to the editor—We read with great interest the article published by Nickbakhsh et al [1] describing epidemiological evidence of interaction between seasonal coronaviruses and other co-circulating viruses in a United Kingdom population. The authors have suggested that prior exposure of children to coronavirus OC43 offers protection against severe COVID-19 phenotype by possible cross-immunity. These observations encouraged us to investigate the possible role of Plasmodium infection on coronavirus disease 2019 (COVID-19) infection or severity.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and mortality rates are variable in different countries. Several factors may help account for the variability in the virus infection or mortality rate, such as age, sex, comorbidity, or genetic makeup. Recent studies indicated that protozoan infections may offer some protection against various positive-strand RNA viruses. Prior exposure to plasmodia significantly suppressed chikungunya virus–associated pathogenesis, characterized by reduced viral load and improved joint inflammation [2]. Furthermore, coinfections of a rodent Plasmodium strain and lactate dehydrogenase–elevating virus offered protection against experimental cerebral malaria and experimental autoimmune encephalomyelitis [3]. Based on these observations on Plasmodium infection and positive-strand RNA viruses, we hypothesized that there could be a possible association between malaria and SARS-CoV-2 infection. To validate our observation, we investigated the prevalence of COVID-19 in the Plasmodium falciparum–endemic area of Odisha, India,
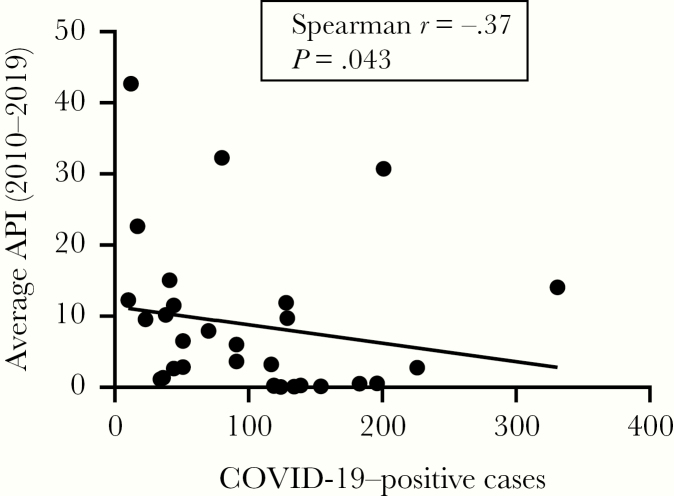
Odisha is highly endemic for P. falciparum infection. We obtained the annual parasite index (API) of P. falciparum for the last 10 years (2010–2019) from the National Vector Borne Disease Control Program and COVID-19 infection status in Odisha from the government of Odisha website (see https://health.odisha.gov.in/covid19-dashboard.html). API and COVID-19 data from 30 districts were analyzed and shown in Figure 1. A significant negative correlation (Spearman r = –0.37; P = .04; n = 30) was observed between 10-year average API scores and the number of COVID-19 cases detected. Malaria is known to stimulate B cells resulting in hyper-gammaglobulinemia [4] and multiple cross-reactive antibodies are often produced, which could be protective. A recent study further highlighted the production of immunoglobulin G (IgG) and immunoglobulin M (IgM) against P. falciparum merozoite, which persists for an extended period and protects the host from clinical malaria [5]. It has also been well established that infection by some enveloped viruses triggers naturally occurring antibodies to activate the complement system, leading to lysis of the virus [6]. Antibodies against disaccharide galactose α-(1,3)-galactose (α-Gal) are most prevalent and constitute about 2% of total IgG and IgM. Previously, we have demonstrated high levels of antibodies to α-Gal (IgG and IgM) in healthy subjects in malaria-endemic areas (IgG: 144.62 ± 47.23; IgM: 19.93 ± 15.08 enzyme-linked immunosorbent assay [ELISA] units) compared to residents of nonendemic regions (IgG: 30.15 ± 9.3; IgM: 8.5 ± 6.1 ELISA units) [7]. These are polyspecific antibodies capable of interacting with multiple antigens. Although the presence of α-Gal on the surface of SARS-CoV-2 has not been reported, the anti-Gal antibodies, being polyspecific, can cross-react with multiple epitopic determinants [8]. Furthermore, in our earlier study (unpublished), we observed high levels of specific antimalarial antibodies (PfP0, PfEMP, RESA, MSP-1, and MSP-3) in residents of malaria-endemic areas. The cross-reactivity of these antibodies, along with anti-Gal antibodies and COVID-19, needs to be investigated for possibly demonstrating a cause-effect relationship. Further validation of this hypothesis might be established from other P. falciparum–endemic areas of the world.
Figure 1.
Correlation between the number of coronavirus disease 2019 (COVID-19) cases reported and the average annual malarial parasite index (API) during 2010–2019. Data from all districts of Odisha state were analyzed, and each dot represents 1 district. An inverse correlation was observed between API and number of COVID-19 cases reported through 19 June 2020 (n = 30).
Notes
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Nickbakhsh S, Ho A, Marques DFP, McMenamin J, Gunson RN, Murcia PR. Epidemiology of seasonal coronaviruses: establishing the context for the emergence of coronavirus disease 2019. J Infect Dis 2020; 222:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Teo TH, Lum FM, Ghaffar K, et al. Plasmodium co-infection protects against chikungunya virus-induced pathologies. Nat Commun 2018; 9:3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hassan A, Wlodarczyk MF, Benamar M, et al. A virus hosted in malaria-infected blood protects against T cell-mediated inflammatory diseases by impairing DC function in a type I IFN-dependent manner. mBio 2020; 11:e03394-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silveira ELV, Dominguez MR, Soares IS. To B or not to B: understanding B cell responses in the development of malaria infection. Front Immunol 2018; 9:2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boyle MJ, Chan JA, Handayuni I, et al. IgM in human immunity to Plasmodium falciparum malaria. Sci Adv 2019; 5:eaax4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol 2007; 81:3487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Das BK. Immunological correlates in Plasmodium falciparum infection with special reference to cerebral malaria. PhD dissertation. Bhubaneswar, Odisha: Utkal University, 2003. [Google Scholar]
- 8. Ravindran B, Satapathy AK, Das MK. Naturally-occurring anti-alpha-galactosyl antibodies in human Plasmodium falciparum infections—a possible role for autoantibodies in malaria. Immunol Lett 1988; 19:137–41. [DOI] [PubMed] [Google Scholar]
- 9. Worldometer. COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus/#countries. Accessed 2 June 2020.
- 10.Department of Health and Family Welfare, Government of India. District wise dashboard. https://health.odisha.gov.in/covid19-dashboard.html. Accessed 19 June 2020.