Abstract
Coronavirus disease 2019 (Covid-19) has affected millions of people and may disproportionately affect those with hypertension and diabetes. Because of inadequate methods in published systematic reviews, the prevalence of diabetes and hypertension and associated risks of poor outcomes in Covid-19 patients are unknown. We searched databases from December 1, 2019, to April 6, 2020, and selected observational peer-reviewed studies in English of patients with Covid-19. Independent reviewers extracted data on study participants, interventions, and outcomes and assessed risk of bias, and the certainty of evidence. We included 65 (15 794 participants) observational studies at moderate to high risk of bias. Overall prevalence of diabetes and hypertension was 12% (95% confidence interval [CI], 10-15; n = 12 870; I2: 89%), and 17% (95% CI, 13-22; n = 12 709; I2: 95%), respectively. In severe Covid-19, the prevalence of diabetes and hypertension were 18% (95% CI, 16-20; n = 1099; I2: 0%) and 32% (95% CI, 16-54; n = 1078; I2: 63%), respectively. Unadjusted relative risk for intensive care unit admission and mortality were 1.96 (95% CI, 1.19-3.22; n = 8890; I2: 80%; P = .008) and 2.78 (95% CI, 1.39-5.58; n = 2058; I2: 75%; P = .0004) for diabetics; and 2.95 (95% CI, 2.18-3.99; n = 1737; I2: 0%; P < .001) and 2.39 (95% CI, 1.54-3.73; n = 3107; I2: 66%; P < .001) for hypertensives. Neither diabetes (1.50; 95% CI, 0.90-2.50; n = 1991; I2: 74%; P = .119) nor hypertension (1.48; 95% CI, 0.99-2.23; n = 2023; I2: 69%; P = .058) was associated with severe Covid-19. In conclusion, the risk of intensive care unit admission and mortality for patients with diabetes or hypertension who developed Covid-19 is increased compared with those without these comorbidities.
PROSPERO registration number
CRD42020176582.
Keywords: Covid-19, SARS-CoV-2, diabetes mellitus, hypertension, endocrinology
Coronavirus disease 2019 (Covid-19) is the worst pandemic in the past 100 years spanning more than 200 countries [1] and affecting millions of individuals worldwide [2]. The novel severe acute respiratory syndrome coronavirus (SARS-CoV-2) was identified as the causative agent of Covid-19, with angiotensin-converting enzyme 2 (ACE2) as one of its cellular receptors [3]. Covid-19 has a spectrum of clinical manifestations ranging from asymptomatic or mildly symptomatic in about 80% of those affected according to community surveys to an approximate 2% case fatality rate in the hospitalized populations [4-7]. Although the statistical estimations are changing daily, more than 11 million people have been affected by Covid-19, resulting in more than half a million deaths across the world by July 7, 2020 [1, 6].
A great risk of severe Covid-19 has been reported in patients with diabetes and hypertension [8]. One study of 191 patients reported a mortality risk of 2.85-fold and 3.05-fold for those with diabetes and hypertension, respectively [9]. Furthermore, the Chinese Center for Disease Control reported a higher case fatality rate for persons with diabetes compared with those without (7.3%. vs 2.3%, respectively) [7]. This risk may be explained by a dysregulated immune response, a higher comorbidity burden, and alterations of ACE2 cellular expression [6, 10-12]. The latter has been the subject of intense scrutiny, given the lack of evidence against the use of renin-angiotensin system blocking agents and their known benefits in diabetes and hypertension [12-14], as well as other cardiovascular conditions that have been shown to enhance ACE2 expression [15].
Previous systematic reviews reported a prevalence of diabetes and hypertension in patients with Covid-19 ranging from 9.7% to 11.9% and 17.1% to 20%, respectively [16-19]. The risks of severe Covid-19 in patients with diabetes and hypertension were ~3 and ~2-fold, respectively [16-18, 20]. However, these reports failed to address the high probability of including repeated information and patient duplicates in the analysis and thus may lead to inaccurate effect sizes and misleading results [16, 18-21]. This has been listed by authors as a major limitation [20], and has raised major editorial concerns [22-24]. Ultimately, risk estimates remain uncertain. Therefore, we systematically assessed the prevalence of diabetes and hypertension in patients with Covid-19 after excluding repeated patients across studies and analyzed the associated risks for Covid-19 severity, intensive care unit (ICU) admission and mortality.
Methods
Protocol registration
This systematic review adheres to the standards set in the Meta-analysis of Observational Studies in Epidemiology and Preferred Reported Items for Systematic Reviews and Meta-Analysis [25, 26]. Registration ID is CRD42020176582 and is available at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=176582.
Eligibility criteria
For our first aim, we included observational and interventional studies that reported the frequency of diabetes and/or hypertension in adult population with Covid-19. For our second aim, we included studies that reported exposure-outcome association as univariate or multivariate analysis, with diabetes, hypertension being the exposure, and severe Covid-19 through ICU admissions or mortality being the outcome of interest. We excluded case reports (n < 2) and studies including pregnant women and pediatric populations (age < 18 years). We did not set a criterion based on Covid-19 diagnosis definition, exposure ascertainment, or outcome definition because these were expected to be different and/or with limited rigor.
Search strategy
An experienced librarian (N.A.V.), with input from investigators, searched several databases for peer-reviewed manuscripts in English published between December 1, 2019, and April 6, 2020, including Ovid Medline In-Process & Other Non-Indexed Citations, Ovid Medline, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. Manual screening of references from the included studies was performed [27]. All supplementary material and figures are located in adigital research materials repository [27].
