Abstract
Clinical laboratory testing routinely provides actionable results, which help direct patient care in the inpatient and outpatient settings. Since December 2019, a novel coronavirus (SARS-CoV-2) has been causing disease (COVID-19 [coronavirus disease 2019]) in patients, beginning in China and now extending worldwide. In this context of a novel viral pandemic, clinical laboratories have developed multiple novel assays for SARS-CoV-2 diagnosis and for managing patients afflicted with this illness. These include molecular and serologic-based tests, some with point-of-care testing capabilities. Herein, we present an overview of the types of testing available for managing patients with COVID-19, as well as for screening of potential plasma donors who have recovered from COVID-19.
Beginning in December 2019, an outbreak of “pneumonia of unknown cause” was detected in Wuhan City, Hubei Province, China. Ultimately, the 2019 novel coronavirus, or SARS-CoV-2, was identified as the causative agent and subsequently isolated and sequenced.1 Since that time, SARS-CoV-2 has spread worldwide, causing a severe illness known as COVID-19 (coronavirus disease 2019), which led the World Health Organization (WHO) to declare it a pandemic on March 11, 2020.2 Since the beginning of the outbreak, clinical laboratories have been developing various assays to aid in detecting SARS-CoV-2 and clinically managing patients with COVID-19.
The 3 categories of tests used to detect current or past viral infection are molecular, serologic, and antigen-detection assays (Table 1). In this context, a molecular assay is used to determine whether a patient is actively infected with the pathogen of interest. Reverse transcription polymerase chain reaction (RT-PCR) is a common laboratory technique used to detect respiratory viral pathogens, such as influenza and respiratory syncytial virus (RSV).3 Currently, this is the main type of test being utilized to determine whether patients are infected with SARS-CoV-2.
Table 1.
Clinical Laboratory Testing Types
| Test Type | Patient Specimen Type | Detection | Clinical Utility | Necessary Reagents | Development Time |
|---|---|---|---|---|---|
| Nucleic acid amplification (ie, RT-PCR, isothermal amplification) | NP swaba | RNA | Active infection | Oligo primers | Fastest: oligonucleotide production and molecular assay development (takes days to weeks) |
| Serology | Serum | IgM and/or IgG, or total antibodies | Past exposure; immune status | Recombinant/purified protein | Intermediate: production of viral protein (recombinant/purified) and assay development/optimization (takes 2 to several weeks) |
| Protein detection | NP swab and/or other clinical fluidsa | Viral antigen | Active infection | Antibody to viral protein(s) | Slowest: requires antibody production, assay development, and optimization (takes several weeks to months) |
Abbreviations: RT-PCR, reverse transcription polymerase chain reaction; NP, nasopharyngeal; Ig, immunoglobulin.
aNP swabs are difficult to access using this method.
RT-PCR is a sensitive technique for RNA detection, whereby RNA is reverse transcribed into complementary DNA (cDNA) and cDNA targets specific for the pathogen of interest are amplified. If SARS-CoV-2 RNA is present in a patient specimen, typically collected as a nasopharyngeal (NP) or anterior nasal swab,4 it will be detected by this assay. Depending on the platform, these assays can be completed in less than 1 hour to several hours, once the specimen arrives in the laboratory and is loaded onto the platform.
The caveats to interpreting results from this assay type are that doing so does not inform us whether a patient previously had the infection; rather, this type of assay only detects patients actively shedding virus (current infection or carriage state) or those who have residual viral RNA present. Therefore, these assays are most useful in acute settings to detect patients with COVID-19, where the results can inform appropriate isolation protocols and ensure that appropriate personal protective equipment (PPE) protocols are utilized when treating these patients. As of the date of publication of this article, of the 102 commercial laboratories and/or test kit manufacturers approved for emergency use by the United States Food and Drug Administration (FDA) for SARS-CoV-2 testing, 81 of them were molecular assays (Table 2). We note that many of these commercial assays require a laboratory to have vendor-specific instrumentation and equipment to utilize these test kits. The FDA also has authorized 37 molecular-based laboratory developed tests (LDTs) that can be used in the single laboratory that developed the test.
