Abstract
Worldwide, there is an array of clinical trials under way to evaluate treatment options against coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2. Concurrently, several nutritional therapies and alternative supportive treatments are also being used and tested to reduce the mortality associated with acute respiratory distress in patients with COVID-19. In the context of COVID-19, improved nutrition that includes micronutrient supplementation to augment the immune system has been recognized as a viable approach to both prevent and alleviate the severity of the infection. The potential role of micronutrients as immune-boosting agents is particularly relevant for low- and middle-income countries, which already have an existing high burden of undernutrition and micronutrient deficiencies. A systematic literature review was performed to identify nutritional interventions that might prevent or aid in the recovery from COVID-19. The PubMed, ScienceDirect, Cochrane, Scopus, Web of Science, and Google Scholar databases were searched electronically from February to April 2020. All abstracts and full-text articles were examined for their relevance to this review. The information gathered was collated under various categories. Deficiencies of micronutrients, especially vitamins A, B complex, C, and D, zinc, iron, and selenium, are common among vulnerable populations in general and among COVID-19 patients in particular and could plausibly increase the risk of mortality. Judicious use of need-based micronutrient supplementation, alongside existing micronutrient fortification programs, is warranted in the current global pandemic, especially in low- and middle-income economies.
Keywords: COVID-19, immune system, influenza, micronutrients, nutrition, SARS-CoV-2
INTRODUCTION
The recent coronavirus disease 2019 (COVID-19) outbreak, caused by the severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2), originated in China before becoming a global pandemic. As of June 23, 2020, more than 8,99 million cases of COVID-19 and more than 469 587 COVID-19–associated deaths were reported worldwide. The rate of new infections seemed to outpace both the scale of preparedness and the public health response, especially in resource-constrained economies.1 The COVID-19 containment strategy implemented by countries like South Korea, Taiwan, and Singapore offers an example for the rest of the world2,3 but may be challenging for many countries.
The possible emergence of new strains of SARS viruses that cause flu-like disease, along with the measures needed to prevent the spread of such strains, has been reported in a number of retrospective studies.4,5 Despite these alerts, the world was unprepared for the COVID-19 pandemic. While current research is focused on the development of a vaccine and effective therapeutic agents, many scientists have also emphasized the importance of boosting the immune system through various nutritional interventions.6 Given the potential usefulness of Bacillus Calmette-Guérin (BCG) vaccine and the worldwide clinical trials initiated to counter the COVID-19 pandemic, BCG vaccination has been suggested as a strategy to trigger immunity in patients with COVID-19.7,8 Moreover, the use of chloroquine and hydroxychloroquine as chemoprophylaxis in COVID-19 has been extensively debated in the literature, although evidence is insufficient to recommend the routine use of any drug. Agrawal et al9 have comprehensively summarized the various ongoing clinical trials for chemoprophylaxis in COVID-19, eg, NCT04304053, NCT04318444, NCT04251871, NCT04303507, NCT04321174, NCT04312243, and NCT04308668. Yao et al,10 in an in vitro study, have validated antiviral activity elicited by these drugs against SARS-CoV-2. Similarly, Singh et al11 proposed chloroquine and hydroxychloroquine to be potentially beneficial in the context of low-income and lower-middle-income countries, especially in the absence of any effective treatment option against COVID-19.
In their recent review, Zhang and Liu12 examined a variety of treatment options for novel coronavirus infection, placing increased focus on the role of micronutrients as supportive and complementary components of treatment regimens. In a very recent report, Grant et al13 recommended vitamin D3 intake at 10 000 IU/d for a few weeks, followed by 5000 IU/d until the serum 25(OH)D concentration reaches 100 to 150 nmol/L, as a preventive strategy against COVID-19 among people at risk. There is existing evidence for the potential role of improved nutrition to augment the immune system. Vitamins A, B complex, C, D, and E and many trace elements, such as iron, zinc, selenium, magnesium, and copper, have been shown to elicit immune-boosting properties,14–16 and thus deficiencies of these micronutrients could be detrimental to immune function in viral infections.17 Likewise, supplementing diets with micronutrients has been reported as a way to improve or optimize immune function against viral infections; therefore, public health officials must consider nutritional interventions as a means to combat emerging viral infections.6,16
The scientific community, the various governments worldwide, and the global pharmaceutical industry, along with social and healthcare foundations and nongovernmental organizations, have endeavored to find a possible solution for COVID-19, whether a medication or a safe vaccine, yet thus far their efforts have not borne fruit. The aim of this review is to highlight the potential role of nutrition in preventing and reducing the severity of COVID-19, with a focus on the role of various micronutrients.
NUTRITION AND IMMUNE DEFENSE
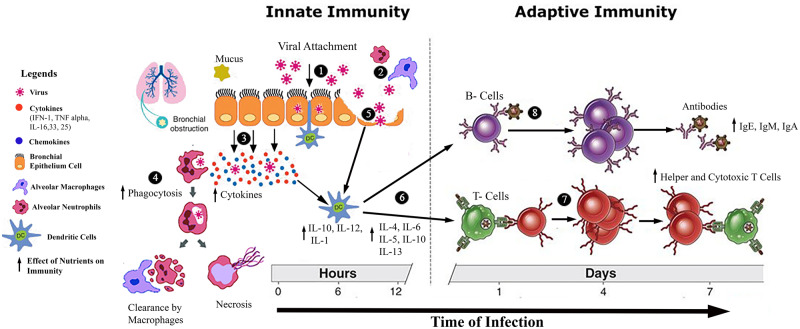
Micronutrients are dietary components that may contribute substantially to a robust immune system.18 Essential micronutrients like vitamins A, D, E, C, B6, B12, and folate and trace elements such as iron, zinc, and selenium, available in a variety of fresh animal- and plant-based foods, aid the body’s ability to fight infections.19,20 Health and survival are increasingly dependent on the functioning of the immune system. Mechanistically, a rapid innate immune response occurs through phagocytes when a pathogen assaults the living system, but an adaptive immune response more specifically identifies the invading pathogen. Basically, these immune responses are controlled and coordinated by T cells, which recognize the antigens and are classified as cytotoxic T cells. Cytotoxic T cells kill infected, damaged cells and the T helper cells Th1 and Th2. These cells are involved in antiviral and cellular immune responses (Figure 1) as well as humoral and antiparasitic responses.21 A strong immune system ensures host defense against pathogens and neoplastic cells, and balanced nutrition augments the immune system to provide optimal defense against infectious agents. Childs et al18 explained the critical role of the immune system as well as the defense mechanisms involved in protecting the body from invading agents, particularly in the presence of appropriate nutrition. Thevarajan et al22 reported their findings on the kinetics of the immune response to COVID-19, describing higher concentrations of follicular helper T cells, antibody-secreting cells, activated CD4+ and CD8+ T cells, and immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies, all of which were observed to bind to coronavirus SARS-CoV-2. The results thus validate the role of a strong immune defense in patients COVID-19.