Selection process and data extraction
Search results were uploaded into an online software program (DistillerSR; Evidence Partners, Ottawa, ON, Canada). Reviewers, working independently and in duplicate, screened studies for eligibility using standardized and prepiloted instructions in a round of title and abstract and one of full-text screening. In round 1, disagreements were included; and in round 2, disagreements were resolved by consensus or arbitration by a third investigator (F.H.S.). To identify articles with high probability of patient repetition, studies that were included after full-text screening followed a preliminary data extraction conducted by 2 pairs of investigators. We extracted the timeframes of each study, hospital(s), location(s), country of origin, and the list of authors. Next, the enrollment timeframes from the studies were plotted with the information of the hospital(s). Studies without overlap in the plot were included for all outcomes. If studies overlapped, we analyzed outcomes reported by each study. If outcomes were repeated in overlapping studies, we included the data for outcomes (or frequency of comorbidities) from the study with the largest sample [27]. Data extraction was also performed in an independent and duplicated manner using a standardized and prepiloted.
Risk of bias and confidence in the body of evidence
For case series, we modified 2 tools and analyzed: selection, ascertainment of outcomes and exposures, causality, and reporting [28, 29]. For case control studies, the Newcastle-Ottawa Scale was used [30]. The quality of evidence for each outcome was determined using the Grading of Recommendations Assessment, Development and Evaluation approach [31]. Both risk of bias and overall quality of evidence assessment were performed independently and in duplicate. Disagreements were resolved by consensus between the 2 reviewers, or by arbitration by a third author (R.R.G.) [27]. Full details are listed elsewhere [27].
Data synthesis
We estimated the full-text screening inter-rater reliability with the Cohen’s kappa statistic. To estimate the prevalence, we used a binomial-normal model for meta-analysis of proportions (i.e., generalized linear mixed model) [32, 33]. We calculated the relative risk (RR) for each outcome and performed a meta-analysis results using a random-effect models and the restricted maximum-likelihood estimator [34]. Meta-analysis of unadjusted and adjusted estimates was not combined. We were unable to calculate adjusted estimates because of scarcity of data. Inconsistency for each outcome, not attributable to chance, was assessed visually using forest plots and estimated using the percentage of variance in a meta-analysis that is attributable to study heterogeneity (I2) statistic: I2 < 25% and > 75% reflects low and high inconsistency, respectively [35]. All statistical analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria) [36].
Analysis of subgroups and sensitivity
Details on all predefined and nonpredefined subgroup and sensitivity analyses are listed separately [27]. To identify confounders, we designed a directed acyclic graph (DAG) in R [27, 37-41].
Results
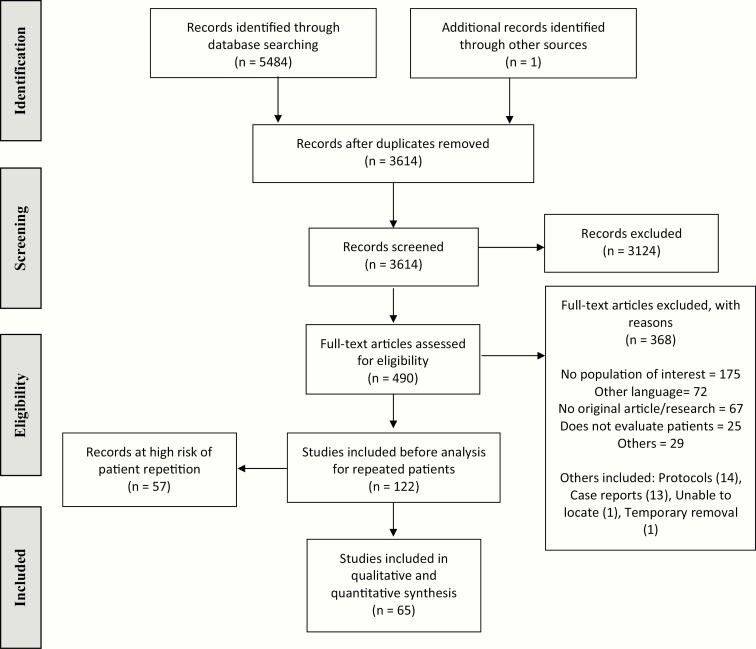
Search strategy yielded 5484 studies. After deduplication and screening, 122 studies fulfilled our selection criteria (Fig. 1). Full-text screening inter-observer agreements were substantial (k = 0.77, 0.80, and 0.84 for each pair of reviewers). We identified 93 articles at high probability of repeating patients. From those, we fully excluded 57 (47%), and partially (some outcomes included) excluded some outcomes in 36 (30%) [27]. Ultimately, 65 (15 794 patients) were included in our analysis. Overall, 18 (28%) studies had low risk of bias, 3 (4%) had some concerns, and 44 (68%) had high risk. For prevalence, 40 (62%) studies resulted at low risk of bias, 1 (1%) at some concerns, and 24 (37%) at high risk. Overall confidence in the body of evidence is graded as low [27]. We did not assess risk of publication bias through the funnel plot because of the limited number of studies [22].
Figure 1.
Flow chart of the selection process.
Characteristics of included studies
Most studies were retrospective case series (97%) performed at a single center (63%) in China (71%), with inpatients (43%) diagnosed with Covid-19 using RT-PCR (Table 1) (97%). Most articles described treatments, which included standard of care (38%) and supplemental antiviral therapy (42%). Study length was reported in most studies (75%; 9-65 days). Percentage of males varied between 0 and 88, and the mean age ranged from 33 to 75 years. A total of 5 studies (8%) reported ethnicity.
Table 1.