Table 2.
Current FDA Emergency Use Authorized SARS-CoV-2 Assays, as of May 26, 2020
| Molecular | ||
|---|---|---|
| Manufacturer | Test Name | Assay Type |
| 1drop Inc. | 1copy COVID-19 qPCR Multi Kit | RT-PCR |
| Abbott Diagnostics Scarborough, Inc. | ID NOW COVID-19 | Isothermal nucleic acid amplification |
| Abbott Molecular | Abbott RealTime SARS-CoV-2 assay | RT-PCR |
| Abbott Molecular Inc. | Alinity m SARS-CoV-2 assay | RT-PCR |
| Altona Diagnostics GmbH | RealStar SARS-CoV02 RT-PCR Kits U.S. | RT-PCR |
| Applied BioCode, Inc. | BioCode SARS-CoV-2 Assay | RT-PCR |
| Applied DNA Sciences, Inc. | Linea COVID-19 Assay Kit | RT-PCR |
| Assurance Scientific Laboratories | Assurance SARS-CoV-2 Panel | RT-PCR |
| Atila BioSystems, Inc. | iAMP COVID-19 Detection Kit | Isothermal amplification test |
| Avellino Lab USA, Inc. | AvellinoCoV2 test | RT-PCR |
| Becton, Dickinson & Company | BD SARS-CoV-2Reagents for BD MAX System | RT-PCR |
| Becton, Dickinson & Company (BD) | BioGX SARS-CoV-2 Reagents for BD MAX System | RT-PCR |
| BGI Genomics Co. Ltd | Real-Time Fluorescent RT-PCR Kit for Detecting SARS-2019-nCoV | RT-PCR |
| BioCore Co., Ltd. | BioCore 2019-nCoV Real Time PCR Kit | RT-PCR |
| Bio-Rad Laboratories, Inc | Bio-Rad SARS-CoV-2 ddPCR Test | RT-PCR |
| BioFire Defense, LLC | BioFire COVID-19 Test | RT-PCR |
| BioFire Defense, LLC | BioFire Respiratory Panel 2.1 (RP2.1) *panel includes 20 other viral or bacterial pathogens | RT-PCR |
| BioMérieux SA | SARS-COV-2 R-GENE | RT-PCR |
| Centers for Disease Control and Prevention’s (CDC) | CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel (CDC) | RT-PCR |
| Cepheid | Xpert Xpress SARS-CoV-2 test | RT-PCR |
| ChromaCode Inc. | HDPCR SARS-CoV-2 Assay | RT-PCR |
| Co-Diagnostics, Inc. | Logix Smart Coronavirus Disease 2019 (COVID-19) Kit | RT-PCR |
| Cue Health Inc. | Cue COVID-19 Test | Isothermal nucleic acid amplification |
| dba SpectronRx | Hymon SARS-CoV-2 Test Kit | RT-PCR |
| DiaCarta, Inc | QuantiVirus SARS-CoV-2 Test kit | RT-PCR |
| DiaSorin Molecular LLC | Simplexa COVID-19 Direct assay | RT-PCR |
| Everlywell, Inc. | Everlywell COVID-19 Test Home Collection Kit | RT-PCR |
| Euroimmun US Inc. | EURORealTime SARS-CoV-2 | RT-PCR |
| Fast Track Diagnostics Luxembourg S.á.r.l. | FTD SARS-CoV-2 | RT-PCR |
| Fosun Pharma USA Inc. | Fosun COVID-19 RT-PCR Detection Kit | RT-PCR |
| Fulgent Therapeutics, LLC | Fulgent COVID-19 by RT-PCR Test | RT-PCR |
| GeneMatrix, Inc. | NeoPlex COVID-19 Detection Kit | RT-PCR |
| Genetron Health (Beijing) Co., Ltd. | Genetron SARS-CoV-2 RNA Test | RT-PCR |
| GenMark Diagnostics, Inc. | ePlex SARS-CoV-2 Test | PCR, electrochemical detection (voltammetry) |
| GenoSensor, LLC | GS™ COVID-19 RT-PCR KIT | RT-PCR |
| Gnomegen LLC | Gnomegen COVID-19 RT-Digital PCR Detection Kit | RT-PCR |
| Gnomegen LLC | Gnomegen COVID-19-RT-qPCR Detection Kit | RT-PCR |
| Gravity Diagnostics, LLC | Gravity Diagnostics COVID-19 Assay | RT-PCR |
| Hologic, Inc. | Aptima SARS-CoV-2 assay | Target capture, transcription mediated amplification and dual kinetic assay |
| Hologic, Inc. | Panther Fusion SARS-CoV-2 | RT-PCR |
| Illumina, Inc. | Illumina COVIDSeq Test | Next-Generation Sequencing (NGS) |