Figure 1.
Effect of nutrients on the mechanisms of innate and adaptive immunity. (1) Virus attaches to bronchial epithelial cell layer. (2) Alveolar neutrophils and macrophages attack the virus. (3) Infected bronchial epithelial cells release cytokines (IFN-1, TNF-α, IL-16, IL-33, IL-25) to alert the neighboring cells and activate the innate immune cells, ie, neutrophils, natural killer cells, dendritic cells, and macrophages. (4) Alveolar neutrophils and macrophages engulf the virus for phagocytosis. (5) After multiple viral replications, epithelial cells burst and release new daughter viral copies, which results in activation of the dendritic cells. (6) Dendritic cells activate T-lymphocyte helper cells and B-lymphocyte cells. (7) T-helper 12 cells activate the cytotoxic T-lymphocyte cells. (8) B lymphocytes divide into memory and plasma cells, and plasma cells produce antibodies against the virus. Abbreviations: IFN, interferon; Ig, immunoglobulin; IL, interleukin; TNF, tumor necrosis factor.
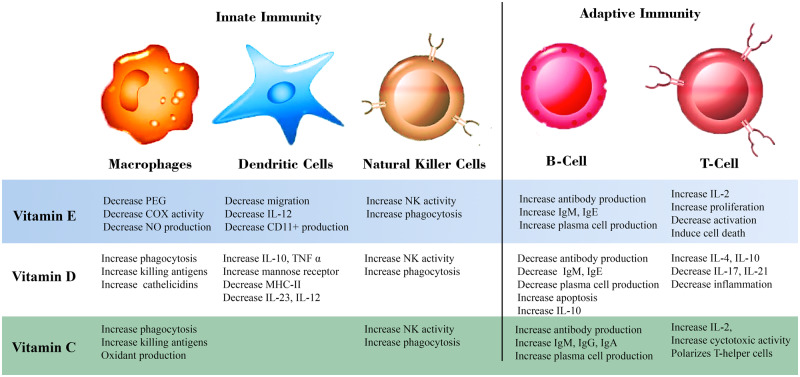
An optimally functioning immune system is closely linked to an adequate supply of micronutrients to the body, while severe deficiencies of these micronutrients lead to weakened immune responses and vulnerability to infections. Vitamins A, C, E, and B complex, along with folic acid, zinc, selenium, iron, and copper, all play important roles in boosting the immune system of the population.16,23 Several studies have confirmed that micronutrient deficiencies are associated with a weakened immune system that predisposes individuals to increased vulnerability to infections.24,25 Gombart et al16 demonstrated the critical role of essential vitamins and trace elements in boosting the immune system. They emphasized that micronutrients such as vitamins A, B6, B12, C, D, and E, (Figure 2) in addition to iron, selenium, and zinc (Table 1),26–36 might work synergistically to help immune cells function appropriately. Recent research also supports a role of certain minerals and vitamins as adjunct therapeutic agents to treat microbiological infections as well as immunological and nonimmunological chronic diseases (Table 2).37–45 More recently, Calder et al6 reviewed the association between optimal nutrition and the immune system in providing better protection against viral infections. They suggested that essential micronutrients and the omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic have the capacity to boost immunity against viral infections. Similarly, Chaturvedi et al46 described the complex relationship between trace elements and viral infections, highlighting the immunomodulatory properties and antiviral activities of certain micronutrients such as iron, zinc, selenium, and copper. Apart from functioning as antioxidants, these trace elements were shown to inhibit viral replication in host cells.
Figure 2.
Role of vitamins E, D, and C in innate and adaptive immunity. Abbreviations: COX, cyclooxygenase; Ig, immunoglobulin; IL, interleukin; MHC, macrophage histocompatibility complex; NK, natural killer; NO, nitric oxide; PEG, polyethylene glycol; TNF, tumor necrosis factor.
Table 1.
Role of selected minerals in innate and adaptive immunity
| Mineral | Role in innate immunity | Role in adaptive immunity | References |
|---|---|---|---|
| Folate |
|
|
Haryanto et al (2015)27; McClung & Peterson (2010)28; Saeed et al (2016)29 |
| Iron |
|
Increases T-cell proliferationImproves cytotoxic T-cell function | Haryanto et al (2015)27; Weiss (2002)30; Xu et al (2016)31 |
| Zinc |
|
|
Saeed et al (2016)29; Wintergerst et al (2006)32; Maares & Haase (2016)33 |
| Copper |
|
|
McClung & Peterson (2010)28; Maggini et al (2007)34 |
| Selenium |
|
|
Diwakar et al (2016)26; Haryanto et al (2015)27; Avery & Hoffmann (2018)35; Ivory et al (2017)36 |
Table 2.
