Abstract
The COVID-19 pandemic has led to a major setback in both the health and economic sectors across the globe. The scale of the problem is enormous because we still do not have any specific anti-SARS-CoV-2 antiviral agent or vaccine. The human immune system has never been exposed to this novel virus, so the viral interactions with the human immune system are completely naive. New approaches are being studied at various levels, including animal in vitro models and human-based studies, to contain the COVID-19 pandemic as soon as possible. Many drugs are being tested for repurposing, but so far only remdesivir has shown some positive benefits based on preliminary reports, but these results also need further confirmation via ongoing trials. Otherwise, no other agents have shown an impactful response against COVID-19. Recently, research exploring the therapeutic application of mesenchymal stem cells (MSCs) in critically ill patients suffering from COVID-19 has gained momentum. The patients belonging to this subset are most likely beyond the point where they could benefit from an antiviral therapy because most of their illness at this stage of disease is driven by inflammatory (over)response of the immune system. In this review, we discuss the potential of MSCs as a therapeutic option for patients with COVID-19, based on the encouraging results from the preliminary data showing improved outcomes in the progression of COVID-19 disease.
Keywords: Stem cells, COVID-19, SARS-CoV-2 Virus, pandemic, vaccine, clinical trials
As of June 3, 2020, there were 6,551,389 global COVID-19 cases with 386,196 deaths. Although approximately 98% of patients experience mild disease, 2% experience severe disease often requiring critical care support.1,2
To date, physicians and intensivists treating patients with COVID-19 have limited treatment options in the absence of an effective antiviral agent or vaccine.3-5 Therapeutic options in the form of convalescent plasma therapy, remdesivir, tocilizumab, and repurposing of various drugs are being explored.6-10 Recently, a few studies have examined the role of mesenchymal stem cells (MSCs) in critically ill patients with COVID-19. These studies have shown some early promise as therapeutic possibilities for the treatment of severe COVID-19.11-14
Basic Principles of MSC Therapy
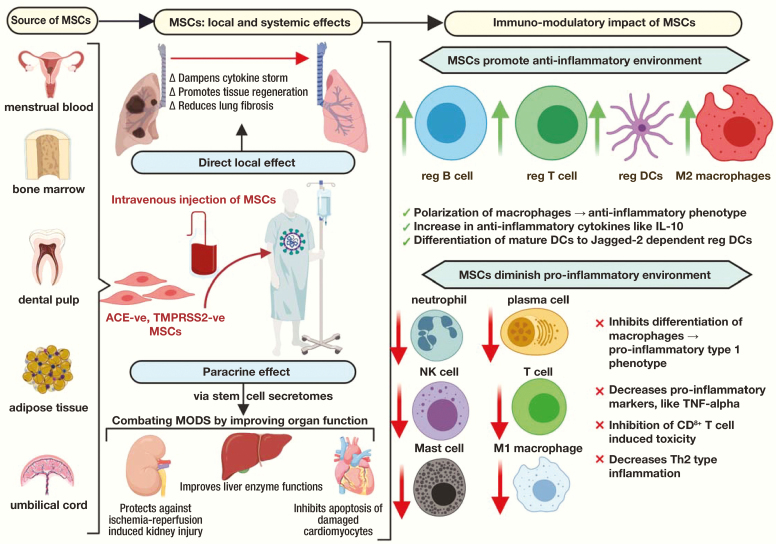
In multiple and mostly regenerative clinical settings, MSCs have been evaluated to either treat or prevent a disease or condition characterized by tissue damage. Regenerative treatment models have so far suggested the following major methods of repair:15,16 anti-inflammatory effect; stem cell homing to damaged networks, with recruitment of other cells; inhibition of apoptosis; and differentiation capability. The molecules and exosomes emitted from stem cells promote tissue healing and regeneration. In addition to the direct effect, stem cells also possess a paracrine function that works by releasing the soluble factors termed stem cell secretomes.17,18 This property allows the systemic distribution of the positive immunomodulatory and regenerative effects of MSCs throughout the body, thereby ensuring a systemic effect in addition to local modulation.18
Stem Cell Therapies and Concerns
The role of MSCs in regenerative medicine has been explored in the past. Many studies have shown the beneficial effects of MSC-based therapies.14,16,19 Such therapies have been studied in various neurological disorders, endocrinopathies, and bone and cartilage diseases. Contrary to these results, some studies have showed that MSCs may promote cancer pathogenesis.20,21 Therefore, the role of MSCs in regenerative medicine and their therapeutic potential needs more study and clarification.22 There is thus a constant effort from research bodies and other agencies such as the U.S. Food and Drug Administration (FDA), the International Society for Cellular Therapy, and the International Society for Stem Cell Research to actively monitor stem cell clinic industries across the globe to minimize unapproved and unproven cell-based therapies.23,24 With regard to the current pandemic, the main challenge is the novelty of the disease, its unfamiliar pathophysiology, and the lack of an anti-SARS-CoV-2 antiviral agent or vaccine. Realizing the pandemic as a time-sensitive matter, the FDA has been relatively liberal in clearing pathways to study MSCs and natural killer (NK) cells for their potential immune activity in response to COVID-19.25,26
Rationale of Using MSCs in COVID-19
Research has shown that MSCs have specific cytokines that drive immunomodulation, which may be useful against SARS-CoV-2.27 The available literature so far suggests that the hallmark of SARS-CoV-2 infection is a cytokine-induced storm. SARS-CoV-2 induces an acute release of cytokines such as granulocyte colony-stimulating factor, IP10, Monocyte Chemoattractant Protein-1, interleukin (IL)-2/6/7, and tumor necrosis factor (TNF) in large amounts.28,29 These cytokines lead to increased vasculature permeability, pulmonary edema, vascular congestion, and impairment of air exchange across the membranes, and in severe cases they may lead to acute respiratory distress syndrome (ARDS) and death.