| InBios International, Inc | Smart Detect SARS-CoV-2 rRT-PCR Kit | RT-PCR |
| Ipsum Diagnostics, LLC | COV-19 IDx assay | RT-PCR |
| Kaiser Permanente Mid-Atlantic States | KPMAS COVID-19 Test | RT-PCR |
| KorvaLabs Inc. | Curative-Korva SARS-Cov-2 Assay | RT-PCR |
| LabGenomics Co., Ltd. | LabGun COVID-19 RT-PCR Kit | RT-PCR |
| Laboratory Corporation of America (LabCorp) | COVID-19 RT-PCR Test | RT-PCR |
| Luminex Corporation | ARIES SARS-CoV-2 Assay | RT-PCR |
| Luminex Molecular Diagnostics, Inc. | NxTAG CoV Extended Panel Assay | RT-PCR |
| Maccura Biotechnology (USA) LLC | SARS-CoV-2 Fluorescent PCR Kit | RT-PCR |
| Mesa Biotech Inc. | Accula SARS-Cov-2 Test | PCR and lateral flow assay |
| NeuMoDx Molecular, Inc. | NeuMoDx SARS-CoV-2 Assay | RT-PCR |
| OPTI Medical Systems, Inc. | OPTI SARS-CoV-2 RT PCR Test | RT-PCR |
| OSANG Healthcare | GeneFinder COVID-19 Plus RealAmp Kit | RT-PCR |
| P23 Labs, LLC. | P23 Labs TaqPath SARS-CoV-2 Assay | RT-PCR |
| PerkinElmer, Inc. | PerkinElmer New Coronavirus Nucleic Acid Detection Kit | RT-PCR |
| Phosphorus Diagnostics LLC | Phosphorus COVID-19 RT-qPCR Test | RT-PCR |
| Primerdesign Ltd. | Primerdesign Ltd COVID-19 genesig Real-Time PCR assay | RT-PCR |
| PrivaPath Diagnostics, Inc. | LetsGetChecked Coronavirus (COVID-19) Test | RT-PCR |
| QIAGEN GmbH | QIAstat-Dx Respiratory SARS-CoV-2 Panel *panel includes 22 other viral or bacterial pathogens | RT-PCR |
| Quest Diagnostics Infectious Disease, Inc. | Quest SARS-CoV-2 rRT-PCR | RT-PCR |
| Quidel Corporation | Lyra SARS-CoV-2 Assay | RT-PCR |
| Quidel Corporation | Lyra Direct SARS-CoV-2 Assay | RT-PCR |
| Rheonix, Inc. | Rheonix COVID-19 MDx Assay | RT-PCR |
| Roche Molecular Systems, Inc. (RMS) | cobas SARS-CoV-2 | RT-PCR |
| RTA Laboratories Biological Products Pharmaceutical and Machinery Industry | Diagnovital SARS-CoV-2 Real-Time PCR Kit | RT-PCR |
| Rutgers Clinical Genomics Laboratory at RUCDR Infinite Biologics - Rutgers University | Rutgers Clinical Genomics Laboratory TaqPath SARS-CoV-2-Assay | RT-PCR |
| Sansure BioTech Inc. | Novel Coronavirus (2019-nCoV) Nucleic Acid Diagnostic Kit (PCR-Fluorescence Probing) | RT-PCR |
| ScienCell Research Laboratories | ScienCell SARS-CoV-2 Coronavirus Real-time RT-PCR (RT-qPCR) Detection Kit | RT-PCR |
| SD Biosensor, Inc. | STANDARD M nCoV Real-Time Detection Kit | RT-PCR |
| Seasun Biomaterials | U-TOP COVID-19 Detection Kit | RT-PCR |
| Seasun Biomaterials, Inc. | AQ-TOP COVID-19 Rapid Detection Kit | RT-LAMP |
| Seegene, Inc. | Allplex 2019-nCoV Assay | RT-PCR |
| Sherlock BioSciences, Inc. | Sherlock CRISPR SARS-CoV-2 Kit | CRISPR |
| SolGent Co., Ltd. | DiaPlexQ Novel Coronavirus (2019-nCoV) Detection Kit | RT-PCR |
| TBG Biotechnology Corp. | ExProbe SARS-CoV-2 Testing Kit | RT-PCR |
| Thermo Fisher Scientific, Inc. | TaqPath COVID-19 Combo Kit | RT-PCR |
| Tide Laboratories, LLC | DTPM COVID-19 RT-PCR Test | RT-PCR |
| Trax Management Services Inc. | PhoenixDx 2019-CoV | RT-PCR |
| Wadsworth Center, New York State Department of Public Health’s (CDC) | New York SARS-CoV-2 Real-time Reverse Transcriptase (RT)-PCR Diagnostic Panel | RT-PCR |