Vitamin and mineral adjunctive therapies in viral, bacterial, and oncologic diseases
| Disease | Study design | Study year | Therapy | Findings | Reference |
|---|---|---|---|---|---|
| Glioma/glioblastoma | Phase 2 interventional (clinical trial) | 2023 | Radiation, temozolomide, vitamin C | Ongoing; ending in 2023 | Clinical trial NCT0234435537 |
| COVID-19 | Randomized, open-label phase 4 trial | 2020 | Hydroxychloroquine, azithromycin, zinc sulfate, doxycycline | Ongoing; ending in 2020 | Clinical trial NCT0437078238 |
| Leukemia | Phase 2 clinical trial | 2020 | Azacitidine, vitamin C | Ongoing; ending in 2020 | Clinical trial NCT0339717339 |
| Sepsis | – | 2019 | Vitamin D, standard antibiotic therapy | Vitamin D supplementation improved sepsis score and decreased levels of high-sensitivity C-reactive protein | Hagag et al (2019)40 |
| Severe pneumonia | Retrospective cohort study | 2018 | Vitamin C, hydrocortisone, thiamine | Compared with control group, adjunctive therapy group had lower hospital mortality and higher rate of recovery from severe pneumonia | Kim et al (2018)41 |
| Atopic dermatitis | Randomized, double-blind, placebo-controlled clinical trial | 2018 | Vitamin D3 (5000 IU/d), baseline therapy (topical steroid, soap substitute, and emollient) | Treatment group had reduced atopic dermatitis severity score | Sánchez-Armendáriz et al (2018)42 |
| Hepatitis C | Controlled randomized clinical trial | 2017 | Vitamin C (orange juice), pegylated interferon combined with ribavirin | Levels of liver enzyme aspartate aminotransferase decreased in patients who had high levels before the study | Gonçalves et al (2017)43 |
| Ehrlich ascites carcinoma | Rodent study | 2017 | Selenium, cyclophosphamide | Selenium improved chemotherapeutic effect of cyclophosphamide | Bhattacharjee et al (2017)44 |
| Sensorineural hearing loss | Nonblinded, noncontrolled study | 2015 | Vitamin A (26 000 IU), vitamin C (200 mg), vitamin E (200 IU), selenium (50 μg), methylprednisolone (1 mg/kg), trimetazidine dihydrochloride (20 mg, 3 times daily) | Treatment with vitamins A, C, and E and selenium was effective in restoring function after sudden idiopathic sensorineural hearing loss | Kaya et al (2015)45 |
Sufficient literature suggests that vitamin and mineral supplementation in conjunction with a balanced and diversified diet can be used to meet the Recommended Dietary Allowance (RDA) for essential micronutrients. Further studies have proposed supplementation with vitamin C47,48 beyond the RDA, ie, above 0.2 g/d, as a strategy to reduce both upper and lower respiratory tract infections among older patients. In a recent report, Grant et al13 speculated that doses of vitamin D3 above 10 000 IU/d may be useful for treatment of patientswith COVID-19.
IMMUNE-BOOSTING ROLE OF VITAMINS
Vitamin A
Vitamin A is capable of defending the body against a variety of infections, primarily by regulating the proliferation and differentiation of immune cells. Supplementation with preformed vitamin A has been suggested to downregulate the secretion of proinflammatory cytokines like tumor necrosis factor α and interleukin 6 in response to infections.49,50 The anti-infective role of vitamin A has been described in a number of studies that suggest all-trans retinoic acid to function via the nuclear retinoid acid receptor.51,52 Retinoic acid has also been shown to exhibit immune-regulating properties, notably in humoral defense in viral infections.53
Vitamin A is essential for maintaining normal bodily functions, including defense against infections. An inadequate intake of vitamin A–rich food results in vitamin A deficiency, thus necessitating supplementation. In chickens infected with infectious bronchitis virus, a type of coronavirus, there was a marked difference in plasma retinol, retinol-binding protein, albumin, and transthyretin levels between those fed vitamin A–adequate diets and those fed marginally deficient diets.54,55 The protective role of vitamin A supplementation against a variety of infections such as malaria, lung infections, and HIV has been widely reported in the literature.49 In addition, the compromised efficacy of inactivated bovine coronavirus vaccine was attributed to vitamin A deficiency in infected calves.56 Evidence further suggests that vitamin A supplementation in children reduces deaths associated with diarrheal and respiratory diseases.57,58 Supplementation with preformed retinoids at a dose 100 times higher than the RDA, ie, up to 500 000 IU, may cause hypervitaminosis in adults, while an intake of more than 10 000 IU of preformed vitamin A in first 60 days after conception may cause congenital abnormalities in infants.59 In the current COVID-19 pandemic, researchers have suggested vitamin A supplementation as part of a tailored multivitamin solution to satisfy the RDA as a promising option to combat COVID-19 in noncritical patients.12,60
B vitamins
As cofactors to enzymes, B vitamins are central to the formation of, and the energy metabolism in, certain organic molecules. Multiple studies have suggested a significant role of B vitamins, eg, folic acid, B12, and B6, in the function of the immune system. These vitamins have the ability to operate as one-carbon donors in nucleotide synthesis, and one-carbon metabolism is involved primarily in complex biochemical pathways that are responsible for donating and regenerating one-carbon units. Natural killer cells and cytotoxic CD8+ lymphocytes are also influenced by these vitamins; hence, a balance of B12 and folate must be maintained for immune responses.61,62 Vitamin B6 in the form of pyridoxal phosphate is particularly important, playing an active role as a cofactor in at least 160 catalytic pathways, with some metabolites shown to exhibit immune-modulating properties.63,64
Scientific reports validate the significant role of vitamin B in normal function of the immune system, including the direct regulatory effects of vitamin B on immune response.65,66 Among its myriad immune-promoting features, vitamin B3 has been shown to inhibit neutrophil infiltration in the lungs, indicating an anti-inflammatory effect during ventilator-associated lung injury.67 Zhang and Liu12 reported that deficiency of B vitamins negatively influences the immune system and suggested that supplementation with B vitamins provides defense against viral infections. Deficiency of B vitamins has been shown to weaken the host immune response; hence, B vitamins may have a role in enhancing the immune system of COVID-19 patients.