Recently, Huang et al30 studied the cytokine profile of 41 patients with COVID-19. They found higher plasma levels of proinflammatory cytokines in patients in the intensive care unit (ICU) than in patients who were not. Chen, Wu, et al31 found markedly lower absolute numbers of T lymphocytes, CD4+ T cells, and CD8+ T cells in patients with severe cases.31 The patients with high cytokine levels, lower CD4+ T cells, and lower CD8+ T cells became more sick, required ICU care, and had a higher likelihood of developing ARDS.
Current efforts with MSC therapeutics are focusing on the capability of MSCs to abort or minimize this cytokine storm, thereby reducing lung damage and promoting the restoration of tissue function through their inherent reparative properties.32 The MSCs express angiotensin-converting enzyme 2 (ACE-2) and transmembrane protease serine 2 (TMPRSS2) cells and have the potential to induce mature dendritic cells (DCs) to novel Jagged-2 dependent regulatory DCs that possess an immunosuppressive capacity to generate specific immune tolerance and diminish Th2-type inflammation.33,34 The MSCs also help in the preferable differentiation of human CD34+ cells to regulatory DCs over classical DCs.35 Based on these immunomodulatory functions, Leng et al11 found MSC infusion beneficial in their patients with COVID-19. Similarly, Zhao36 also suggested that MSCs could help patients who are critically ill with COVID-19.
Biological Characteristics of MSCs and Identification of the Best Stem Cell Source
Stem cells derived from various tissues in the body are being evaluated for therapies in numerous degenerative disorders.16,37 The common stem cells used in clinical practice originate from bone marrow (BM), adipose tissue (AT), amnion, the umbilical cord (UC), dental pulp, menstrual blood, the buccal fat pad, and fetal liver.37 The MSCs hold much promise to change the dynamics of incurable diseases for many reasons: (i) easy accessibility, (ii) multipotency, (iii) ease of expansion to required clinical volume, (iv) storage potential for repetitive therapeutic usage, (v) immune evasive property indicating a minimal chance of rejection with allogeneic MSCs, and (vi) easy route of administration (via intravenous [IV]).
For patients with COVID-19, among the various stem cells, UC mesenchymal stem cells (UC-MSCs) have recently gained more attention because of their ready availability, compatibility, potency, and plasticity:19,38-41
-
i.
When compared with BM, UC harvests have a higher concentration of stem cells.
-
ii.
The UC-MSCs have a faster doubling time.
-
iii.
A higher proliferation rate of UC-MSCs allows for a scalable expansion that may benefit a larger population of critically ill patients.
-
iv.
The harvesting tissue for UC-MSCs is a byproduct during delivery and does not require any invasive procedure unlike BM stem cell extraction.
-
v.
The UC-MSCs have the advantage of being immune tolerant because of low expression of major histocompatibility complex (MHC) class I and no expression of MHC class II. These characteristics allow the use of even allogeneic MSCs.
Similarly, another potential source of MSCs with an advantage over other harvesting sites is AT. Using AT-derived MSCs has the following benefits:38,39
-
i.
Easily accessible site for extraction that poses minimum discomfort to the donor.
-
ii.
Comparatively easy to isolate MSCs from the harvested AT.
-
iii.
Comparatively, a higher fraction of MSCs can be extracted from harvested AT compared with BM.
-
iv.
Comparatively, a higher success rate of isolating MSCs from AT than UC.
Reviving COVID-19-Induced Lung Damage via MSCs
Studies on animal models have shown that pneumocyte type II cells support coronavirus replication better than pneumocyte type I cells and alveolar macrophages.42,43 In addition, postmortem histopathology examination of lung tissue from patients with COVID-19 has shown significant lung damage with evidence of diffuse alveolar damage, type II pneumocyte hyperplasia, and intra-alveolar fibrinous exudates.44 Recent studies have provided ample evidence that stem cells promote lung tissue healing and regeneration by differentiating to pulmonary epithelial cells. Once injected intravenously, a significant amount of MSCs accumulate in the lung and exhibit an immunomodulatory effect, thereby protecting the alveolar epithelial cells, restoring the pulmonary alveolar niche, preventing fibrosis, and improving overall pulmonary function.45,46 This phenomenon may benefit critically ill patients with COVID-19 and help them recover from ARDS, pulmonary edema, and diffuse alveolar damage.
Clinical Use of MSCs in COVID-19: Paucity of Data
The COVID-19 pandemic is evolving, and we are still in the learning phase. There is a lack of impactful data on MSC therapeutics for COVID-19. Physicians can extrapolate the potential clinical benefits of MSCs in COVID-19 pneumonia based on studies from previous viral outbreaks with positive outcomes.47 Chen et al infused MSCs extracted from allogeneic menstrual blood from healthy female donors into 17 patients with H7N9-induced ARDS. They found a significant mortality benefit in the MSC-infused arm (17.6% died) as compared with the control arm (54.5% died). The major limitations of the study were the small sample size, high attrition rate, and not-ideal comparison sample because patients were receiving other drugs as well.
Because H7N9 and SARS-CoV-2 share similar complications—ARDS, hypoxic respiratory failure, severe inflammation, overt immune response, and multiorgan dysfunction syndrome—MSCs therapy may be beneficial for patients with COVID-19 pneumonia as well.27,28 A recent meta-analysis on MSC use in ARDS (study period 1990 to March 31, 2020)7 showed an improvement in radiographic shadows, pulmonary function, and biochemical marker levels. This meta-analysis also showed a mortality benefit with the use of MSCs but not to a significant level.
