the activation of c-Jun N-terminal kinase.90 Another randomized, double-blinded, placebo-controlled crossover study found that an 8-week HCQ treatment decreased insulin resistance, total cholesterol, and LDL-c among 23 RA patients.91 A small open-label clinical trial administered HCQ to 13 obese participants for 6 weeks, which significantly increased the insulin sensitivity index.92 Another RCT with 135 patients with sulfonylurea-refractory Type 2 diabetes proved that HCQ could decrease glycated hemoglobin and improve glucose tolerance.93
In animal studies, CQ could lower blood pressure through TLR signaling and prevent the subsequent recruitment of immune cells to the vasculature in spontaneously hypertensive rats.94 In rat hepatocytes, CQ was shown to be an effective inhibitor of cholesterol synthesis.95 It has also been reported that CQ improved the cardiac diastolic function by inhibiting autophagy in streptozotocin-induced heart failure with preserved ejection fraction in mice.96 Evidence was also found that activation of ataxia telangiectasia mutated with low-dose CQ decreased features of the metabolic syndrome including atherosclerosis in mice.97 Taken together, the claimed CV benefits of CQ/HCQ are mostly generated from animal studies or observational studies in humans.
In rare cases, CQ/HCQ treatment presents cardiotoxicity including hypotension, arrhythmia, atrioventricular block,98 cardiomyopathy,23,99 and heart failure,23,100 which could be serious.101,102 The cardiotoxicity of CQ/HCQ may be under-recognized.23 Among the 25 episodes of intentional CQ overdosage, 19% died and 50% had cardiac arrest.103Ex vivo acute CQ treatment decreased heart function, and in vivo chronic low-dose CQ treatment significantly decreased aortic output and total work in hearts.104 A systemic review of patients with cardiac complications attributed to CQ/HCQ found that for the 78 patients reported to have been withdrawn from CQ/HCQ treatment, 44.9% recovered normal heart function, while 12.9% had suffered irreversible damage and 30.8% died.102
The mechanisms underlying the effects of CQ/HCQ on the CV system are not fully understood (Figure 2). CQ could improve insulin sensitivity by increasing the affinity of insulin receptors, inhibiting insulin degradation, and increasing insulin secretion.103 CQ/HCQ could also increase the lipid clearance rate and expression of LDL receptors.106 HCQ is thought to protect against accelerated atherosclerosis, targeting TLR signaling, cytokine production, T-cell and monocyte activation, oxidative stress, and endothelial dysfunction.107 HCQ can also reduce the induction of endosomal NADPH oxidase (NOX) by TNFα, IL-1β, and antiphospholipid antibodies through the inhibition of the translocation of the catalytic subunit of NOX2 into the endosome, which is involved in many inflammatory and pro-thrombotic signaling pathways.108 However, chronic use of CQ/HCQ can result in an acquired lysosomal storage disorder, leading to cardiomyopathy characterized by concentric hypertrophy and conduction abnormalities associated with increased adverse clinical outcomes and mortality.109 HCQ is structurally and mechanistically similar to the Class IA antiarrhythmic quinidine,110 and may, therefore, inhibit voltage-gated sodium and potassium channels, prolonging the QT interval and increasing the risk of “torsades de pointes” (a specific type of abnormal heart rhythm) and sudden cardiac death.111 An animal study found that high-dose CQ significantly impaired mitochondrial antioxidant buffering capacity and accentuated oxidative stress and mitochondrial dysfunction in pressure-overload hypertrophy.104 In addition, CQ may increase CV risk by impairing the terminal glycosylation of ACE2,8 which possibly amplifies ACE/AngII/AT1 axis signaling and depresses ACE2/Ang1–7/MasR axis signaling.