| Zymo Research Corporation | Quick SARS-CoV-2rRT-PCR Kit | RT-PCR |
| Serology | ||
| Manufacturer | Test Name | Assay Type |
| Abbott Laboratories Inc. | SARS-CoV-2 IgG assay | Chemiluminescent microparticle immunoassay |
| Autobio Diagnostics Co. Ltd. | Anti-SARS-CoV-2 Rapid Test IgM and IgG | Lateral flow immunoassay |
| Bio-Rad Laboratories, Inc | Platelia SARS-CoV-2 Total Ab assay | Enzyme-Linked Immunosorbent Assays (ELISA) |
| Cellex Inc. | qSARS-CoV-2 IgG/IgM Rapid Test | Lateral flow immunoassay |
| Chembio Diagnostic System, Inc. | DPP COVID-19 IgM/IgG System | Immunochromatography |
| DiaSorin Inc. | LIAISON SARS-CoV-2 S1/S2 IgG | Chemiluminescent immunoassay |
| Emory Medical Laboratories | SARS-CoV-2 RBD IgG test | Enzyme-Linked Immunosorbent Assays (ELISA) |
| EUROIMMUN US Inc. | Anti-SARS-CoV-2 ELISA (IgG) | Enzyme-Linked Immunosorbent Assays (ELISA) |
| Hangzhou Biotest Biotech Co., Ltd. | RightSign COVID-19 IgG/IgM Rapid Test Cassette | Lateral flow chromatographic immunoassay |
| Healgen Scientific LLC | COVID-19 IgG/IgM Rapid Test Cassette | Lateral flow immunoassay |
| InBios International, Inc. | SCoV-2 Detect IgG ELISA | ELISA |
| Mount Sinai Laboratory | COVID-19 ELISA IgG Antibody Test | Enzyme-Linked Immunosorbent Assays (ELISA) |
| Ortho Clinical Diagnostics, Inc. | VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total Reagent Pack | Immunometric luminescence |
| Ortho-Clinical Diagnostics, Inc. | VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgG Reagent Pack | Immunometric luminescence |
| Roche Diagnostics | Elecsys Anti-SARS-CoV-2 | Electrochemiluminescence Immunoassay |
| Siemens Healthcare Diagnostics Inc. | Dimension Vista SARS-CoV-2 Total antibody assay (COV2T) | Chemiluminescent immunoassay |
| Dimension EXL SARS-CoV-2 Total antibody assay (CV2T) | Chemiluminescent immunoassay | |
| Atellica IM SARS-CoV-2 Total (COV2T) | Chemiluminescent immunoassay | |
| ADVIA Centaur SARS-CoV-2 Total (COV2T) | Chemiluminescent immunoassay | |
| Vibrant America Clinical Labs | Vibrant COVID-19 Ab Assay | Chemiluminescence immunoassay |
| Wadsworth Center, New York State Department of Health | New York SARS-CoV Microsphere Immunoassay for Antibody Detection | Microsphere Immunoassay |
| Antigen | ||
| Manufacturer | Test Name | Assay Type |
| Quidel Corporation | Sofia 2 SARS Antigen FIA | Lateral flow immunofluorescent sandwich assay |
Abbreviations: FDA, United States Food and Drug Administration; qPCR, quantitative polymerase chain reaction; RT-PCR, reverse transcriptase polymerase chain reaction; ddPCR, droplet digital polymerase chain reaction; CDC, Centers for Disease Control and Prevention; PCR, polymerase chain reaction; Ig, immunoglobulin; ELISA, enzyme-linked immunosorbent assay; FIA, fluorescence immunoassay analyzer.
aAs of May 26, 2020. For the most up-to-date list, please refer to: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations. We also note that based on the FDA policy for Diagnostic Tests for Coronavirus Disease–2019 during the Public Health Emergency issued on March 16, 2020, commercial manufacturers can develop and distribute serology tests without an emergency-use authorization (EUA), as long as the test has been validated and the FDA is notified.