With regard to COVID-19, Leng et al11 recently used BM-derived MSCs intravenously in 7 patients with COVID-19 (1 critically severe illness, 4 severe illnesses, and 2 common illnesses). Significant clinical benefit was noted in all patients with symptomatic improvement and reduced oxygen requirement after 2 to 4 days of MSC transplantation. Mass cytometry and cytokine analysis of the patients’ peripheral blood also showed disappearance of overactivated T cells and NK cells, an increase in anti-inflammatory cytokines like IL-10, and a decrease in proinflammatory cytokines like TNF-alpha. However, the small sample size, the lack of a control arm, patients who were also receiving other drugs, and a patient cohort with only 1 patient with critical serious illness are possible limitations to consider before analyzing the results.
The above-discussed studies regarding the use of MSCs in COVID-19 have provided a much-needed clinical platform for further research on MSCs in COVID-19 and other viral disorders. Therapy using MSC in high-risk populations including pregnant women, patients with human immunodeficiency virus, patients with malignancies, and transplant recipients would need further refinement before being executed.48-52 In addition, it is too early to suggest that MSCs are safe to use in patients with COVID-19 and requires further confirmation.53,54
Current Literature on Ongoing Trials of MSCs for COVID-19
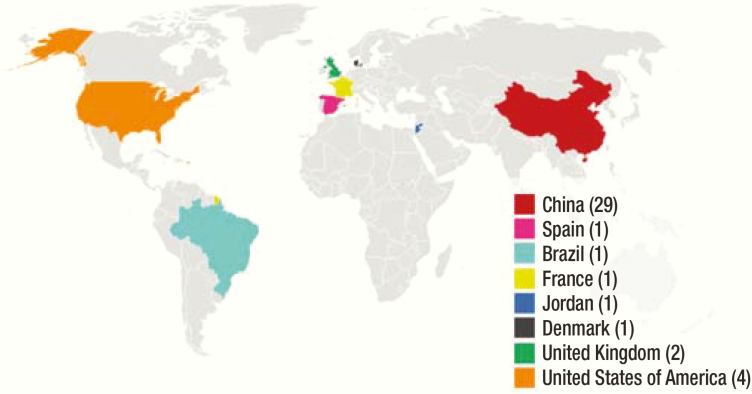
Recently, clinical trials studying various aspects of MSC use in COVID-19 are underway in several clinical phases (Figure 1). China and the United States are the 2 leading countries studying cell-based therapy–related clinical trials, from which some reports have been published as well (Table 1).55,56 As of April 21, 2020, 40 clinical trials were active across the globe (http://www.chictr.org.cn/index.aspx and https://clinicaltrials.gov/). Zhongnan Hospital in China and its collaborator, Tuohua Biological Technology Co. Ltd., are studying UC-MSC treatment (IV on day 1, day 3, day 5, and day 7) in patients with serious and critical pneumonia. The primary outcome is to study the improvement in oxygenation index on day 14 after enrollment (NCT04269525). Similarly, Renmin Hospital of Wuhan University is planning to evaluate the safety and efficacy of allogeneic human dental pulp MSCs in severe pneumonia caused by COVID-19 (ChiCTR2000031319). Elsewhere, scientists from other countries including the United Kingdom, Brazil, Jordan, and France are conducting similar MSC therapy clinical trials for COVID-19.
Figure 1.
Number of studies currently enrolled regarding MSCs in COVID-19 by country (as of April 22, 2020).
Table 1.
List of Registered Cell-Based Clinical Trials for Treating COVID-19 Worldwide
| ClinicalTrials.gov Identifier | Title | Study Characteristics | Primary Outcome Measures | Responsible Party |
|---|---|---|---|---|
| United States | ||||
| NCT04299152 | Clinical Application of Stem Cell Educator Therapy for the Treatment of Viral Inflammation Caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) | —Interventional study —Estimated participants: 20 —Study design: parallel |
Determine the number of patients with COVID-19 who were unable to complete SCE therapy (time frame: 4 weeks) | Tianhe Stem Cell Biotechnologies Inc., China |
| NCT04348435 | A Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Allogeneic Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19 | —Interventional study —Estimated participants: 100 —Study design: parallel |
—Incidence of hospitalization for COVID-19 (time frame: week 0 through week 26 [end of study]) —Incidence of symptoms associated with COVID-19 (time frame: week 0 through week 26 [end of study]) |
Hope Biosciences Stem Cell Research Foundation, Sugar Land, TX |
| NCT04355728 | Umbilical Cord-derived Mesenchymal Stem Cells for COVID-19 Patients with Acute Respiratory Distress Syndrome (ARDS) | —Interventional study —Estimated participants: 12 —Study design: parallel |
—Incidence of prespecified infusion-associated AEs (time frame: day 5) —Incidence of SAEs (time frame: 90 days) |
Diabetes Research Institute, University of Miami Miller School of Medicine, Miami, FL |
| NCT04345601 | Single Donor Banked Bone Marrow Mesenchymal Stromal Cells for the Treatment of SARS-CoV-2 Induced Acute Respiratory Failure: A Pilot Study | —Interventional study —Estimated participants: 30 —Intervention model: single group |
—Incidence of unexpected AEs (time frame: 28 days post—cell infusion) —Improved oxygen saturations ≥ 93% (time frame: within 7 days of cell infusion) |
Houston Methodist Hospital, Houston, TX |
| China | ||||
| ChiCTR2000031319 | Safety and Efficacy Study of Allogeneic Human Dental Pulp Mesenchymal Stem Cells to Treat Severe Pneumonia of COVID-19: a Single-center, Prospective, Randomised Clinical Trial | —Interventional study —Estimated participants: 20 —Study design: parallel |
… | Center for Regenerative Medicine, Renmin Hospital of Wuhan University, China |
| ChiCTR2000031735 | Clinical study for natural killer (NK) cells from umbilical cord blood in the treatment of viral pneumonia include novel coronavirus pneumonia (COVID-19) | —Interventional study —Estimated participants: 20 —Study design: parallel |