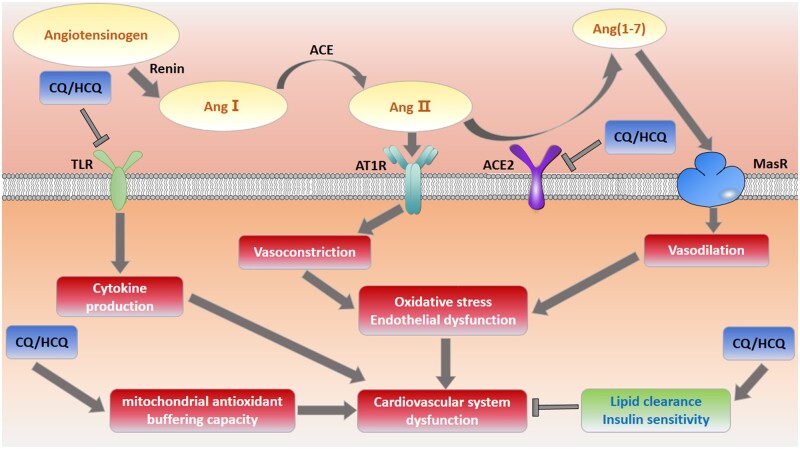
Figure 2.
The effects of chloroquine and hydroxychloroquine on the CV system. CQ/HCQ could protect against accelerated atherosclerosis targeting TLR signaling, cytokine production, T-cell and monocyte activation, oxidative stress, and endothelial dysfunction. However, CQ/HCQ interferes with the glycosylation of ACE2 and this leads to dysregulation of the RAS, which eventually causes imbalance of the ACE/AngII/AT1 axis and the ACE2/Ang1–7/MasR axis. Meanwhile, CQ/HCQ can cause cardiotoxicity which may increase the risk of CV disease. Therefore, CQ/HCQ may have a paradoxical effect on the CV system.
Concluding Remarks
The role of ACE2 in the action of CQ/HCQ needs to be further studied. Based on the existing studies, CQ/HCQ may be potential drugs for treatment of COVID-19 and other ACE2-related virus infections. However, the use of CQ/HCQ should be dealt with cautiously and careful monitoring of potential cardiotoxicity is required in clinical practice and research. The use of high doses and long-term CQ/HCQ treatment requires particular care and serious consideration.
Understanding how the virus enters host cells, and the details surrounding how it binds to the receptor on the host cell, are critical for facilitating the development of detection methods, antiviral therapeutics, and vaccines. Reliable information on the molecular mechanisms underlying viral entry and proliferation will enable us to target and combat the virus.
Finally, it is challenging to interpret the extensive amount of COVID-19-related research that has been published within a very short space of time. This is a highly unusual situation in the routine life cycles of any research topic. We, therefore, need to maintain a degree of healthy skepticism when interpreting the COVID-19-related scientific literature.
Search Strategy
We carried out electronic searches using PubMed, Web of Science, ResearchGate, and Google. The search terms were “virus,” “coronavirus,” “angiotensin-converting enzyme 2,” “chloroquine,” “hydroxychloroquine,” “cardiovascular system,” and others, alone and in combination. Many firstly identified references were investigated further to find the original primary research articles that were then cited in the review.
Funding
H.W. is supported by the National Natural Science Foundation of China (81871542 and 81670359). S.P. is supported by the Medical Scientific Research Foundation of Guangdong Province, China (A2019205 and A2020121), and the Fundamental Research Funds for the Central Universities (21620424).
Conflict of interest statement
None declared.