Vitamin C
A plethora of scientific literature supports the role of vitamin C as an immune booster. Besides exerting antioxidant activity, supplementation with ascorbic acid significantly affects epigenetic regulation and cell signaling.19 The potential role of vitamin C as an antiviral agent against coronavirus has been observed in animal models by Atherton et al.68 Carr and Maggini14 outlined several immune-supporting features of vitamin C, including involvement in phagocytosis, antibody production, growth and functioning of immune cells, and transitioning of leukocytes at infection sites. There is also evidence for the role of vitamin C as a weak antihistamine agent to reduce symptoms of stuffy nose and swollen sinuses.69
An ameliorative role of vitamin C supplementation in upper respiratory tract infections has been well described in numerous studies.47,70,71 Vitamin C has shown potential to restore the damage caused by impaired phagocytosis and respiratory burst.14 It is also proven to reduce the duration and severity of the common cold in adults and children.47 High-dose intravenous vitamin C, administered at 50 to 200 mg/kg/d as treatment in patients with virus-induced acute respiratory distress syndrome on extracorporeal membrane oxygenation, was reported by Fowler et al.72 Previous studies have shown that vitamin C supplementation is associated with reduced incidence of pneumonia and lower respiratory tract infections and also offers protection against coronavirus infection by boosting the immune system.73,74 Zhang and Liu12 suggested supplementation with vitamin C to reduce the incidence of severe lower respiratory tract infections, such as pneumonia, and as a treatment option for COVID-19. The study by Hemilä73 considers the findings of 3 controlled trials in humans, wherein vitamin C supplementation at doses between 0.05 and 2 g/d were reported to result in significantly fewer cases of pneumonia. In general, no adverse effects of large doses of intravenously or orally administrated vitamin C have been documented, except in patients with glucose 6-phosphate deficiency, renal insufficiency, or renal failure.75,76
Vitamin D
Vitamin D receptors are present on monocytes, macrophages, T- and B-lymphocytes, and other immune cells. The 25(OH)D-1α-hydroxylase available on these cells converts 25-hydroxyvitamin D [25(OH)D] to its active form, 1,25-dihydroxyvitamin D.77 Vitamin D status is correlated with several autoimmune and inflammatory diseases. Evidence of this association could be demonstrated by the north-south gradient observed with respect to the prevalence of diabetes mellitus type I, inflammatory bowel disease, and multiple sclerosis, suggesting reduced intracutaneous synthesis of vitamin D at higher latitudes.78,79 Vitamin D has the ability to foster differentiation of monocytes to macrophages, which destroy invading agents. The formation of special antimicrobial proteins is regulated by certain vitamin D metabolites, and these antimicrobial proteins play a substantial role in combating infections, including lung infections, by destroying pathogens.80,81
Respiratory tract infections can severely exacerbate chronic diseases, leading to increased risk of death. Vitamin D can act through several mechanisms to decrease the risk of respiratory infections, including pneuomonia.13 These findings are supported by the findings of Zhou et al,82 whose meta-analysis suggests that vitamin D deficiency is associated with a heightened risk of pneumonia. A meta-analysis of individual participant data showed that vitamin D supplementation is safe and protective against acute respiratory tract infection and is most beneficial in patients with vitamin D deficiency.15 Caccialanza et al60 recently proposed supplementation with 25 000 IU or 50 000 IU of cholecalciferol per week to noncritical COVID-19 patients with serum 25-hydroxycholecalciferol levels of 20 to 30 ng/mL or ≤ 20 ng/mL, respectively. Likewise, Grant et al13 suggested administrating 10 000 IU of vitamin D3 per day to prevent infection among individuals at risk of influenza or COVID-19. Vitamin D3 supplementation also seems to decrease mortality in elderly people living independently or in institutional care.83 Recent data from countries like China have demonstrated a high number of COVID-19–infected individuals to have low serum 25(OH)D levels.12 Likewise, fortification of foods with vitamin D is lacking in these countries, likely leading to vitamin D deficiency.84,85 A correlation between increased incidence of COVID-19 and insufficient vitamin D concentrations in patients was further supported by Zhang and Liu,12 who confirmed that middle-aged to elderly people recently affected by COVID-19 in China had low vitamin D levels. However, Zisi et al86 has highlighted the need to validate whether vitamin D supplementation is beneficial for COVID-19 patients, as conflicting results were noted in some studies, while Grant et al13 suggested vitamin D supplementation as a therapeutic option for the treatment of COVID-19.
Vitamin E
As a potential antioxidant, vitamin E has the capacity to protect cells and their functional components from injury caused by the release of reactive oxygen species that occurs during immune reactions to invading pathogens in respiratory infections.87 Vitamin E is involved in multiple aspects of the immune response, including phagocytosis, the production of antibodies, and T-cell proliferation. The role of vitamin E supplementation in enhancing T-cell function is well documented in the literature.88 Vitamin E has a direct effect on T cells.89,90 Han et al91 confirmed that vitamin E exerts gene transcription–mediated immune-modulating effects. Vitamin E deficiency leads to a dampened immune response, though deficiency is rare in humans. Kim et al92 recently used disease-canceling technology to investigate the role of vitamin E in attenuating coronavirus-induced patterns of gene expression. The study reported vitamin E as a top hit compound, alongside ruxolitinib and glutamine, to induce gene expression signals counteracting disease-associated signals. Double-blind, large population studies suggest a few side effects of vitamin E oral supplementation at a dose of 3200 mg/d.93
TRACE MINERALS FOR A WELL-FUNCTIONING IMMUNE SYSTEM
Trace minerals are an essential component of the diet. Their regulatory effects on immune function have been well defined, and inadequate levels of trace elements have been reported to alter immune competence in humans.94,95 Prolonged dietary deficiencies of trace minerals may result in impaired immune function by influencing one or more components of the immune system.96 Although the specific functions of minerals in protecting or boosting human immunity are not well understood, several trace elements such as zinc, magnesium, iron, copper, selenium, and manganese have gained wide recognition for their roles in maintaining optimal health.