Monitoring of AEs within 24 hours after infusion (including infusion-related events and SAEs associated with nonprimary disease) | Huzhou Central Hospital, Zhejiang, China |
| ChiCTR2000031139 | Safety and Effectiveness of Human embryonic stem cell-derived M cells (CAStem) for Pulmonary Fibrosis Correlated with novel coronavirus pneumonia (COVID-19) | —Interventional study —Estimated participants: 20 —Study design: sequential |
—Pulmonary function evaluation —Changes in blood gas analysis —Evaluation of activity —Evaluation of dyspnea |
Wuhan Jinyintan Hospital (Wuhan Infectious Diseases Hospital), Wuhan, Hubei, China |
| ChiCTR2000030509 | Clinical Study of NK Cells in the Treatment of Novel Coronavirus Pneumonia (COVID-19) | —Interventional study —Estimated participants: 40 —Study design: parallel |
Time and rate of novel coronavirus become negative | The First Hospital of Harbin Medical University, Harbin, Heilongjiang, China |
| ChiCTR2000030329 | Clinical trial for umbilical cord blood CIK and NK cells in the treatment of mild and general patients infected with novel coronavirus pneumonia (COVID-19) | —Interventional study —Estimated participants: 90 —Study design: parallel |
—Status of immune function —Nucleic acid test is negative —Length of stay in hospital |
The Second Affiliated Hospital of Xi’an Medical University, Xi’an, Shaanxi, China |
| ChiCTR2000030224 | Clinical study of mesenchymal stem cells in treating severe novel coronavirus pneumonia (COVID-19) | —Interventional study —Estimated participants: 32 —Study design: parallel |
—Inflammatory biomarkers —Blood routine —Temperature —Lesions of lung seen in CT scan |
Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China |
| ChiCTR2000030173 | Key techniques of umbilical cord mesenchymal stem cells for the treatment of novel coronavirus pneumonia (COVID-19) and clinical application demonstration | —Interventional study —Estimated participants: 60 —Study design: parallel |
—Pulmonary function —Novel coronavirus pneumonic nucleic acid test |
Nanhua Hospital, affiliated with Nanhua University, Hengyang, Hu’nan, China |
| ChiCTR2000030088 | Umbilical cord Wharton’s Jelly derived mesenchymal stem cells in the treatment of severe novel coronavirus pneumonia (COVID-19) | —Interventional study —Estimated participants: 40 —Study design: parallel |
—Nucleic acid test for novel coronavirus is negative —CT scan of ground glass shadow disappeared |
The Sixth Medical Center of PLA General Hospital, Beijing, China |
| ChiCTR2000030020 | The clinical application and basic research related to mesenchymal stem cells to treat novel coronavirus pneumonia (COVID-19) | —Interventional study —Estimated participants: 40 —Study design: sequential |
—Coronavirus nucleic acid markers have negative rate —Inflammation (per chest CT) —Symptoms improved after 4 treatments |
Second Hospital of University of South China, Hengyang, China |
| ChiCTR2000029816 | Clinical Study of Cord Blood Mesenchymal Stem Cells in the Treatment of Acute Novel Coronavirus Pneumonia (COVID-19) | —Interventional study —Estimated participants: 60 —Study design: parallel |
Time to disease recovery | Guangzhou Reborn Health Management Consultation Co, Ltd, Guangzhou, Guangdong, China |
| ChiCTR2000029812 | Clinical Study for Umbilical Cord Blood Mononuclear Cells in the Treatment of Acute Novel Coronavirus Pneumonia (NCP) | —Interventional study —Estimated participants: 60 —Study design: parallel |
Time to disease recovery | Guangzhou Reborn Health Management Consultation Co, Ltd, Guangzhou, Guangdong, China |
| ChiCTR2000029606 | Clinical Study for Human Menstrual Blood-derived Stem Cells in the Treatment of Acute Novel Coronavirus Pneumonia (COVID-19) | —Interventional study —Estimated participants: 63 —Study design: parallel |
Mortality in patients | The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China |
| ChiCTR2000029580 | Severe novel coronavirus pneumonia (COVID-19) patients treated with ruxolitinib in combination with mesenchymal stem cells: a prospective, single blind, randomized controlled clinical trial | —Interventional study —Estimated participants: 70 —Study design: parallel |
Safety | Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China |
| ChiCTR2000029572 | Safety and efficacy of umbilical cord blood mononuclear cells in the treatment of severe and critically novel coronavirus pneumonia (COVID-19): a randomized controlled clinical trial | —Interventionalstudy —Estimated participants: 30 —Study design: parallel |
PSI | Xiangyang First People’s Hospital, Xiangyang, Hubei, China |
| ChiCTR2000029569 | Safety and efficacy of umbilical cord blood mononuclear cells conditioned medium in the treatment of severe and critically novel coronavirus pneumonia (COVID-19): a randomized controlled trial | —Interventional study —Estimated participants: 30 —Study design: parallel |
PSI | Xiangyang First People’s Hospital, Xiangyang, Hubei, China |
| CTR2000030116 | Safety and effectiveness of human umbilical cord mesenchymal stem cells in the treatment of acute respiratory distress syndrome of severe novel coronavirus pneumonia (COVID-19) | Interventional study —Estimated participants: 16 —Study design: dose comparison |
Time to leave ventilator on day 28 after receiving MSC infusion | The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China |