bPanel includes 20 other viral or bacterial pathogens.
Given that true NP specimens are often difficult to obtain, the FDA has stated that oropharyngeal (OP), nasal midturbinate, and anterior nares swabs are acceptable when using an NP swab is not possible.4 Also, the FDA recently granted an Emergency-Use Authorization (EUA) to Rutgers Clinical Genomics Laboratory-Rutgers University for an RT-PCR LDT for qualitative detection of SARS-CoV-2 in saliva specimens as well as OP, NP, anterior nasal, and midturbinate nasal swabs. Saliva testing presents potential benefits of eliminating the need for swabs and decreasing the risk posed to the health-care workers collecting these specimens. However, this specimen type might require additional dilution or pretreatment due to its viscosity. Also, viral RNA might be more difficult to detect in this specimen type, although the results of a previous study5 found that saliva and NP specimens were comparable for detection of respiratory viruses by RT-PCR.
The possibility of false negative results with molecular assays should also be considered. One report6 documents a patient with multiple RT-PCR NP/OP specimens that tested negative; this patient ultimately had SARS-CoV-2 detected in a bronchoalveolar lavage (BAL) fluid specimen. For lower respiratory tract specimens, currently, the FDA recommends testing BAL fluid only under certain clinical circumstances such as invasive mechanical ventilation. Sputum should be tested from patients who develop a productive cough, although the FDA does not recommend induction of sputum for SARS-CoV-2 testing.4
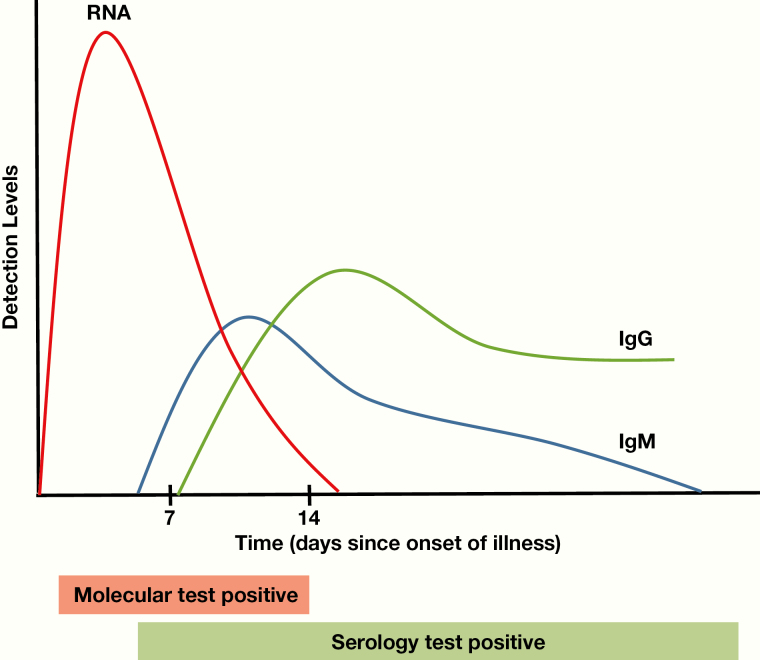
The other main type of assay is serological. These assays determine the exposure history and/or immune status of a patient. They detect the presence of antibodies against SARS-CoV-2 antigens in serum, plasma, or whole blood specimens. After initial viral infection, there is a delay before the production of antibodies by the immune system (Figure 1). During this time, known as the window period, a patient who is infected with SARS-CoV-2 but has no detectable antibodies, would have negative test results on a serologic assay. Typically, when the immune system mounts a response against a virus, short-lived immunoglobulin (Ig)M antibodies are initially produced, followed by a more durable IgG antibody response. However, there are limited data thus far in the literature regarding the longevity of anti–SARS-CoV-2 antibodies.
Figure 1.
SARS-CoV-2 estimated seroconversion rates.
The graph in Figure 1 demonstrates estimated viral RNA, IgM, and IgG detection levels for SARS-CoV-2 based on the limited published literature to date. The estimated median seroconversion time is 7 to 12 days; most patients with COVID-19 have detectable antibodies approximately 15 days after the onset of symptoms. Due to the subjectivity in determining symptom onset, these dates can be highly variable. Also, viral RNA has been shown to peak in the first week of illness and then gradually decline.