Zinc
Zinc, as a cofactor, is an integral component of more than 300 enzymes that exert secondary effects on the human immune system.97 Effects of zinc on the immune system are multifaceted. For example, in vivo studies on zinc deficiency have unveiled weaker immunological responses, as evidenced by reduced recruitment of neutrophils and decreased neutrophil chemotaxis, which might result in impaired function of natural killer cells, poor phagocytic activity by macrophages and neutrophils, and rapid production of reactive oxygen species, ie, oxidative burst.98 The impact of zinc on immune mediators like enzymes, cytokines, and thymic peptides has also been reported, suggesting that recommended dietary intake or supplementation of zinc is essential to prevent functional loss.99
Zinc deficiency in the elderly can lead to decreased or diminished T-cell response, reduced natural killer cell activity, and depressed thymic hormone levels, thus creating substantial risk for respiratory infections and their associated morbidity and mortality.99–102 Zinc supplementation has also been suggested to reduce the incidence of lower respiratory tract infections in zinc-deficient children,103,104 and zinc administration within 24 hours of the onset of symptoms reduces the duration of common cold symptoms.105 Zinc at doses of more than 75 mg/d has been suggested to have promising antiviral effects against common cold viruses, including influenza viruses, with significant reductions in the duration of the common cold reported.106 Mild adverse effects of zinc supplementation have been reported with dosages above 200 mg/d107,108; hence, relatively lower dosages may also reduce the severity of COVID-19. Increasing the intracellular levels of zinc with zinc ionophores can effectively impair the replication of viral RNA. Studies have illustrated the effectiveness of zinc combined with pyrithione at lower concentrations, ie, 2µM Zn2+ and 2µM pyrithione, in inhibiting SARS coronavirus.109 A recent report by Zhang and Liu12 provides support for the immune-promoting properties of zinc, suggesting that zinc supplementation can ameliorate COVID-19–induced diarrhea and respiratory symptoms, ie, cough, sore throat, and shortness of breath. The synergistic effect of oral zinc sulfate together with BCG vaccine has been well described in the literature, providing a promising immunotherapeutic approach in communities increasingly prone to infection with the SARS-CoV-2 virus.8
Iron
The substantial role of iron in the immune response has been widely documented in the literature, with iron deficiency shown to lead to impairment of the host immune system. Studies have suggested that iron levels in humans must be carefully controlled to limit the availability of iron to pathogens, since iron regulates the growth and activity of a wide range of microorganisms, including viruses. Even though some information on iron regulation in COVID-19 patients is currently available, Liu et al110 have suggested limiting the iron supply to COVID-19 patients in order to inhibit viral replication and reduce the risk and severity of infections. Iron overload in the host creates oxidative stress that increases the risk of virus mutation.111 Mullick et al112 considered the immune-modulating effect of iron and its deficiency to be a potential risk factor for the development of recurrent respiratory tract infections, a notion later confirmed by Jayaweera et al.113
Adequate iron intake influences the innate immune response of the host by mediating the nuclear factor κB (NF-κB) and interferon γ (IFN-γ) signaling pathways in macrophages. The metal enhances the host’s ability to resist intracellular pathogens.114 Iron deficiency leads to a T-cell–mediated immune response that may be associated with reduced activity of ribonucleotidyl reductase, which in turn regulates DNA synthesis. Iron-binding proteins like transferrin and lactoferrin have been shown to have a high affinity for metal ions. Thus, iron-binding proteins do not merely lower free iron in infections but may also exhibit bactericidal properties.115 A recent report by Wessling-Resnick116 suggested the aforementioned mechanism as an innate immune response in humans that controls iron metabolism by limiting the availability of iron in infections. Higher concentrations of free iron are reported in people with protein energy malnutrition. Such concentrations are potentially linked to lower levels of transferrin, suggesting prudent use of oral iron therapy in infected individuals with anemia.115 In contrast to the recommendation of Liu et al110 to restrict iron supply in COVID-19 patients, recent updates on the management of anemia in high-risk COVID-19 patients like pregnant women and cancer patients suggest iron replacement therapy as a more promising approach than transfusion to promote erythropoiesis.117,118 Worsening of anemia concurrent with COVID-19 in hospitalized premenopausal women not in the intensive care unit was reported to necessitate iron replacement therapy as a viable treatment approach to prevent transfusion-associated complications.117
Selenium
Selenium, present within selenoproteins in humans, influences cellular function by regulating redox-active proteins, antioxidant activity, and thyroid hormone metabolism.119 The majority of the 25-member family of selenoproteins function as enzymes to catalyze redox-based reactions. However, some selenoproteins do not exert enzymatic activity; for example, selenoprotein K plays an essential role in the activation and proliferation of immune cells.120 Selenium, as selenoproteins, supports efficient functioning of both the adaptive and the nonadaptive immune systems.121,122 Selenium deficiency is characterized by a reduced rate of mitogen-induced lymphocyte proliferation, while leukotriene B4 synthesis, essential in neutrophil chemotaxis, is also impaired. Several studies reported selenium-deficient study participants to have a weakened humoral immune response, as demonstrated by decreased IgG and IgM titers.26,119,121,122
Dietary selenium deficiency increases oxidative stress, which in turn increases the virulence of benign or mildly pathogenic viruses (eg, influenza viruses) by genetic mutation and impairs the immune response.123–125 Selenium also acts as an antioxidant for a group of enzymes that inhibit the production of free radicals and prevent oxidative damage to host cells.125 Ma et al126 reported an increased antibody response in chickens immunized with a live bivalent vaccine of Newcastle virus and infectious bronchitis virus administrated in conjunction with selenium and ginseng stem-leaf saponins (Se-GSLS), suggesting that Se-GSLS enhances both the proliferation of lymphocytes and the production of interleukin 4 and IFN-γ. Consistent with the above reports of the antiviral effects of selenium, a recent update on the correlation between selenium status, determined by measurement of selenium concentrations in hair, and the COVID-19 cure rate in a city population showed a significant association between poor selenium status and lower cure rates in COVID-19 patients.127 Nevertheless, more individual-level data are needed to establish an association between infection severity and selenium status. Selenium supplementation in selenium-deficient patients, particularly those who are elderly, may be an effective option for treating COVID-19 and preventing or reducing its severe outcomes.
OMEGA-3 POLYUNSATURATED FATTY ACIDS AND IMMUNITY
Polyunsaturated fatty acids (PUFAs), like selenoproteins, also exert major effects on both the innate and adaptive immune systems. In addition to their role as a constitutive part of the cell membrane, omega-3 PUFAs and their derivatives act as signaling molecules.128 Metabolites of omega-3 and omega-6 PUFAs are known as proresolving mediators, which are classified as prostaglandins, protectins, thromboxanes, resolvins, leukotrienes, and maresins. The synthesis of these metabolites is managed by a group of enzymes that include lipoxygenase, cyclooxygenase, and cytochrome P450.129,130 Omega-3 PUFAs, mainly α-linolenic acid, docosahexaenoic acid, and eicosapentaenoic acid, inhibit the activation of immune cells while also supporting specific immune functions like phagocytosis and neutrophil differentiation. This suggests that omega-3 PUFAs do not repress nonspecific immunity.128
Protectin D1, an omega-3 PUFA–derived lipid mediator, has been reported to markedly attenuate replication of influenza virus.131 Morita et al131 suggested that protectin D1 levels were inversely related to the pathogenicity of influenza viruses (eg, H5N1). Infected mice treated with protectin D1 plus peramivir were completely rescued from death due to influenza. In light of these findings on the role of PUFA derivatives in mediating immune function, omega-3 PUFA metabolites like protectin D1 may be useful as supportive dietary therapy for prevention and treatment of flu-like viral infections, including COVID-19.