| ChiCTR2000030484 | HUMSCs and Exosomes Treating Patients with Lung Injury following Novel Coronavirus Pneumonia (COVID-19) | —Interventional study —Estimated participants: 90 —Study design: parallel |
—PaO2/FiO2 or respiratory rate (without oxygen) —Frequency of respiratory exacerbation —Observe physical signs and symptoms and record clinical recovery time —Number and range of lesions indicated by CT and X-ray of lung —Time for cough to become mild or absent —Time for dyspnea to become mild or no dyspnea —Frequency of oxygen inhalation or noninvasive ventilation, frequency of mechanical ventilation —Inflammatory cytokines (eg, CRP/PCT/SAA) —Frequency of SAE |
Hubei Shiyan Taihe Hospital, Shiyan, Hubei, China |
| ChiCTR2000030866 | Open-label, observational study of human umbilical cord derived mesenchymal stem cells in the treatment of severe and critical COVID-19. | Observational study —Estimated participants: 30 Study design: single arm |
-Oxygenation index (PaO2/ FiO2) —Conversion rate from serious to critical patients —Conversion rate and conversion time from critical to serious patients —Mortality in serious and critical patients |
Changsha First Hospital, Changsha, Hu’nan, China |
| ChiCTR2000030835 | Clinical study on the efficacy of Mesenchymal stem cells (MSC) in the treatment of severe novel coronavirus pneumonia (COVID-19) | —Interventional study —Estimated participants: 20 —Study design:single arm |
NA | The First Affiliated Hospital of Xinxiang Medical University, Xinxiang, He’nan, China |
| ChiCTR2000030138 | Clinical Trial for Human Mesenchymal Stem Cells in the Treatment of Severe Novel Coronavirus Pneumonia (COVID-19) | —Interventional study —Estimated participants: 60 —Study design: parallel |
Clinical index | Chinese PLA General Hospital, Haidian Distract, Beijing, China |
| NCT04302519 | Clinical Study of Novel Coronavirus Induced Severe Pneumonia Treated by Dental Pulp Mesenchymal Stem Cells | —Interventional study —Estimated participants: 24 Study design: single group |
Disappear time of ground-glass shadow in lungs (time frame: 14 days) | CAR-T (Shanghai) Biotechnology Co, Ltd, Shanghai, China |
| NCT04288102 | Multicenter, Randomized, Double-blind, Placebo-controlled Study Evaluating the Efficacy and Safety of Human Mesenchymal Stem Cells in Combination with Standard Therapy in the Treatment of COVID-19 Patients With Severe Convalescence | —Interventional study —Estimated participants: 90 —Study design: parallel |
Size of lesion area and severity of pulmonary fibrosis by chest CT (time frame: at baseline, day 6, day 10, day 14, day 28, day 90] | Maternal and Child Hospital of Hubei Province, Wuhan, Hubei, China, and Wuhan Huoshenshan Hospital, Wuhan, Hubei, China |
| NCT04273646 | Clinical Study of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Severe COVID-19 | —Interventional study —Estimated participants: 48 —Study design: parallel |
—Pneumonia severity index (time frame: from baseline to 12 weeks after treatment) —Oxygenation index (PaO2/FiO2; time frame: from baseline to 12 weeks after treatment) |
Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China |
| NCT04252118 | Safety and Efficiency of Mesenchymal Stem Cell in Treating Pneumonia Patients Infected With COVID-19 | —Interventional study —Estimated participants: 20 —Study design: parallel |
—Size of lesion area by chest radiograph or CT (time frame: at baseline, day 3, day 6, day 10, day 14, day 21, day 28) —Side effects in MSC treatment group (time frame: at baseline, day 3, day 6, day 10, day 14, day 21, day 28, day 90, day 180) |
Beijing 302 Military Hospital of China, Beijing, China |
| NCT04269525 | Clinical Research Regarding the Availability and Safety of UC-MSCs Treatment for Serious Pneumonia and Critical Pneumonia Caused by the 2019-nCOV Infection | —Interventional study —Estimated participants: 10 —Intervention model: single group |
Oxygenation index (time frame: on day 14 after enrollment) | Zhongnan Hospital, Wuhan, Hubei, China |
| NCT04276987 | A Pilot Clinical Study on Aerosol Inhalation of the Exosomes Derived From Allogenic Adipose Mesenchymal Stem Cells in the Treatment of Severe Patients With Novel Coronavirus Pneumonia | —Interventional study —Estimated participants: 30 —Intervention model: single group |
—Time to clinical improvement (time frame: up to 28 days) —AE and SAE (time frame: up to 28 days) |
Ruijin Hospital, Wuhan, China |
| NCT04346368 | Safety and Efficacy of Intravenous Infusion of Bone Marrow-Derived Mesenchymal Stem Cells in Severe Patients with Coronavirus Disease 2019 (COVID-19): A Phase 1/2 Randomized Controlled Trial | —Interventional study —Estimated participants: 20 —Intervention model: Single group |
—AEs in BM-MSC treatment group (time Frame: baseline–6 months) —Changes in oxygenation index (PaO2/FiO2; time frame: baseline, 6hours, day 1, day 3, week 1, week 2, week 4, month 6) |
Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China |
| NCT04339660 | Clinical Research of Human Mesenchymal Stem Cells in the Treatment of COVID-19 Pneumonia | —Interventional study —Estimated participants: 30 —Study design: parallel |
—Observe immune function (TNF-α, IL-1β, IL-6, TGF-β, IL-8, PCT, CRP; time frame: within 4 weeks) —Monitor blood oxygen saturation (time frame: within 4 weeks) |
Puren Hospital, affiliated with Wuhan University of Science and Technology, Wuhan, Hubei, China |