References
- 1. Zhu N, Zhang D, Wang W, et al. China novel coronavirus I and research T. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382(18):727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579(7798):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020;5(4):536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan WJ, Ni ZY, Hu Y, et al. , China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV Infection from an asymptomatic contact in Germany. N Engl J Med 2020;382(10):970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ooi EE, Chew JS, Loh JP, Chua RC. In vitro inhibition of human influenza A virus replication by chloroquine. Virol J 2006;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yan Y, Zou Z, Sun Y, et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res 2013;23(2):300–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis 2003;3(11):722–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30(3):269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000;87(5):E1–E–9.. [DOI] [PubMed] [Google Scholar]
- 13. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 2000;275(43):33238–332. [DOI] [PubMed] [Google Scholar]
- 14. Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 2003;100(14):8258–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111(20):2605–26. [DOI] [PubMed] [Google Scholar]
- 16. Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383(Pt 1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426(6965):450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11(8):875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yogasundaram H, Hung W, Paterson ID, Sergi C, Oudit GY. Chloroquine-induced cardiomyopathy: a reversible cause of heart failure. ESC Heart Fail 2018;5(3):372–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Donnell VB, Thomas D, Stanton R, et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function 2020;1:zqaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bosch BJ, van der Zee R, de Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol 2003;77(16):8801–8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181(2):271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 2020;58(7):1116–1120. [DOI] [PubMed] [Google Scholar]
- 28. Bosch BJ, Bartelink W, Rottier PJ. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J Virol 2008;82(17):8887–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci USA 2009;106(14):5871–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madu IG,, Roth SL, Belouzard S, Whittaker GR. Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide. J Virol 2009;83(15):7411–7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020;367(6483):1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020;181(2):281–292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tse LV, Hamilton AM, Friling T, Whittaker GR. A novel activation mechanism of avian influenza virus H9N2 by furin. J Virol 2014;88(3):1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020;367(6485):1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 2020;94(7):e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020;581(7807):215–220. [DOI] [PubMed] [Google Scholar]
- 37. Sungnak W, Huang N, Becavin C, et al. , Network HCALB. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020;26(5):681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci USA 2005;102(33):11876–11881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park JE, Li K, Barlan A, et al. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc Natl Acad Sci USA 2016;113(43):12262–12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsuyama S, Shirato K, Kawase M, et al. Middle East respiratory syndrome coronavirus spike protein is not activated directly by cellular furin during viral entry into target cells. J Virol 2018;92(19):e00683–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu Y, Wang F, Shen C, et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020;368(6496):1274–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rogers TF, Zhao F, Huang D, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016;19(2):181–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Herold S, Steinmueller M, von Wulffen W, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med 2008;205(13):3065–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hogner K, Wolff T, Pleschka S, et al. Macrophage-expressed IFN-beta contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLoS Pathog 2013;9(2):e1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rodrigue-Gervais IG, Labbe K, Dagenais M, et al. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe 2014;15(1):23–35. [DOI] [PubMed] [Google Scholar]
- 48. Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol 1998;8(24):1335–1338. [DOI] [PubMed] [Google Scholar]
- 49. Mauthe M, Orhon I, Rocchi C, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018;14(8):1435–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shibata M, Aoki H, Tsurumi T, et al. Mechanism of uncoating of influenza B virus in MDCK cells: action of chloroquine. J Gen Virol 1983;64(Pt 5):1149–11. [DOI] [PubMed] [Google Scholar]