IMMUNE DEFENSE AND NUTRITIONAL NEEDS OF OLDER ADULTS
Immunosenescence, or the progressive deterioration of the immune response in aging, affects both innate and adaptive immunity in various pathological conditions, resulting not only in increased susceptibility of older adults to infections but also a reduced response to various treatment regimens, including vaccines.132,133 In 2019, the worldwide population of persons over 65 years of age was 703 million. Of this number, 260 million were from East Asia and South-East Asia, and more than 200 million were from Europe.134 Undoubtedly, immunocompromised older adults with additional comorbidities constitute a population at high risk of infection and severe morbidity. More drastic outcomes have been observed during the ongoing COVID-19 pandemic: data confirm older adults as the most vulnerable population, with mortality reaching up to an estimated 15%.135,136
Older adults, compared with younger populations, are more susceptible to COVID-19–like viral infections and their associated serious outcomes. This increased susceptibility is attributable to aging-associated physiological changes, a weakened immune response, malnutrition, and multimorbidities.137 Prolonged hospitalization to ensure the stabilization and recovery of COVID-19 patients increases the risk of malnutrition and severe loss of lean body mass and muscle function. Nutritional screening and treatment of malnutrition in older patients is therefore mandated as part of COVID-19 patient care.138A recent cross-sectional study from Wuhan, China, reported that 52.7% of the 182 older adult patients with COVID-19 were malnourished, with the mean Mini Nutritional Assessment score being below 17.139 Advanced age is associated with a high risk of nutritional frailty, characterized by sudden weight loss, loss of lean body mass, and loss of physiological nutritional reserves. Nutritional frailty compromises an individual’s ability to meet their nutritional needs and increases their susceptibility to disability.140 The European Society for Clinical Nutrition and Metabolism138 proposed several considerations for the nutritional care of older COVID-19 patients: nutritional screening; optimization of nutritional status by dietary counseling; supplementation with essential vitamins and minerals, oral nutritional supplements, and enteral and parenteral nutritional support when nutritional needs are not met; and regular physical activity in quarantine. Adverse clinical outcomes of viral infections have been linked to low intakes of micronutrients. Thus, providing the RDA of vitamins A, D, E, C, B6, and B12 and iron, zinc, selenium, and omega-3 PUFAs to malnourished older adults may help prevent or treat adverse clinical outcomes of COVID-19.
SUGGESTIONS AND RECOMMENDATIONS
With the current emphasis on exploring therapeutic options to treat COVID-19, more than 100 clinical trials are under way to develop a vaccine, design effective drugs, and test novel and repurposed compounds against SARS-CoV-2. However, data from longitudinal and observational studies on the extent of micronutrient deficiencies in COVID-19 patients, along with infection severity scores, are needed. Factors predicted to be associated with high risk of severe COVID-19 include age above 50 years, male gender, smoking, chronic kidney disease, diabetes, cardiovascular disease, chronic obstructive pulmonary disease, and cerebrovascular disease. Individuals with these risk factors should be screened for micronutrient deficiencies. Supplementation to achieve adequate serological levels of the deficient nutrients may be provided in accordance with in-practice guidelines. The impact of supplementation should be evaluated relative to reductions in the severity of infection and improvements in the recovery index.
CONCLUSION
The role of optimal nutrition for managing the current COVID-19 pandemic cannot be underestimated. Nutrition has a demonstrable role in the prevention and treatment of moderate to severe respiratory and nonrespiratory infections. Adequate nutrition is even more essential for marginalized communities and in low- and middle-income countries, where deficiencies in key vitamins and minerals expose individuals to greater morbidity and mortality. Low- and middle-income countries should strategize to ensure the population at large has access to optimal nutrition to boost the immune system and should provide specific supplementation for treatment of COVID-19 patients, especially those with severe disease. Older adults represent a high-risk population and may be prioritized to receive care in nursing facilities and to receive specialized nutritional support to improve physical and mental outcomes of the COVID-19 pandemic.
Acknowledgments
Author contributions. S.A and T.I. conceived the review and drafted the paper. All authors provided input for the draft of the manuscript. W.S. performed the literature review and organized the results. Z.A.B. and J.K.D. read the paper critically and contributed important revisions. M.W. designed the figures.