Characteristics of the Included Studies
| No. | ID | Author | Publishing Date | Country | Study Design | Selection Criteria | Setting | Centers | Sample Size | Males, n (%) | Age, Mean ± SD | Molecular Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | Grasselli et al. | 4/6/20 | Italy | Retrospective case series | Severe (ICU) | Inpatient | Multicenter | 1043 | 1304 (82) | 63 ± 10 | RT-PCR |
| 2 | 44 | Wang et al. | 4/3/20 | China | Retrospective case series | General | Outpatient | Single | 1012 | 524 (52) | 50 ± 14 | RT-PCR |
| 3 | 489 | COVID-19 NIRST | 3/4/20 | Australia | Retrospective case series | General | Community | Multicenter | 23 | 13 (52) | 48 ± 18 | NA |
| 4 | 1405 | Fried et al. | 4/3/20 | USA | Retrospective case series | General | Inpatient | NR | 4 | 2 (50) | 54 ± 12 | NA |
| 5 | 7916 | Zhang et al. | 2/17/20 | China | Retrospective case series | General | Inpatient | Single | 140 | 71 (51) | 57 ± 10 | RT-PCR |
| 6 | 7929 | Liu et al. | 2/9/20 | China | Retrospective case series | General | Inpatient | Single | 12 | 8 (67) | 53 ± 18 | RT-PCR |
| 7 | 7942 | Chan et al. | 1/24/20 | China | Retrospective case series | General | Inpatient | Single | 6 | 3 (50) | 46 ± 22 | RT-PCR |
| 8 | 7949 | Xu et al. | 2/28/20 | China | Retrospective case series | General | Inpatient | Single | 90 | 39 (43) | 50 ± 11 | RT-PCR |
| 9 | 8070 | Pung et al. | 3/28/20 | Singapore | Retrospective case series | General | Outpatient | Multicenter | 17 | 7 (41) | 40 ± 11 | NA |
| 10 | 8149 | KSID et al. | 3/24/20 | Republic of Korea | Retrospective case series | Death patients | Community | Multicenter | 54 | 33 (61) | 75 ± 10 | NA |
| 11 | 8236 | Long et al. | 3/15/20 | China | Retrospective case series | General | Inpatient | Single | 10 | 3 (30) | 54 ± 27 | RT-PCR |
| 12 | 8264 | Qui et al. | 4/2/20 | China | Retrospective case series | Severe (ICU) | Inpatient | Single | 10 | 0 (0) | 65 ± 9 | RT-PCR |
| 13 | 8405 | Wang et al. | 3/31/20 | China | Retrospective case series | General | Inpatient | Single | 116 | 67 (58) | 54 ± 22 | RT-PCR |
| 14 | 8424 | Zhang et al. | 3/31/20 | China | Retrospective case series | Severe (critical) | Inpatient | Multicenter | 4 | 2 (50) | 57 ± 18 | RT-PCR |
| 15 | 8525 | Meng et al. | 3/31/20 | China | Retrospective case series | General | Inpatient | Single | 42 | 24 (57) | 64 ± 10 | RT-PCR |
| 16 | 8542 | Escalera et al. | 4/2/20 | Bolivia | Retrospective case series | General | Both in- and out-patient | Multicenter | 12 | 6 (50) | 36 ± 15 | RT-PCR |
| 17 | 8581 | Kim et al. | 4/6/20 | Republic of Korea | Retrospective case series | General | Inpatient | Multicenter | 28 | 15 (54) | 43 ± 13 | RT-PCR |
| 18 | 8586 | Lescure et al. | 3/27/20 | France | Retrospective case series | General | Inpatient | Multicenter | 5 | 3 (60) | 47 ± 20 | RT-PCR |
| 19 | 8606 | Wang et al. | 3/30/20 | China | Retrospective case series | General | Inpatient | Single | 339 | 166 (49) | 69 ± 8 | RT-PCR |
| 20 | 8609 | Mo et al. | 3/16/20 | China | Retrospective case series | General | Inpatient | Single | 155 | 86 (55) | 54 ± 18 | NA |
| 21 | 8645 | Wang et al. | 3/31/20 | China | Retrospective case series | General | Inpatient | Single | 5 | 3 (60) | 61 ± 8 | RT-PCR |
| 22 | 8680 | Young et al. | 3/3/20 | Singapore | Retrospective case series | General | Inpatient | Multicenter | 18 | 9 (50) | 47 ± 10 | RT-PCR |
| 23 | 8691 | Shen et al. | 3/27/20 | China | Prospective case series | Severe (ARDS) | Inpatient | Single | 5 | 3 (60) | 54 ± 15 | RT-PCR |
| 24 | 8816 | To et al. | 3/23/20 | Hong Kong | Retrospective case series | General | Inpatient | Multicenter | 23 | 13 (57) | 62 ± 19 | RT-PCR |
| 25 | 8844 | Yuan et al. | 3/19/20 | China | Retrospective case series | General | Inpatient | Single | 27 | 12 (44) | 60 ± 16 | RT-PCR |
| 26 | 8898 | Fang et al. | 3/21/20 | China | Retrospective case series | General | Inpatient | Single | 32 | 16 (50) | 41 ± 15 | RT-PCR |
| 27 | 8920 | Liu et al. | 3/12/20 | China | Retrospective case series | General | Inpatient | Single | 10 | 4 (40) | 43 ± 10 | RT-PCR |
| 28 | 8965 | Zhao et al. | 3/12/20 | China | Retrospective case series | General | Inpatient | Multicenter | 19 | 11 (58) | 48 ± 21 | RT-PCR |
| 29 | 8969 | Lu et al. | 3/17/20 | China | Retrospective case series | General | Inpatient | Single | 5 | 1 (20) | 52 ± 9 | RT-PCR |
| 30 | 9041 | Wang et al. | 2/7/20 | China | Retrospective case series | General | Inpatient | Single | 138 | 75 (54) | 56 ± 19 | RT-PCR |
| 31 | 9056 | Chen et al. | 3/30/20 | China | Retrospective case series | General | Inpatient | Single | 22 | 14 (64) | 37 ± 18 | RT-PCR |
| 32 | 9094 | Ye et al. | 4/2/20 | China | Retrospective case series | General | Inpatient | Single | 5 | 3 (60) | 40 ± 14 | RT-PCR |