Serological tests can be unique to one class of immunoglobulins; can detect IgM and IgG antibodies simultaneously; or can be total antibody tests, which detect IgA antibodies as well. Depending on the exact protocol and platform, such assays can typically be completed in 1 to 2 hours once a specimen arrives in the laboratory and is loaded onto the relevant platform. We note, however, that at the time of publication of this article, there are a few commercial assays available on large, automated analyzers, from diagnostic manufacturers including Roche Diagnostics, Abbott Diagnostics, and Ortho Clinical Diagnostics (with offerings more recently available from Beckman Coulter, Inc and Siemens AG). However, to our knowledge, there are no objective, peer-reviewed data on their performance characteristics.
Many available commercial serological assays use a lateral flow assay format; for many of these assays, there are unsubstantiated, or even false, claims about test performance.7The estimated median seroconversion time is 7 to 12 days, with virtually all patients with COVID-19 having detectable antibodies approximately 15 days after onset of symptoms.8–10 However, the results of a recent acute antibody response study11 demonstrated simultaneous or sequential IgM and IgG seroconversion, with a slight decrease in IgM antibody titers 3 weeks after symptom onset. Therefore, these assays will be most helpful in determining the exposure status of an individual and in assessing the immune response of that person to SARS-CoV-2.
Because SARS-CoV-2 is a new virus, it is not clear whether an immune response confers immunity and how long the immune response will last—that is, whether it is durable and sustained (years) or if it is short-lived (1–2 months). These assays can be particularly helpful for individuals who may have had symptoms consistent with COVID-19 but who were never tested with an RT-PCR test (due to the severe limitations in testing capabilities in many areas) and now have recovered from their illness. Currently, the Centers for Disease Control and Prevention (CDC) does not recommend using serology testing to determine eligibility to return to the in-person workforce. Individuals who were symptomatic can stop self-isolation as long as their symptoms have improved with 3 days of no fever and at least 10 days have elapsed since the onset of their symptoms.12
Finally, these tests can also be used in serosurveillance, in which data are obtained to calculate the prevalence of anti–SARS-CoV-2 antibodies in the community. Such information can help epidemiologists better understand the true burden of disease and to model continued viral-transmission dynamics based on the percentage of the population that is immune vs susceptible. Given that approximately 80% of COVID-19 cases are mild to moderate in severity10,13 and that molecular testing has been restricted to the most severely ill patients, the true number of cases has likely not been revealed by molecular-based assays. Thus, serological testing can provide a more accurate enumeration of the number of past infections. As with all laboratory testing, however, the results of these assays will only be accurate and maximally useful based on their performance characteristics, including sensitivity and specificity.