| NCT04331613 | Safety and Efficacy Study of Human Embryonic Stem Cells Derived M Cells (CAStem) for the Treatment of Severe COVID-19 Associated With or Without Acute Respiratory Distress Syndrome (ARDS) | —Interventional study —Estimated participants: 09 —Study design: single group |
—AE and SAE (time frame: within 28 days after treatment) —Changes in lung imaging examinations (time frame: within 28 days after treatment) |
Beijing YouAn Hospital, Capital Medical University Beijing, Beijing, China, |
| Brazil | ||||
| NCT04315987 | Exploratory Clinical Study to Assess the Efficacy of NestCell Mesenchymal Stem Cell to Treat Patients with Severe COVID-19 Pneumonia | —Interventional study —Estimated participants :66 —Study design: single group |
Change in clinical condition (time frame: 10 days) | Hospital Vera Cruz Campina Grande, São Paulo, Brazil |
| Jordan | ||||
| NCT04313322 | Treatment of COVID-19 Patients Using Wharton’s Jelly-Mesenchymal Stem Cells | —Interventional study —Estimated participants: 5 —Intervention model: single group |
—Improvement of clinical symptoms (time frame: 3 weeks) —AEs measured by chest radiograph/CT scan (time frame: 3 weeks) —RT-PCR results of viral RNA turning negative (time frame: 3 weeks) |
Stem Cells Arabia, Amman, Jordan |
| France | ||||
| NCT04333368 | Cell Therapy Using Umbilical Cord-derived Mesenchymal Stromal Cells in SARS-CoV-2-related ARDS | —Interventional study —Estimated participants: 60 —Intervention model: single group |
Respiratory efficacy evaluated by increase in PaO2/FiO2 ratio from baseline to day 7 in experimental group compared with placebo group | Hôpital Pitié-Salpêtrière—APHP, Paris, France, and Hôpital Européen Georges Pompidou—APHP, Paris, France |
| United Kingdom | ||||
| NCT04349540 | A Prospective Non-Interventional Study to Evaluate the Role of Immune and Inflammatory Response in Recipients of Allogeneic Haematopoietic Stem Cell Transplantation (SCT) Affected by Severe COVID19 Infection | —Observational study —Estimated participants: 40 —Intervention model: case-only |
… | Great Ormond Street Hospital, NHS Foundation Trust, London, United Kingdom |
| NCT03042143 | Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration (REALIST): An Open Label Dose Escalation Phase 1 Trial Followed by a Randomized, Double-blind, Placebo-controlled Phase 2 Trial (COVID-19) | —Interventional study —Estimated participants: 75 —Study design: parallel |
—Oxygenation index (time frame: day 7) —Incidence of SAEs (time frame: 28 days) |
Belfast Health and Social Care Trust, Royal Hospitals Belfast, Northern Ireland, United Kingdom |
| Spain | ||||
| NCT04348461 | Two-treatment, Randomized, Controlled, Multicenter Clinical Trial to Assess the Safety and Efficacy of Intravenous Administration of Expanded Allogeneic Adipose Tissue Adult Mesenchymal Stromal Cells in Critically Ill Patients COVID-19 | —Interventional study —Estimated participants: 100 —Study design: parallel |
—Efficacy of administration of allogeneic MSCs derived from AT assessed by survival rate (time frame: 28 days) —Safety of administration of allogeneic MSCs derived from AT assessed by AE rate (time frame: 6 months) |
Instituto de Investigación, Sanitaria de la Fundación Jiménez Díaz, Madrid, Spain |
| Denmark | ||||
| NCT04341610 | Allogeneic Adipose Tissue Derived Mesenchymal Stromal Cell Therapy for Treating Patients with Severe Respiratory COVID-19. A Danish, Double-blind, Randomized Placebo-controlled Study | —Interventional study —Estimated participants: 40 —Study design: parallel |
Changes in clinical critical treatment index (time frame: day 7 from randomization) | Department of Cardiology, The Heart Centre, University Hospital Rigshospitalet, Copenhagen, Denmark |
SCE, stem cell educator; AE, adverse event; SAE, serious adverse event; CT, computed tomography; PSI, patient safety indicators; MSCs, mesenchymal stem cells; PaO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen; PCT, procalcitonin; SAA, serum amyloid A; CRP, C-reactive protein; BM-MSC, bone marrow mesenchymal stem cells; RT-PCR, real-time polymerase chain reaction; AT, adipose tissue
Side Effects and Concerns Over Practical Usability During COVID-19 Pandemic
To prove MSC therapy successful, in addition to evaluating its efficacy it is equally important to assess other practical aspects of community use and accessibility.57,58 With regard to MSCs in COVID-19, although the experience so far has been encouraging, the key barriers still include the following:59-61
-
i.
Data to date are preliminary and based on compassionate use of MSCs, with final results from clinical trials still pending.
-
ii.
The downstream effects of MSCs on the lungs are unclear and unknown. For example, we lack an understanding of MSC impact on the expression of ACE-2 and TMPRSS2, which are known facilitators of viral entry into cells and subsequent replication (Figure 2).
-
iii.
Similarly, there is a differential expression of ACE-2 receptors in various organ systems. It would be noteworthy to determine whether there would be a difference in the recovery/response of different organs upon MSC infusion.
-
iv.
There is concern over the commercialization and subsequent abuse of unproven cell-based therapies by unauthorized stem cell clinics.
-
v.
Therapy with MSCs is not a readily available resource, especially in developing countries.