- 51. Misinzo G, Meerts P, Bublot M, Mast J, Weingartl HM, Nauwynck HJ. Binding and entry characteristics of porcine circovirus 2 in cells of the porcine monocytic line 3D4/31. J Gen Virol 2005;86(Pt 7):2057–2068. [DOI] [PubMed] [Google Scholar]
- 52. Long J, Wright E, Molesti E, Temperton N, Barclay W. Antiviral therapies against Ebola and other emerging viral diseases using existing medicines that block virus entry. F1000Research 2015;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA 1978;75(7):3327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020;71(15):732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 2011;186(8):4794–4804. [DOI] [PubMed] [Google Scholar]
- 57. Misinzo G, Delputte PL, Nauwynck HJ. Inhibition of endosome-lysosome system acidification enhances porcine circovirus 2 infection of porcine epithelial cells. J Virol 2008;82(3):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sakurai Y, Kolokoltsov AA, Chen CC, et al. Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 2015;347(6225):995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Calcraft PJ, Ruas M, Pan Z, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009;459(7246):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gerasimenko JV, Charlesworth RM, Sherwood MW, et al. Both RyRs and TPCs are required for NAADP-induced intracellular Ca(2)(+) release. Cell Calcium 2015;58(3):237–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020;11(1):1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morgan AJ, Galione A. Fertilization and nicotinic acid adenine dinucleotide phosphate induce pH changes in acidic Ca(2+) stores in sea urchin eggs. J Biol Chem 2007;282(52):37730–3773. [DOI] [PubMed] [Google Scholar]
- 63. Cosker F, Cheviron N, Yamasaki M, et al. The ecto-enzyme CD38 is a nicotinic acid adenine dinucleotide phosphate (NAADP) synthase that couples receptor activation to Ca2+ mobilization from lysosomes in pancreatic acinar cells. J Biol Chem 2010;285(49):38251–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Petersen OH, Gerasimenko OV, Gerasimenko JV. Endocytic uptake of SARS-CoV-2: the critical roles of pH, Ca2+, and NAADP. Function 2020;1:zqaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anderson ED, VanSlyke JK, Thulin CD, Jean F, Thomas G. Activation of the furin endoprotease is a multiple-step process: requirements for acidification and internal propeptide cleavage. EMBO J 1997;16(7):1508–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Molloy SS, Bresnahan PA, Leppla SH, Klimpel KR, Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem 1992;267(23):16396–16402. [PubMed] [Google Scholar]
- 67. Gunaratne GS, Yang Y, Li F, Walseth TF, Marchant JS. NAADP-dependent Ca(2+) signaling regulates Middle East respiratory syndrome-coronavirus pseudovirus translocation through the endolysosomal system. Cell Calcium 2018;75:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gehring G, Rohrmann K, Atenchong N, et al. The clinically approved drugs amiodarone, dronedarone and verapamil inhibit filovirus cell entry. J Antimicrob Chemother 2014;69(8):2123–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Johansen LM, DeWald LE, Shoemaker CJ, et al. A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity. Sci Transl Med 2015;7(290):290ra89. [DOI] [PubMed] [Google Scholar]
- 70. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020;56(1):105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71. Huang M, Li M, Xiao F, et al. Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. Natl Sci Rev 2020. doi: 10.1093/nsr/nwaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 2020;369:m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv 2020:2020.04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. National Library of Medicine (US). ClinicalTrials.gov. Available at: https://clinicaltrials.gov/. Accessed 14 March 2020.
- 75. Letko M, Munster V. Functional assessment of cell entry and receptor usage for lineage B β-coronaviruses, including 2019-nCoV. bioRxiv 2020:2020.01.22.915660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet 2020;395(10223):470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun 2004;323(1):264–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Glowacka I, Bertram S, Herzog P, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol 2010;84(2):1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zou Z, Yan Y, Shu Y, et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun 2014;5:3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yang P, Gu H, Zhao Z, et al. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep 2014;4:7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Liu D, Li X, Zhang Y, et al. Chloroquine and hydroxychloroquine are associated with reduced cardiovascular risk: a systematic review and meta-analysis. Drug Des Devel Ther 2018;12:1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2018;77(1):98–103. [DOI] [PubMed] [Google Scholar]
- 83. Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997;145(5):408–415. [DOI] [PubMed] [Google Scholar]
- 84. Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthrit Rheum 2001;44(10):2331–2337. [DOI] [PubMed] [Google Scholar]