| 33 | 9113 | Wang et al. | 3/23/20 | China | Retrospective case series | General | Inpatient | Single | 114 | 58 (51) | 53 ± 9 | RT-PCR |
| 34 | 9122 | Guo et al. | 3/31/20 | China | Retrospective case series | General | Inpatient | Single | 174 | 20 (11) | 61 ± 9 | RT-PCR |
| 35 | 9123 | Zhang et al. | 3/20/20 | China | Retrospective case series | General | Inpatient | Multicenter | 645 | 328 (51) | 41 ± 15 | RT-PCR |
| 36 | 9125 | Wang et al. | 3/5/20 | China | Retrospective case series | General | Inpatient | Single | 18 | 10 (56) | 39 ± 19 | RT-PCR |
| 37 | 9151 | Zhu et al. | 3/13/20 | China | Retrospective case series | General | Inpatient | Multicenter | 32 | NA | 46 ± 13 | RT-PCR |
| 38 | 9171 | Cai et al. | 4/2/20 | China | Retrospective case series | General | Inpatient | Single | 298 | 145 (48) | 48 ± 21 | RT-PCR |
| 39 | 9174 | Sun et al. | 3/25/20 | Singapore | Case control | General | Inpatient | Single | 54 | 29 (54) | 42 ± 15 | RT-PCR |
| 40 | 9175 | Cao et al. | 4/2/20 | China | Retrospective case series | General | Inpatient | Single | 102 | 53 (52) | 54 ± 22 | RT-PCR |
| 41 | 9198 | Ren et al. | 2/11/20 | China | Retrospective case series | Severe (ARDS) | Inpatient | Single | 5 | 3 (60) | 54 ± 10 | RT-PCR |
| 42 | 9307 | Guo et al. | 3/27/20 | China | Retrospective case series | General | Inpatient | Single | 187 | 91 (49) | 59 ± 15 | RT-PCR |
| 43 | 9314 | Arentz et al. | 3/19/20 | USA | Retrospective case series | Severe (ICU) | Inpatient | Single | 21 | 11 (52) | 70 ± 12 | RT-PCR |
| 44 | 9321 | Zhang et al. | 3/26/20 | China | Retrospective case series | General | Inpatient | Multicenter | 28 | 17 (61) | 65 ± 10 | RT-PCR |
| 45 | 9332 | NCCE et al. | 3/13/20 | Iran | Retrospective case series | Death patients | Community | Multicenter | 514 | 6629 (58) | 54 ± 16 | RT-PCR |
| 46 | 9339 | Ding et al. | 3/20/20 | China | Retrospective case series | General | Inpatient | Single | 5 | 2 (40) | 50 ± 10 | NA |
| 47 | 9340 | Albarello et al. | 2/26/20 | Italy | Retrospective case series | General | Inpatient | Single | 2 | 1 (50) | 66 ± 1 | RT-PCR |
| 48 | 9377 | Zhang et al. | 3/26/20 | China | Retrospective case series | General | Inpatient | Single | 17 | 8 (47) | 49 ± 13 | RT-PCR |
| 49 | 9400 | Wei et al. | 2/28/20 | China | Retrospective case series | General | Inpatient | Multicenter | 78 | 39 (50) | 33 ± 18 | RT-PCR |
| 50 | 9431 | Shi et al. | 3/25/20 | China | Retrospective case series | General | Inpatient | Single | 416 | 205 (49) | 64 ± 12 | RT-PCR |
| 51 | 9446 | Wang et al. | 3/30/20 | China | Retrospective case series | Severe (ARDS) | Inpatient | Multicenter | 17 | 7 (41) | 65 ± 14 | RT-PCR |
| 52 | 9496 | Xin et al. | 3/30/20 | China | Retrospective case series | General | Inpatient | Single | 8 | 6 (75) | 64 ± 18 | NA |
| 53 | 9608 | CDC COVID-19 RT | 4/3/20 | USA | Retrospective case series | General | Inpatient, outpatient, and community | Multicenter | 7162 | NA | NA | RT-PCR |
| 54 | 9609 | Iwasawa et al. | 3/31/20 | Japan | Retrospective case series | General | Inpatient | Single | 6 | 2 (33) | 69 ± 3 | RT-PCR |
| 55 | 9622 | Liu et al. | 3/27/20 | China | Retrospective case series | General | Inpatient | Single | 56 | 31 (55) | 58 ± 13 | RT-PCR |
| 56 | 9667 | Guan et al. | 3/26/20 | China | Retrospective case series | General | Inpatient | Multicenter | 1590 | 904 (57) | 49 ± 16 | RT-PCR |
| 57 | 9679 | Wong et al. | 3/27/20 | Hong Kong | Retrospective case series | General | Inpatient | Multicenter | 64 | 26 (41) | 56 ± 19 | RT-PCR |
| 58 | 9695 | Hu et al. | 3/4/20 | China | Retrospective case series | General | Inpatient | Single | 24 | 8 (33) | 33 ± 28 | RT-PCR |
| 59 | 9702 | Xie et al. | 4/2/20 | China | Retrospective case series | General | Inpatient | Single | 79 | 44 (56) | 60 ± 13 | NA |
| 60 | 9764 | Xu et al. | 3/13/20 | China | Retrospective case series | General | Inpatient | Single | 51 | 25 (49) | 42 ± 20 | RT-PCR |
| 61 | 10641 | Liu et al. | 3/23/20 | China | Retrospective case series | Severe (ICU) | Inpatient | Single | 8 | 7 (88) | 63 ± 11 | RT-PCR |
| 62 | 10782 | Gao et al. | 3/17/20 | China | Retrospective case series | General | Inpatient | Single | 43 | 26 (60) | 44 ± 12 | RT-PCR |
| 63 | 10860 | McMichael et al. | 3/27/20 | USA | Retrospective case series | General | Both in- and outpatient | Multicenter | 167 | 55 (33) | 72 ± 13 | RT-PCR |
| 64 | 10861 | Bai et al. | 3/10/20 | China | Case control | General | Inpatient | Multicenter | 219 | 119 (54) | 45 ± 15 | RT-PCR |
| 65 | 1 | CDC COVID-19 RT | 4/8/20 | USA | Retrospective case series | General | Inpatient | Multicenter | 159 | NA | NA | NA |
General selection criteria were patients hospitalized because of pneumonia caused by SARS-CoV-2.