Funding/support. No external funds supported this work.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1. Culp WC., Jr Coronavirus disease: in-home isolation room construction. AA Pract; 2020;14:e1218. doi: 10.1213/xaa.0000000000001218 [DOI] [PMC free article] [PubMed]
- 2. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 3. Ji Y, Ma Z, Peppelenbosch MP, et al. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health. 2020;8:E480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng VCC, Lau SKP, Woo PCY, et al. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev. 2007;20:660–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuen K-Y, Wong SSY, Peiris JSM.. The severe acute respiratory syndrome In: Fong IW, Alibek K, eds. New and Evolving Infections of the 21st Century. Springer; 2007:163–193. [Google Scholar]
- 6. Calder PC, Carr AC, Gombart AF, et al. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. doi: 10.3390/nu12041181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ayoub BM. COVID-19 vaccination clinical trials should consider multiple doses of BCG. Pharmazie. 2020;75:159. [DOI] [PubMed] [Google Scholar]
- 8. Sharquie IK. BCG is a good immunotherapeutic agent for viral and autoimmune diseases: is it a new weapon against coronavirus (COVID-19)? Electron J Gen Med. 2020;17:em229. doi: 10.29333/ejgm/7892 [DOI] [Google Scholar]
- 9. Agrawal S, Goel AD, Gupta N.. Emerging prophylaxis strategies against COVID-19. Monaldi Arch Chest Dis. 2020;90:169–172. [DOI] [PubMed] [Google Scholar]
- 10. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [published online March 27, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh AK, Singh A, Shaikh A, et al. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr. 2020;14:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang L, Liu Y.. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grant W, Lahore H, McDonnell S, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carr AC, Maggini S.. Vitamin C and immune function. Nutrients. 2017;9:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. [doi:10.1136/bmj.i6583] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gombart AF, Pierre A, Maggini S.. A review of micronutrients and the immune system—working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta S, Read SA, Shackel NA, et al. The role of micronutrients in the infection and subsequent response to hepatitis C virus. Cells. 2019;8:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Childs CE, Calder PC, Miles EA.. Diet and immune function. Nutrients. 2019;11:1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elmadfa I, Meyer AL.. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2 suppl 2):S3–S23. doi: 10.1016/j.jaci.2009.12.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Romagnani S. T-cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol. 2000;85:9–18. [DOI] [PubMed] [Google Scholar]
- 22. Thevarajan I, Nguyen THO, Koutsakos M, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26:453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chandra RK. Nutrition and the immune system from birth to old age. Eur J Clin Nutr. 2002;56(suppl 3):S73–S76. [DOI] [PubMed] [Google Scholar]
- 24. Alpert PT. The role of vitamins and minerals on the immune system. Home Health Care Manag Pract. 2017;29:199–202. [Google Scholar]
- 25. Milner JJ, Beck MA.. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diwakar BT, Finch ER, Liao C, et al. The role of selenoproteins in resolution of inflammation In: Hatfield DL, Schweizer U, Tsuji PA, Gladyshev VN, eds. Selenium: Its Molecular Biology and Role in Human Health. 4th ed.Springer International Publishing; 2016:499–510. [Google Scholar]
- 27. Haryanto B, Suksmasari T, Wintergerst E, et al. Multivitamin supplementation supports immune function and ameliorates conditions triggered by reduced air quality. Vitam Miner. 2015;4:2–15. doi: 10.4172/2376-1318.1000128 [DOI] [Google Scholar]
- 28. McClung JP, Peterson DG.. Trace elements and immune function In: Watson RR, Zibadi S, Preedy VR, eds. Dietary Components and Immune Function. Humana Press; 2010:253–262. [Google Scholar]
- 29. Saeed F, Nadeem M, Ahmed RS, et al. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—a review. Food Agric Immunol. 2016;27:205–229. [Google Scholar]
- 30. Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest. 2002;32(suppl 1):70–78. [DOI] [PubMed] [Google Scholar]
- 31. Xu Y, Sherwood JA, Lackey KH, et al. The responses of immune cells to iron oxide nanoparticles. J Appl Toxicol. 2016;36:543–553. [DOI] [PubMed] [Google Scholar]
- 32. Wintergerst ES, Maggini S, Hornig DH.. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50:85–94. [DOI] [PubMed] [Google Scholar]
- 33. Maares M, Haase H.. Zinc and immunity: an essential interrelation. Arch Biochem Biophys. 2016;611:58–65. [DOI] [PubMed] [Google Scholar]
- 34. Maggini S, Wintergerst ES, Beveridge S, et al. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98(suppl 1)29–S35. [DOI] [PubMed] [Google Scholar]
- 35. Avery JC, Hoffmann PR.. Selenium, selenoproteins, and immunity. Nutrients. 2018;10:1203. doi: 10.3390/nu10091203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ivory K, Prieto E, Spinks C, et al. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin Nutr. 2017;36:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.A phase 2 trial of high-dose ascorbate in glioblastoma multiforme. ClinicalTrials.gov identifier: NCT02344355. https://clinicaltrials.gov/ct2/show/NCT02344355. Updated April 7, 2020. Accessed May 12, 2020.
- 38.Hydroxychloroquine and zinc with either azithromycin or doxycycline for treatment of COVID-19 in outpatient setting. ClinicalTrials.gov identifier: NCT04370782. https://clinicaltrials.gov/ct2/show/NCT04370782?term=NCT04370782&draw=2&rank=1. Updated May 18, 2020. Accessed May 12, 2020.
- 39.TET2 mutations in myelodysplastic syndromes and acute myeloid leukemia with azacitidine + ascorbic acid. ClinicalTrials.gov identifier: NCT03397173. https://clinicaltrials.gov/ct2/show/NCT03397173?term=NCT03397173&draw=2&rank=1. Updated January 21, 2020. Accessed May 12, 2020.
- 40. Hagag AA, El Frargy MS, Houdeeb HA.. Therapeutic value of vitamin D as an adjuvant therapy in neonates with sepsis [published online June 25, 2019]. Infect Disord Drug Targets. doi: 10.2174/1871526519666190626141859 [DOI] [PubMed] [Google Scholar]
- 41. Kim W-Y, Jo E-J, Eom JS, et al. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: propensity score–based analysis of a before–after cohort study. J Crit Care. 2018;47:211–218. [DOI] [PubMed] [Google Scholar]
- 42. Sánchez-Armendáriz K, García-Gil A, Romero CA, et al. Oral vitamin D3 5000 IU/day as an adjuvant in the treatment of atopic dermatitis: a randomized control trial. Int J Dermatol. 2018;57:1516–1520. [DOI] [PubMed] [Google Scholar]
- 43. Gonçalves D, Lima C, Ferreira P, et al. Orange juice as dietary source of antioxidants for patients with hepatitis C under antiviral therapy. Food Nutr Res. 2017;61:1296675.doi: 10.1080/16546628.2017.1296675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhattacharjee A, Basu A, Biswas J, et al. Chemoprotective and chemosensitizing properties of selenium nanoparticle (Nano-Se) during adjuvant therapy with cyclophosphamide in tumor-bearing mice. Mol Cell Biochem. 2017;424:13–33. [DOI] [PubMed] [Google Scholar]