- 85. Sharma TS, Wasko MC, Tang X, et al. Hydroxychloroquine use is associated with decreased incident cardiovascular events in rheumatoid arthritis patients. J Am Heart Assoc 2016;5(1):e002867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthrit Care Res 2014;66(11):1619–16. [DOI] [PubMed] [Google Scholar]
- 87. Petri M, Lakatta C, Magder L, Goldman D. Effect of prednisone and hydroxychloroquine on coronary artery disease risk factors in systemic lupus erythematosus: a longitudinal data analysis. Am J Med 1994;96(3):254–25. [DOI] [PubMed] [Google Scholar]
- 88. Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA 2007;298(2):187–193. [DOI] [PubMed] [Google Scholar]
- 89. Reese T, Dickson AL, Shuey MM, Gandelman JS, Barnado A. Increased blood pressure visit-to-visit variability in patients with systemic lupus erythematosus: association with inflammation and comorbidity burden. Lupus2019;28(8):954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McGill JB, Johnson M, Hurst S, et al. Low dose chloroquine decreases insulin resistance in human metabolic syndrome but does not reduce carotid intima-media thickness. Diabetol Metab Syndr 2019;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Solomon DH, Garg R, Lu B, et al. Effect of hydroxychloroquine on insulin sensitivity and lipid parameters in rheumatoid arthritis patients without diabetes mellitus: a randomized, blinded crossover trial. Arthrit Care Res 2014;66(8):1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mercer E, Rekedal L, Garg R, Lu B, Massarotti EM, Solomon DH. Hydroxychloroquine improves insulin sensitivity in obese non-diabetic individuals. Arthrit Res Ther 2012;14(3):R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gerstein HC, Thorpe KE, Taylor DW, Haynes RB. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas–a randomized trial. Diabetes Res Clin Pract 2002;55(3):209–2. [DOI] [PubMed] [Google Scholar]
- 94. McCarthy CG, Wenceslau CF, Goulopoulou S, Baban B, Matsumoto T, Webb RC. Chloroquine suppresses the development of hypertension in spontaneously hypertensive rats. Am J Hypertens 2017;30(2):173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Beynen AC, van der Molen AJ, Geelen MJ. Inhibition of hepatic cholesterol biosynthesis by chloroquine. Lipids 1981;16(6):472–47. [DOI] [PubMed] [Google Scholar]
- 96. Yuan X, Xiao YC, Zhang GP, et al. Chloroquine improves left ventricle diastolic function in streptozotocin-induced diabetic mice. Drug Des Devel Ther 2016;10:2729–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schneider JG, Finck BN, Ren J, et al. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab 2006;4(5):377–389. [DOI] [PubMed] [Google Scholar]
- 98. Lopez-Ruiz N, Uribe CE. Chloroquine cardiomyopathy: beyond ocular adverse effects. BMJ Case Rep 2014;2014:bcr2014205751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dogar MU, Shah NN, Ishtiaq S, et al. Hydroxychloroquine-induced restrictive cardiomyopathy: a case report. Postgrad Med J 2018;94(1109):185–186. [DOI] [PubMed] [Google Scholar]
- 100. Baguet JP, Tremel F, Fabre M. Chloroquine cardiomyopathy with conduction disorders. Heart 1999;81(2):221–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis 2007;7(8):549–558. [DOI] [PubMed] [Google Scholar]
- 102. Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers YM. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf2018;41(10):919–931. [DOI] [PubMed] [Google Scholar]
- 103. Britton WJ, Kevau IH. Intentional chloroquine overdosage. Med J Australia. 1978;2(9):407–4. [DOI] [PubMed] [Google Scholar]
- 104. Chaanine AH, Gordon RE, Nonnenmacher M, Kohlbrenner E, Benard L, Hajjar RJ. High-dose chloroquine is metabolically cardiotoxic by inducing lysosomes and mitochondria dysfunction in a rat model of pressure overload hypertrophy. Physiol Rep 2015;3(7):e12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bevan AP, Christensen JR, Tikerpae J, Smith GD. Chloroquine augments the binding of insulin to its receptor. Biochem J 1995;311(Pt 3):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lange Y, Duan H, Mazzone T. Cholesterol homeostasis is modulated by amphiphiles at transcriptional and post-transcriptional loci. J Lipid Res 1996;37(3):534–539. [PubMed] [Google Scholar]
- 107. Floris A, Piga M. Protective effects of hydroxychloroquine against accelerated atherosclerosis in systemic lupus erythematosus. Mediators Inflamm2018;2018:3424136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Muller-Calleja N, Manukyan D, Canisius A, Strand D, Lackner KJ. Hydroxychloroquine inhibits proinflammatory signalling pathways by targeting endosomal NADPH oxidase. Ann Rheum Dis 2017;76(5):891–897. [DOI] [PubMed] [Google Scholar]
- 109. Yogasundaram H, Putko BN, Tien J, et al. Hydroxychloroquine-induced cardiomyopathy: case report, pathophysiology, diagnosis, and treatment. Can J Cardiol 2014;30(12):1706–1715. [DOI] [PubMed] [Google Scholar]
- 110. Ballestero JA, Plazas PV, Kracun S, et al. Effects of quinine, quinidine, and chloroquine on alpha9alpha10 nicotinic cholinergic receptors. Mol Pharmacol 2005;68(3):822–829. [DOI] [PubMed] [Google Scholar]
- 111. Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]