ARDS, acute respiratory distress syndrome; ICU, intensive care unit; NA, not available; NR, not reported.
Only 69% of studies reported their definition of severity. Among those that did report severity definitions, 78% of their definitions were derived from established guidelines [27]. Moreover, only 6 of the studies (9%) described the subtype of diabetes (type 2 diabetes), and 1 study (2%) defined the subtype of hypertension (primary hypertension). Finally, none of the included studies provided a definition for diabetes or hypertension.
Prevalence and risks of diabetes and hypertension
Quantitative synthesis.
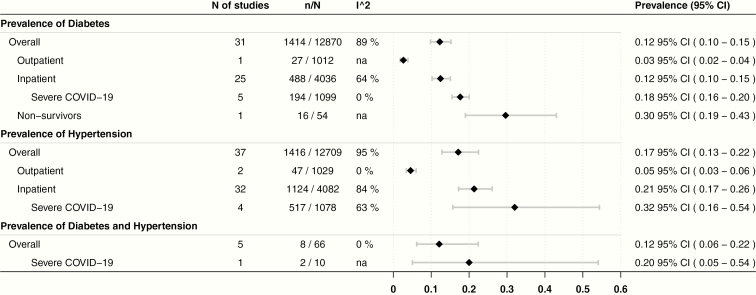
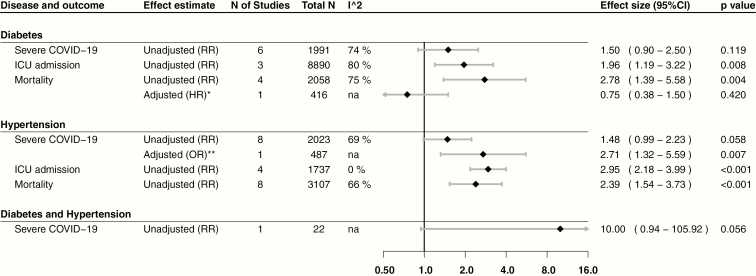
The overall prevalence was 12% (95% confidence interval [CI], 10-15; n = 12 870; I2: 89%) for diabetes, 17% (95% CI, 13-22; n = 12 709; I2: 95%) for hypertension, and 12% (95% CI, 6-22; n = 54; I2: 0%) for coexisting diabetes and hypertension (Fig. 2). The RR of patients with diabetes to develop our outcomes of interest were: Covid-19 severity (1.50; 95% CI, 0.90-2.50; n = 1991; I2: 74%; P = .118), ICU admission (1.96; 95% CI, 1.19-3.22; n = 8890; I2: 80%; P = .007), and mortality (2.78; 95% CI, 1.39-5.58; n = 2058; I2: 75%; P = .004). For patients with hypertension: Covid-19 severity (1.48; 95% CI, 0.99-2.23; n = 2023; I2: 69%; P = .058), ICU admission (2.95; 95% CI, 2.18-3.99; n = 1737; I2: 0%; P < .001), and mortality (2.39; 95% CI, 1.54-3.73; n = 3107; I2: 66%; P < .001). Furthermore, for patients with diabetes and hypertension, RR for Covid-19 severity was 10 (95% CI, 0.94-105.92; n = 22; I2: not applicable; P = .056).
Figure 2.
Prevalence of diabetes and hypertension, overall and by subgroups. Five studies in the diabetes, and 3 in the hypertension overall prevalence were not included in subgroups as they did not specify their setting. Nonsurvivor patients in diabetes were not included in the overall prevalence. Severe Covid-19 patients are also included in the inpatient subgroup prevalence; these patients were those from studies that included only severe, critical, or ICU patients, with or without acute respiratory distress syndrome. All patients included in the overall prevalence for the diabetes and hypertension population were inpatients. Covid-19, coronavirus disease 2019; ICU, intensive care unit.
Narrative synthesis.
Adjusted and unadjusted estimates for mortality (hazard ratio) in patients with diabetes were nonconclusive in 2 studies (0.75; 95% CI, 0.38-1.50; n = 416; P = .420) [42] and (1.09; 95% CI, 0.57-2.08; n = 339; P = .799) [43]. Moreover, an adjusted odds ratio for severe Covid-19 in patients with hypertension reported by a study was 2.71 (95% CI, 1.32-5.59; n = 487; P = .007) [43]. Finally, another study reported an unadjusted hazard ratio that was inconclusive in determining the risk of severe Covid-19 associated with diabetes and hypertension (1.49; 95% CI, 0.92-2.44; n = 339; P = .109) [43]. Adjusted estimates are displayed along with our results for visual comparison in Fig. 3.
Figure 3.
Risk estimates for severe Covid-19, intensive care unit admission, and mortality. RR = relative risk. HR = hazard ratio. *Adjusted for age, preexisting cardiovascular disease (hypertension, coronary heart disease, and chronic heart failure), cerebrovascular disease, chronic obstructive pulmonary disease, renal failure, cancer, acute respiratory distress syndrome, creatine levels, NT-proB-type natriuretic peptide levels, and cardiac injury. **Adjusted for time to admission.
Sensitivity analyses
Predefined sensitivity analyses.
After excluding studies at high risk of bias, the overall prevalence of diabetes (12.4%; 95% CI, 9.5-16; n = 12 077/12 870; I2: 93%; 22/31 studies) and hypertension (16.8%;95% CI, 11.5-23.7; n = 11 912/12 709; I2: 96%; 25/37 studies) was similar. Moreover, in patients with hypertension, RR for severe Covid-19 remained the same but heterogeneity decreased (1.40; 95% CI, 0.65-3.00; n = 53/2023; I2: 0%; P = .389, 2/8 studies), whereas the risk for ICU admission decreased (2.62; 95% CI, 1.45-4.75; n = 143/1733; I2:15%; P = .001; 2/3 studies), and mortality increased (3.24; 95% CI, 1.27-8.28; n = 32/2063; I2: 9%; P = .014; 2/5 studies).