-
vi.
High cost, insurance hurdles, and no standardized treatment protocol are issues of concern. The cost of MSC therapy is extremely variable (between $5000 and $50,000) and depends on the type of stem cells, laboratory location (for extraction), patient location (for infusion), and proliferation character of stem cells. To add to the complexity of the situation, in the United States, Medicare does not cover MSC therapy.
-
vii.
In addition, MSC therapy is technically complex, requiring highly specialized staff and equipment.
-
viii.
Scalability because of these technical challenges may lead to a high discrepancy in the supply-demand chain.
-
ix.
There could be immediate adverse effects from MSC infusion; transfusion reactions such as allergies, anaphylaxis, serum sickness, delayed hypersensitivity reactions, and secondary bacteremia. On a positive note, Leng et al11 did not note any acute infusion-related issues, allergic reactions, or delayed hypersensitivity or secondary infections in their cohort of patients. Researchers have found that MSCs are ACE-2 negative and TMPRSS2 negative and are thereby unlikely to become infected by SARS-CoV-2.
Figure 2.
Pictorial description of the source of stem cells, and impact of mesenchymal stem cells.
Conclusion
During this unprecedented healthcare crisis, researchers and clinicians across the globe are working relentlessly to identify the best strategies and treatment for patients with COVID-19. Although MSCs are a potentially promising therapy, they are still in the early stages of development for COVID-19 treatment. Further data from ongoing clinical trials across the world will help clarify their potential utility in battling the COVID-19 pandemic. LM
Acknowledgments
The article does not contain the participation of any human being or animal. All authors have seen the article and agree to the content and data. All the authors played a significant role in the article.
Glossary
Abbreviations
- MSCs
mesenchymal stem cells
- FDA
U.S. Food and Drug Administration
- NK cells
natural killer cells
- IL
interleukin
- TNF
tumor necrosis factor
- ARDS
acute respiratory distress syndrome
- ICU
intensive care unit
- ACE-2
angiotensin-converting enzyme 2
- TMPRSS2
transmembrane protease, serine 2
- DC
dendritic cell
- BM
bone marrow
- AT
adipose tissue
- UC
umbilical cord
- IV
intravenous
- UC-MSCs
umbilical cord mesenchymal stem cells
- MHC
major histocompatibility complex; SCE, stem cell educator
- AE
adverse event
- SAE
serious adverse event
- CT
computed tomography; PSI, patient safety indicators
- MSCs
mesenchymal stem cells
- PaO2
partial pressure of oxygen
- FiO2
fraction of inspired oxygen; PCT, procalcitonin; SAA, serum amyloid A
- CRP
C-reactive protein
- BM-MSC
bone marrow mesenchymal stem cells
- RT-PCR
real-time polymerase chain reaction
References
- 1. Sahu KK, Kumar R. Current perspective on pandemic of COVID-19 in the United States. J Family Med Prim Care. 2020;9(4):1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, et al. . A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sahu KK, Kumar R. Preventive and treatment strategies of COVID-19: from community to clinical trials. J Family Med Prim Care. 2020;9(5):2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coronavirus update (Live): 6,102,522 cases and 369,127 deaths from COVID-19 virus pandemic – worldometer website updated, June 03, 2020 https://www.worldometers.info/coronavirus/. Accessed June 03 2020.
- 5. Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52(4):583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Douedi S, Miskoff J. Novel coronavirus 2019 (COVID-19): a case report and review of treatments. Medicine (Baltimore). 2020;99(19):e20207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qu W, Wang Z, Hare JM, et al. . Cell-based therapy to reduce mortality from COVID-19: Systematic review and meta-analysis of human studies on acute respiratory distress syndrome [published online ahead of print May 29, 2020]. Stem Cells Transl Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weiss ARR, Dahlke MH. Immunomodulation by Mesenchymal Stem Cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. 2019;10:1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sahu KK, Jindal V, Siddiqui AD, Cerny J, Gerber JM. Convalescent plasma therapy: a passive therapy for an aggressive COVID-19 [published online ahead of print May 21, 2020]. J Med Virol. 2020; doi: 10.1002/jmv.26047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahu KK, Lal A, Mishra AK. Latest updates on COVID-2019: a changing paradigm shift. J Med Virol. 2020;92(6):533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leng Z, Zhu R, Hou W, et al. . Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem Cell Rev Rep. 2020;16(3):427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Metcalfe SM. Mesenchymal stem cells and management of COVID-19 pneumonia. Med Drug Discov. 2020;5:100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu S, Peng D, Qiu H, Yang K, Fu Z, Zou L. Mesenchymal stem cells as a potential therapy for COVID-19. Stem Cell Res Ther. 2020;11(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Atluri S, Manchikanti L, Hirsch JA. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23(2):E71–E83. [PubMed] [Google Scholar]
- 17. Nitkin CR, Rajasingh J, Pisano C, Besner GE, Thébaud B, Sampath V. Stem cell therapy for preventing neonatal diseases in the 21st century: Current understanding and challenges. Pediatr Res. 2020;87(2):265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Öztürk S, Elçin AE, Elçin YM. Mesenchymal stem cells for coronavirus (COVID-19)-induced pneumonia: revisiting the paracrine hypothesis with new hopes? Aging Dis. 2020;11(3):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shetty AK. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)- induced pneumonia. Aging Dis. 2020;11(2):462–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cuiffo BG, Karnoub AE. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adh Migr. 2012;6(3):220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayal TB, Kıratlı B, Şişli HB, Şahin F, Doğan A. Mesenchymal stem cells as regulators of carcinogenesis. Adv Exp Med Biol. 2019;1144:147–166. [DOI] [PubMed] [Google Scholar]
- 22. Marks PW, Witten CM, Califf RM. Clarifying stem-cell therapy’s benefits and risks. N Engl J Med. 2017;376(11):1007–1009. [DOI] [PubMed] [Google Scholar]
- 23. FDA warns about stem cell therapies | FDA. U.S. Food and Drug Administration website, Updated September 03, 2019. https://www.fda.gov/consumers/consumer-updates/fda-warns-about-stem-cell-therapies. Accessed June 06, 2020.