COVID-19 testing is also important for identifying potential patients who have recovered from COVID-19 and have detectable antibodies against SARS-CoV-2 (convalescent donors) for clinical trials. Currently, studies are underway in which convalescent donors can donate plasma, which can then be transfused into critically ill patients with COVID-19. A review of the published literature to date14 indicates that treatment with plasma from convalescent donors demonstrates beneficial effects, although further evaluation with clinical trials remains imperative. Potential donors require a serologic assay to detect the presence of anti–SARS-CoV-2 antibodies in their plasma. For this type of plasma to be beneficial, the antibodies present should have neutralizing activity (ie, the antibodies bind to and neutralize infection by active SARS-CoV-2 virus). Such testing is not currently performed in clinical laboratories but rather in research laboratories. Ideally, convalescent plasma donors would be noninfectious (symptom-free for >14 days) and have high titers of virus-neutralizing antibodies (as determined by serologic testing).
Point-of-care (POC) testing is beginning to be available for SARS-CoV-2. POC testing refers to a broad category of diagnostic tests that can be performed where patient care occurs. Functionally, these tests have a rapid turnaround time (TAT) and can potentially be performed by various nonlaboratory clinical personnel. These assays can be molecular or serologic. One molecular POC test by Abbott Diagnostics uses isothermal nucleic acid amplification (a technique similar to polymerase chain reaction [PCR]) to detect SARS-CoV-2 in approximately 15 minutes. The results of recent studies15–17 have demonstrated low sensitivity for the Abbott ID Now assay with specimens collected in transport media. Thus, the EUA for this test was modified for testing only from direct/dry swabs.
The Cellex serologic POC qSARS-CoV-2 IgG/IgM Rapid Test (Cellex, Inc.) utilizes a lateral flow immunoassay, which qualitatively detects IgM and/or IgG antibodies from whole-blood specimens. A blood specimen flows by capillary action along the cassette and, if anti–SARS-CoV-2 IgM or IgG antibodies are present, they will bind to recombinant SARS-CoV-2 antigens present on the test strip. The presence of these antibody-antigen complexes are then detected by a colorimetric change, which is revealed when the complexes are captured by anti–human IgG or anti–human IgM antibodies. The results are available in approximately 15 to 20 minutes. Of note, currently POC serology tests must be performed in conjunction with a CLIA (Clinical Laboratory Improvement Amendments)–licensed laboratory and cannot be performed in locations with only a CLIA waiver, such as a physician office.
Finally, there is now an antigen detection assay available from Quidel Corporation (Sofia 2 SARS Antigen FIA) that uses a lateral flow immunofluorescent sandwich assay technique for detection of the nucleocapsid protein antigen of SARS-CoV and SARS-CoV-2. In theory, viral proteins can be detected using one of a number of antigen capture methods (eg, antibodies, aptamers). Such tests are used routinely for other viral assays (eg, human immunodeficiency virus [HIV] p24 antigen as part of 4th- and 5th-generation HIV tests), and also for hepatitis B surface antigen.18 Like molecular assays, antigen detection tests can be used to detect active SARS-CoV-2 infection.
In summary, molecular and serological tests provide meaningful data for treating patients with COVID-19. However, each category has different clinical utility, different characteristics, and different limitations. Clinical laboratories continue to develop new assays and implement increased testing capabilities to meet the high demands for patient testing during this pandemic.