After excluding single-center studies from the analysis, RR for severe Covid-19 among patients with diabetes increased (2.1; 95% CI, 1.20-3.66; n = 1613/1991; I2: 33%; P = .009; 2/6 studies). In contrast, RR for ICU admission decreased (1.80; 95% CI, 0.90-3.61; n = 8752/8890; I2: 88%; P = .096; 2/3 studies). Additionally, in patients with hypertension, RR for severe Covid-19 increased (2.55; 95% CI, 2.06-3.16; n = 1613/2023; I2: 0%; P < .0001; 2/8 studies); for ICU admission decreased (2.70; 95% CI, 1.58-4.60; n = 1595/1733; I2: 20%; P = .0002; 2/3 studies); and, for mortality increased (3.32; 95% CI, 1.36-8.10; n = 1595/2063; I2: 13%; P = .008; 2/5 studies).
Non-predefined sensitivity analyses
Because there was high variability in the definition of severe Covid-19 used by authors, we analyzed the risk for severe Covid-19 after including only those studies that defined severity according to the World Health Organization definition [44] or that of the novel coronavirus pneumonia prevention and control program (6th ed.) [27, 45]. The RR resulted in similar estimates but decreased heterogeneity in diabetes (0.97; 95% CI, 0.65-1.46; n = 335; I2: 0%; P = .886), and hypertension (1.03; 95% CI, 0.67-1.57; n = 345; I2: 38%; P = .909). The complete description of sensitivity analysis is provided separately [27].
Minimal sufficient adjustment sets
According to our DAG, conditioning age and obesity is necessary to analyze the effect of diabetes on mortality, whereas for hypertension, age, diabetes, and obesity are necessary [27].
Discussion
Main findings
Our results suggest an overall prevalence of 12% and 17% for diabetes and hypertension (respectively) among nonpregnant, adult patients with Covid-19, respectively. Additionally, these comorbidities were associated with an increased risk for ICU admission and mortality. We found an overwhelming proportion of studies at high risk of data repetition, which indicates a high risk of misrepresentation of estimates in previous systematic reviews that did not address this issue [16, 18-20]. The body of evidence comprises observational studies at moderate to high risk of bias yielding low confidence in the estimates.
Strengths and limitations
We developed a methodology to identify publications at high risk of patient repetition, which, compared with previous systematic reviews, provides a major strength to the current analysis [16, 18-20]. Moreover, we also analyzed and grouped the various definitions used for severe Covid-19. From this, we conclude that this outcome lacks interpretability and therefore clinical significance because of the large heterogeneity in the definitions used. Hence, previous systematic reviews that have analyzed this outcome individually or as part of a composite, suffer from this limitation [16, 18-20]. Furthermore, although we could not perform a thorough isolation of the effect of comorbidities, we identified major confounders of our estimates using DAG [46].
Our study has several limitations. The effects of diabetes and hypertension in univariate analysis cannot be attributed only to these exposures because, aside from possible confounders, patients may have had other comorbidities. To overcome this limitation, we extracted data from reported multivariate analyses. However, because their scarcity, we could not synthesize adjusted estimates. Second, most published studies are sourced from China and may be less generalizable to populations in other parts of the world. Moreover, 3 of the included studies provided data on demographic or biochemical parameters such as blood pressure values, glycemic control markers, duration of disease, or smoking; however, we could not perform an adjusted analysis because these studies did not coincide with the assessed outcomes. Finally, auxiliary reasons for the observed risks could be a higher prevalence of obesity, cardiovascular, and renal disease in these patients. Additionally, elderly individuals are overrepresented among Covid-19 patients requiring hospital admission and critical care, where diabetes and hypertension is highest. Thus, the risks attributed to these comorbidities in relation to Covid-19 might be confounded, as our DAG suggests [27].
Comparison with previous studies
Diabetes.
Two smaller reviews noted a prevalence of diabetes between 10% and 11.9% in Covid-19, comparable to our estimates [16, 17]. However, our estimates are lower than those reported by Shi et al. of 14.3% [43]. Furthermore, compared with the 9.3% global community prevalence of diabetes [47], our study found a 12% prevalence, suggesting a higher figure. In contrast to author-defined severe Covid-19, dichotomous outcomes of disease severity such as ICU admission and mortality were significantly elevated (~2- and ~3-fold, respectively) in diabetes. This is of particular interest because Huang et al. noted an increased risk of a composite poor outcome in patients with diabetes, which included severe Covid-19 as one of the outcomes. However, our analysis suggests that severe Covid-19 is a largely heterogenous outcome that lacks interpretability and may not accurately reflect the outcomes of interest [20].
Additionally, 1 study of 1382 Covid-19 patients with diabetes found a 2.79-fold risk of admission to the ICU [48], higher than our findings of 1.96-fold in 8890 patients. However, the reported risk of Covid-19 mortality in diabetes was 2.85- to 3.21-fold, consistent with our findings [9, 48]. Other meta-analyses did not report ICU admission or mortality risk estimates [16, 49]. Comparatively, the SARS epidemic in 2003, also caused by a betacoronavirus, was associated with a 3-fold risk of poor outcomes in the presence of diabetes, the highest among all comorbidities [50].
The heightened Covid-19 risks in diabetes are multifactorial. Diabetes may facilitate the entry of SARS-CoV-2 by increased expression of ACE2 surface receptors because of the disease itself and the treatment strategies used [10, 11, 51-53]. Furthermore, diabetes leads to dysregulation of immune responses by cytokines such as IL-6 and attenuating anti-inflammatory signaling leading to increased end-organ injury [10, 11, 54-56]. Given that obesity and diabetes often coexist [57], at least part of the heightened Covid-19 risks in diabetes could be attributed to comorbid obesity, an emerging independent risk factor for severe Covid-19 [58]. Furthermore, because diabetes is independently associated with comorbidities, Covid-19 acts as an additional insult to preexisting comorbidities. For instance, hypoglycemia, a comorbidity of diabetes, may be masked by hypoglycemia unawareness in asymptomatic Covid-19 carriers with diabetes mellitus with serious clinical consequences [6].