- 24. Approved cellular and gene therapy products | FDA. U.S. Food and Drug Administration website, Updated September 03, 2019. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products. Accessed June 06, 2020.
- 25. Hope Biosciences. Hope Biosciences gets FDA nod for phase 2 COVID-19 stem cell therapy trial. Updated April 14, 2020. https://pharmaceutical-business-review.com/news/hope-biosciences-covid-19-stem-cell/. Accessed June 06, 2020.
- 26. Slater H. FDA accepts IND for NK cell therapy CYNK-001 to treat patients with COVID-19. Updated April 03, 2020. Accessed June 06, 2020. [Google Scholar]
- 27. Chen Y, Chan VSF, Zheng B, et al. . A novel subset of putative stem/progenitor CD34+ Oct-4 + cells is the major target for SARS coronavirus in human lung. J Exp Med. 2007;204(11):2529–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jamilloux Y, Henry T, Belot A, et al. . Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19(7):102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sahu KK, Siddiqui AD. From Hematologist’s desk: the effect of COVID-19 on the blood system [published online ahead of print May 1, 2020]. Am J Hematol. 2020;doi: 10.1002/ajh.25849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen G, Wu D, Guo W, et al. . Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20(8):822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X. Tumor-educated CD11b high Ia low regulatory dendritic cells suppress T cell response through arginase I. J Immunol. 2009;182(10):6207–6216. [DOI] [PubMed] [Google Scholar]
- 34. Zhang B, Liu R, Shi D, et al. . Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113(1):46–57. [DOI] [PubMed] [Google Scholar]
- 35. Liu X, Qu X, Chen Y, et al. . Mesenchymal stem/stromal cells induce the generation of novel IL-10–dependent regulatory dendritic cells by SOCS3 activation. J Immunol. 2012;189(3):1182–1192. [DOI] [PubMed] [Google Scholar]
- 36. Zhao RC. Stem cell-based therapy for coronavirus disease 2019. Stem Cells Dev. 2020;29(11):679–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rebelatto CK, Aguiar AM, Moretão MP, et al. . Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood). 2008;233(7):901–913. [DOI] [PubMed] [Google Scholar]
- 38. Rogers CJ, Harman RJ, Bunnell BA, et al. . Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J Transl Med. 2020;18(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gentile P, Sterodimas A. Adipose-derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID-19)-induced pneumonia. Expert Opin Biol Ther. 2020;20(7):711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Atluri S, Manchikanti L, Hirsch JA. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23(2):E71–E83. [PubMed] [Google Scholar]
- 41. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. [DOI] [PubMed] [Google Scholar]
- 42. Mossel EC, Wang J, Jeffers S, et al. . SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology. 2008;372(1):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sims AC, Burkett SE, Yount B, Pickles RJ. SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium. Virus Res. 2008;133(1):33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qian Z, Travanty EA, Oko L, et al. . Innate immune response of human alveolar type II cells infected with severe acute respiratory syndrome-coronavirus. Am J Respir Cell Mol Biol. 2013;48(6):742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wichmann D, Sperhake JP, Lütgehetmann M, et al. . Autopsy findings and venous thromboembolism in patients with COVID-19 [published online ahead of print May 6, 2020]. Ann Intern Med. 2020;M20-2003. [Google Scholar]
- 46. Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573–576. [DOI] [PubMed] [Google Scholar]
- 47. Chen J, Hu C, Chen L, et al. . Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID-19 treatment. Engineering. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang X, Yu Y, Xu J, et al. . Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sahu KK, Siddiqui AD, Cerny J. Managing sickle cell patients with COVID-19 infection: the need to pool our collective experience [published online ahead of print May 23, 2020]. Br J Haematol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sahu KK, Siddiqui AD, Cerny J. COVID-19 pandemic and impact on hematopoietic stem cell transplantation [published online ahead of print May 4, 2020]. Bone Marrow Transplant. 2020;1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jindal V, Sahu KK, Gaikazian S, Siddiqui AD, Jaiyesimi I. Cancer treatment during COVID-19 pandemic. Med Oncol. 2020;37(7):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hayes M, Curley G, Ansari B, Laffey JG. Clinical review: stem cell therapies for acute lung injury/acute respiratory distress syndrome—hope or hype? Crit Care. 2012;16(2):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yu F, Jia R, Tang Y, Liu J, Wei B. SARS-CoV-2 infection and stem cells: Interaction and intervention [published online ahead of print June 1, 2020]. Stem Cell Res. 2020;46:101859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. 中国临床试验注册中心 - 世界卫生组织国际临床试验注册平台一级注册机构 Updated May 15, 2020. Available from: http://www.chictr.org.cn/index.aspx. Accessed June 06, 2020
- 56. Home - ClinicalTrials.gov Updated May 20, 2020. Available from: https://clinicaltrials.gov/. Accessed June 06, 2020
- 57. Musiał-Wysocka A, Kot M, Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019;28(7):801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aliotta JM, Keaney P, Passero M, et al. . Bone marrow production of lung cells: the impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotype. Exp Hematol. 2006;34(2):230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Glowacka I, Bertram S, Herzog P, et al. . Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84(2):1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kuba K, Imai Y, Rao S, et al. . A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]