Personal and Professional Conflicts of Interest
None reported.
Glossary
Abbreviations
- COVID-19
coronavirus disease 2019
- WHO
World Health Organization
- RT-PCR
reverse transcription polymerase chain reaction
- RSV
respiratory syncytial virus
- cDNA
complementary DNA
- NP
nasopharyngeal
- PPE
personal protective equipment
- FDA
United States Food and Drug Administration
- LDTs
laboratory-developed tests
- OP
oropharyngeal
- EUA
Emergency-Use Authorization
- BAL
bronchoalveolar lavage
- Ig
immunoglobulin
- CDC
Centers for Disease Control and Prevention
- POC
point-of-care
- TAT
turnaround time
- PCR
polymerase chain reaction
- CLIA
Clinical Laboratory Improvement Amendments
- HIV
human immunodeficiency virus
- qPCR
quantitative polymerase chain reaction
- ddPCR
droplet digital polymerase chain reaction
- ELISA
enzyme-linked immunosorbent assay
- FIA
fluorescence immunoassay analyzer
References
- 1. Severe acute respiratory syndrome coronavirus 2 isolate Wuhan-Hu-1, complete genome, in NCBI Reference Sequence: NC_045512.2. Available from: https://www.ncbi.nlm.nih.gov/nuccore/1798174254. Accessed June 4, 2020.
- 2. World Health Organization Coronavirus disease (COVID-19) Pandemic. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed June 4, 2020.
- 3. Mayer LM, Kahlert C, Rassouli F, Vernazza P, Albrich WC. Impact of viral multiplex real-time PCR on management of respiratory tract infection: a retrospective cohort study. Pneumonia (Nathan). 2017;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19. Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Accessed June 4, 2020.
- 5. Kim Y-G, Yun SG, Kim MY, et al. Comparison between saliva and nasopharyngeal swab specimens for detection of respiratory viruses by multiplex reverse transcription–PCR. J Clin Microbiol. 2017;55(1):226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Winichakoon R, Chaiwarith R, Liwsrisakun C, et al. Negative nasopharyngeal and oropharyngeal swab does not rule out COVID-19 [epub ahead of print]. J Clin Microbiol 2020;58(5):e00297-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration (FDA). Beware of Fraudulent Coronavirus Tests, Vaccines and Treatments Available from https://www.fda.gov/consumers/consumer-updates/beware-fraudulent-coronavirus-tests-vaccines-and-treatments. Accessed June 4, 2020.
- 8. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis 2020;ciaa344:doi.org/10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020;20(5):P565–P574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rokni M, Ghasemi V, Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: Comparison with SARS and MERS. Rev Med Virol. 2020;30(3):e2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Long Q-X, Liu B-Z, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19 [epub ahead of print]. Nat Med. 2020;doi.org/10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention Return to Work Criteria for HCP with Suspected or Confirmed COVID-19 Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/return-to-work.html. Accessed June 4, 2020.
- 13. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A.. Convalescent plasma transfusion for the treatment of COVID-19: systematic review [epub ahead of print]. J Clin Virol. 2020; doi.org/10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smithgall MC, Scherberkova I, Whittier S, Green DA. Comparison of Cepheid Xpert Xpress and Abbott ID Now to Roche cobas for the rapid detection of SARS-CoV-2. J Clin Virol. 2020;128:104428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harrington A, Cox B, Snowdon J, et al. Comparison of Abbott ID Now and Abbott m2000 methods for the detection of SARS-CoV-2 from nasopharyngeal and nasal swabs from symptomatic patients [epub ahead of print]. J Clin Microbiol. 2020;doi:10.1128/JCM.00798-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitchell SL, George KS. Evaluation of the COVID19 ID NOW EUA assay. J Clin Virol. 2020;128:104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gray ER, Bain R, Varsaneux O, Peeling RW, Stevens MM, McKendry RA. p24 revisited: a landscape review of antigen detection for early HIV diagnosis. AIDS. 2018;32(15):2089–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]