Hypertension.
Initial reports indicated a 26% to 30% prevalence of hypertension in Covid-19 patients [9, 59]. Published data from systematic reviews reported a 17.1% to 20% prevalence of hypertension in Covid-19, comparable to our estimates of 17% in our analysis of 12 709 patients [16, 17]. In the inpatient setting, our results suggest that hypertension prevalence could be up to 26%, which is still lower compared with recent data from the United States of ~50% [60, 61]. Moreover, our estimates were lower than the 31.1% global prevalence of hypertension, which could suggest an average (or below average) risk of Covid-19 [62].
We found a nonconclusive risk of severe Covid-19 in hypertension. Other reports suggest a higher risk of severe Covid-19 in hypertensives, of approximately 2.3-fold [17, 49]. However, after analyzing the important variation in the parameters used to define severity, we conclude that these estimates are noninterpretable. In contrast, the risk of ICU admission, which we consider a more reliable proxy of Covid-19 severity, was elevated in our analysis. Finally, we found an elevated risk of Covid-19 mortality associated with hypertension that was not described in other meta-analyses but is comparable to the 2.4- to 3.0-fold risk reported in primary studies [9, 63].
The observed risk of Covid-19 in patients with hypertension is likely multifactorial. The underlying immune dysregulation, with a higher propensity for an exaggerated immune response to viral exposure, resulting in a cytokine storm and end-organ injury could be a major contributor to this risk [64, 65]. Additional contributors may include a higher sympathetic drive, hyperactivity of T-helper cells, increased ACE2 expression, and an enhanced angiotensin II/angiotensin 1-7 ratio reducing anti-inflammatory effects of the latter, and increased pro-inflammatory action of angiotensin II [66-70].
Implications for future research and clinical practice
Future studies of Covid-19 patients with diabetes or hypertension should report on patient characteristics, subtype of hypertension or diabetes, duration of disease, medications used, and disease control markers. This information would not only be valuable for future systematic reviews but also assist frontline clinicians in individualizing the Covid-19 risks faced by their patients. Furthermore, future studies reporting multivariate analyses should consider our proposed minimal sufficient adjustment sets to avoid unnecessary or overadjustment of prognosticators. To date, the risk for severe Covid-19 faced by patients with diabetes and hypertension is unclear because of the large heterogeneity in the author definitions of Covid-19 severity. Until a universal definition of Covid-19 severity is adopted, we propose using the ICU admission rate as a more objective way to define severity.
Conclusion
Compared with previous reviews, our results suggest a lower prevalence of diabetes and hypertension in hospitalized Covid-19 patients. These patients face a higher risk of poor outcomes compared with those without these comorbidities. However, the body of evidence are at high risk of bias and provide low confidence in the estimates.
Acknowledgments
Financial Support: This work was funded by the intramural research program of the National Institutes of Health.
Author Contributions: F.J.B.: figures, study design, data collection, data analysis, data interpretation, writing. S.S.: figures, study design, data collection, data analysis, data interpretation, writing. R.W.: figures, data collection, data interpretation, writing. P.J.M.P.: figures, data collection, data interpretation. O.J.P.: figures, study design, data collection, data analysis, data interpretation. M.H.: data collection, data interpretation. N.A.A.V.: literature search. J.E.H.: study design, data interpretation, approval of final manuscript. E.S.: study design, data interpretation, approval of final manuscript. G.E.: study design, data interpretation, approval of final manuscript. F.P.: study design, data interpretation, approval of final manuscript. J.P.B.: figures, study design, data collection, data analysis, data interpretation, approval of final manuscript. S.R.B.: study design, data interpretation, approval of final manuscript. C.A.S.: study design, data interpretation, approval of final manuscript. J.G.G.G.: study design, data interpretation, approval of final manuscript. R.R.G.: study design, data collection, data analysis, data interpretation, approval of final manuscript. F.H.S.: study design, data collection, data analysis, data interpretation, approval of final manuscript.
Glossary
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- CI
confidence interval
- Covid-19
coronavirus disease 2019
- DAG
directed acyclic graph
- ICU
intensive care unit
- RR
relative risk
- SARS-CoV-2
severe acute respiratory syndrome coronavirus
Additional Information
Disclosure Summary: The authors declare nothing to disclose related to the work described in this article. The funders had no role in the design and conduct of this study or the preparation of this manuscript.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. World Health Organization. Coronavirus disease (COVID-2019) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Published 2020. Updated April 30, 2020. Accessed May 1, 2020.
- 2. WHO Director -General’s Opening Remarks at the Media Briefing on COVID-19 - 11 March 2020 [press release]. 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Google Scholar]
- 3. Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309(5742):1864-1868. [DOI] [PubMed] [Google Scholar]
- 4. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fauci AS, Lane HC, Redfield RR. Covid-19 — navigating the uncharted. N Engl J Med. 2020;382(13):1268-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ademolu AB. Whipple triad its limitations in diagnosis and management of hypoglycemia as a co-morbidity in Covid-19 diabetics and diabetes mellitus in general- a review. Int J Diabetes Endocrinol. 2020;5(2):23-26. [Google Scholar]
- 7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. [DOI] [PubMed] [Google Scholar]
- 8. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? [published correction appears in Lancet Respir Med. 2020 Jun;8(6):e54]. Lancet Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038]. Lancet. 2020;395(10229):1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drucker DJ. Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications. Endocr Rev. 2020;41(3):bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bornstein SR, Dalan R, Hopkins D, Mingrone G, Boehm BO. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol. 2020;16(6):297-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shekhar S, Wurth R, Kamilaris CDC, et al. Endocrine conditions and COVID-19. Horm Metab Res. 2020;52(7):471-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605-2610. [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. ; Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19). Electronic address: https://www.lancovid.org. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ioannidis JPA, Axfors C, Contopoulos-Ioannidis DG. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ Res. 2020;188:109890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ioannidis JPA. Coronavirus disease 2019: The harms of exaggerated information and non-evidence-based measures. Eur J Clin Invest. 2020;50(4):e13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane; 2019. www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bauchner H, Golub RM, Zylke J. Editorial concern-possible reporting of the same patients with COVID-19 in different reports [published online ahead of print March 16, 2020]. JAMA. 2020. doi: 10.1001/jama.2020.3980 [DOI] [PubMed] [Google Scholar]
- 24. Flanagin A. Duplicate publication and submission. In: Christiansen S, Iverson C, Flanagin A, et al. , eds. AMA Manual of Style: A Guide for Authors and Editors. 11th ed. Oxford, United Kingdom: Oxford University Press; 2020:201-211. https://www.amamanualofstyle.com. Accessed May 30, 2020. [Google Scholar]
- 25. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Francisco B, Shekhar S, Ponce O, Rodríguez-Gutiérrez R, Hannah-Shmouni F. Prevalence and impact of diabetes and hypertension on patients with Covid-19 Bethesda, MD: US National Heart, Lung and Blood Institute. https://figshare.com/articles/online_resource/Prevalence_of_Diabetes_and_Hypertension_and_their_Associated_Risks_for_Poor_Outcomes_in_Covid-19_Patients/12636428 Published 2020. Accessed July 7, 2020.
- 28. National Heart LaBI. Study Quality Assessment Tools. Bethesda, MD: US National Heart, Lung and Blood Institute. [Google Scholar]
- 29. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 31. Ryan R, Hill S. How to GRADE the Quality of the Evidence. London, UK: Cochrane Consumers and Communication Group; 2016. [Google Scholar]
- 32. Trikalinos TA, Trow P, Schmid CH. Simulation-Based Comparison of Methods for Meta-Analysis of Proportions and Rates [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. Nov. Available from: https://www.ncbi.nlm.nih.gov/books/NBK179162/ [PubMed] [Google Scholar]
- 33. Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10(3):476-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 35. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 37. Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suttorp MM, Siegerink B, Jager KJ, Zoccali C, Dekker FW. Graphical presentation of confounding in directed acyclic graphs. Nephrol Dial Transplant. 2015;30(9):1418-1423. [DOI] [PubMed] [Google Scholar]
- 39. Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615-625. [DOI] [PubMed] [Google Scholar]
- 40. Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14(3):300-306. [PubMed] [Google Scholar]
- 41. Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45(6):1887-1894. [DOI] [PubMed] [Google Scholar]
- 42. Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China [published online ahead of print, 2020 Mar 25]. JAMA Cardiol. 2020;5(7):802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. WHO. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected Interim guidance. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Published 2020. Updated March 13, 2020. Accessed May 12, 2020.
- 45. Commission CNH. Novel coronavirus pneumonia prevention and control plan (6th ed.). (In Chinese) http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml. Published 2020. Accessed May 12, 2020.
- 46. Hernán MA. A definition of causal effect for epidemiological research. J Epidemiol Community Health. 2004;58(4):265-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- 48. Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen Y, Gong X, Wang L, Guo J. Effects of hypertension, diabetes and coronary heart disease on COVID-19 diseases severity: a systematic review and meta-analysis. medRxiv. 2020:2020.2003.2025.20043133. doi: 10.1101/2020.03.25.20043133 [DOI] [Google Scholar]
- 50. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Jama. 2003;289(21):2801-2809. [DOI] [PubMed] [Google Scholar]
- 51. Roca-Ho H, Riera M, Palau V, Pascual J, Soler MJ. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int J Mol Sci. 2017;18(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang W, Xu YZ, Liu B, et al. Pioglitazone upregulates angiotensin converting enzyme 2 expression in insulin-sensitive tissues in rats with high-fat diet-induced nonalcoholic steatohepatitis. Scient World J. 2014;2014:603409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127-00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999;26(3-4):259-265. [DOI] [PubMed] [Google Scholar]
- 55. Calvet HM, Yoshikawa TT. Infections in diabetes. Infect Dis Clin North Am. 2001;15(2):407-21, viii. [DOI] [PubMed] [Google Scholar]
- 56. Daryabor G, Kabelitz D, Kalantar K. An update on immune dysregulation in obesity-related insulin resistance. Scand J Immunol. 2019;89(4):e12747. [DOI] [PubMed] [Google Scholar]
- 57. Colosia AD, Palencia R, Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes. 2013;6:327-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71(15):896-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China [published online ahead of print March 13, 2020]. JAMA Intern Med. 2020;180(7):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area [published online ahead of print April 22, 2020] [published correction appears in doi:10.1001/jama.2020.7681]. JAMA. 2020;323(20):2052-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus Disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lippi G, Wong J, Henry BM. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130(4):304-309. [DOI] [PubMed] [Google Scholar]
- 64. Amador CA, Barrientos V, Peña J, et al. Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension. 2014;63(4):797-803. [DOI] [PubMed] [Google Scholar]
- 65. Singh MV, Chapleau MW, Harwani SC, Abboud FM. The immune system and hypertension. Immunol Res. 2014;59(1-3):243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020;16(6):305-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peiró C, Moncada S. Substituting angiotensin-(1–7) to prevent lung damage in SARS-CoV-2 infection? Circulation. 2020;141(21):1665-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension. 2012;59(4):755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens. 2020;33(5):373-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li J, Wang X, Chen J, Zhang H, Deng A. Association of Renin-Angiotensin System Inhibitors With Severity or Risk of Death in Patients With Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan, China [published online ahead of print April 23, 2020] [published correction appears in doi: 10.1001/jamacardio.2020.2338]. JAMA Cardiol. 2020;5(7):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]