- 45. Kaya H, Koç AK, Sayın İ, et al. Vitamins A, C, and E and selenium in the treatment of idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2015;272:1119–1125. [DOI] [PubMed] [Google Scholar]
- 46. Chaturvedi UC, Shrivastava R, Upreti RK.. Viral infections and trace elements: a complex interaction. Curr Sci. 2004;87:1536–1554. https://www.jstor.org/stable/24109032 [Google Scholar]
- 47. Hemilä H, Chalker E.. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980.doi: 10.1002/14651858.CD000980.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ran L, Zhao W, Wang J, et al. Extra dose of vitamin C based on a daily supplementation shortens the common cold: a meta-analysis of 9 randomized controlled trials. Biomed Res Int. 2018;2018:1837634. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49. Villamor E, Mbise R, Spiegelman D, et al. Vitamin A supplements ameliorate the adverse effect of HIV-1, malaria, and diarrheal infections on child growth. Pediatrics. 2002;109:E6. [DOI] [PubMed] [Google Scholar]
- 50. Aukrust P, Müller F, Ueland T, et al. Decreased vitamin A levels in common variable immunodeficiency: vitamin A supplementation in vivo enhances immunoglobulin production and downregulates inflammatory responses. Eur J Clin Invest. 2000;30:252–259. [DOI] [PubMed] [Google Scholar]
- 51. Evans RM, Mangelsdorf DJ.. Nuclear receptors, RXR, and the big bang. Cell. 2014;157:255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. di Masi A, Leboffe L, De Marinis E, et al. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol Aspects Med. 2015;41:1–115. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005 : WHO Global Database on Vitamin a Deficiency. World Health Organization; 2009. [Google Scholar]
- 54. West CE, Sijtsma SR, Kouwenhoven B, et al. Epithelia-damaging virus infections affect vitamin A status in chickens. J Nutr. 1992;122:333–339. [DOI] [PubMed] [Google Scholar]
- 55. Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc. 1999;58:719–727. [DOI] [PubMed] [Google Scholar]
- 56. Jee J, Hoet AE, Azevedo MP, et al. Effects of dietary vitamin A content on antibody responses of feedlot calves inoculated intramuscularly with an inactivated bovine coronavirus vaccine. Am J Vet Res. 2013;74:1353–1362. [DOI] [PubMed] [Google Scholar]
- 57. Wu T, Ni J, Wei J.. Vitamin A for non-measles pneumonia in children. Cochrane Database Syst Rev. 2005;(3):CD003700. doi: 10.1002/14651858.cd003700.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Glasziou PP, Mackerras D.. Vitamin A supplementation in infectious diseases: a meta-analysis. Br Med J. 1993;306:366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maia SB, Souza ASR, Caminha MFC, et al. Vitamin A and pregnancy: a narrative review. Nutrients. 2019;11:681. doi: 10.3390/nu11030681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Caccialanza R, Laviano A, Lobascio F, et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74:110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Troen AM, Mitchell B, Sorensen B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136:189–194. [DOI] [PubMed] [Google Scholar]
- 62. Depeint F, Bruce WR, Shangari N, et al. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact. 2006;163:94–112. [DOI] [PubMed] [Google Scholar]
- 63. Percudani R, Peracchi A.. The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Bioinformatics. 2009;10:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bourquin F, Capitani G, Grütter MG.. PLP-dependent enzymes as entry and exit gates of sphingolipid metabolism. Protein Sci. 2011;20:1492–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mikkelsen K, Stojanovska L, Prakash M, et al. The effects of vitamin B on the immune/cytokine network and their involvement in depression. Maturitas. 2017;96:58–71. [DOI] [PubMed] [Google Scholar]
- 66. Wintergerst ES, Maggini S, Hornig DH.. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51:301–323. [DOI] [PubMed] [Google Scholar]
- 67. Jones HD, Yoo J, Crother TR, et al. Nicotinamide exacerbates hypoxemia in ventilator-induced lung injury independent of neutrophil infiltration. PLoS One. 2015;10:E0123460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Atherton JG, Kratzing CC, Fisher A.. The effect of ascorbic acid on infection of chick-embryo ciliated tracheal organ cultures by coronavirus. Arch Virol. 1978;56:195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Field CJ, Johnson IR, Schley PD.. Nutrients and their role in host resistance to infection. J Leukoc Biol. 2002;71:16–32. [PubMed] [Google Scholar]
- 70. Hemilä H, Louhiala P.. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532.doi: 10.1002/14651858.CD005532.pub3 [DOI] [PubMed] [Google Scholar]
- 71. Hemilä H. Vitamin C and infections. Nutrients. 2017;9:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fowler AA III, Kim C, Lepler L, et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J Crit Care Med. 2017;6:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hemilä H. Vitamin C intake and susceptibility to pneumonia. Pediatr Infect Dis J. 1997;16:836–837. [DOI] [PubMed] [Google Scholar]
- 74. Hemilä H. Vitamin C and SARS coronavirus. J Antimicrob Chemother. 2003;52:1049–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cathcart RF. Vitamin C, titrating to bowel tolerance, anascorbemia, and acute induced scurvy. Med Hypotheses. 1981;7:1359–1376. [DOI] [PubMed] [Google Scholar]
- 76. Padayatty SJ, Sun AY, Chen Q, et al. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One. 2010;5:E11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc. 2012;71:50–61. [DOI] [PubMed] [Google Scholar]
- 78. Ghareghani M, Reiter RJ, Zibara K, et al. Latitude, vitamin D, melatonin, and gut microbiota act in concert to initiate multiple sclerosis: a new mechanistic pathway. Front Immunol. 2018;9:2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Altieri B, Muscogiuri G, Barrea L, et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev Endocr Metab Disord. 2017;18:335–346. [DOI] [PubMed] [Google Scholar]
- 80. Gombart AF. The vitamin D–antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Greiller CL, Martineau AR.. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhou Y-F, Luo B-A, Qin L-L, et al. The association between Vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine (Baltimore). 2019;98:e17252. doi: 10.1097/MD.0000000000017252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi: 10.1002/14651858.CD007470.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xie Z, Xia W, Zhang Z, et al. Prevalence of vitamin D inadequacy among Chinese postmenopausal women: a nationwide, multicenter, cross-sectional study. Front Endocrinol (Lausanne). 2019;9:782. doi: 10.3389/fendo.2018.00782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ferrari D, Lombardi G, Strollo M, et al. Association between solar ultraviolet doses and vitamin D clinical routine data in European mid-latitude population between 2006 and 2018. Photochem Photobiol Sci. 2019;18:2696–2706. [DOI] [PubMed] [Google Scholar]
- 86. Zisi D, Challa A, Makis A.. The association between vitamin D status and infectious diseases of the respiratory system in infancy and childhood. Hormones. 2019;18:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Knight JA. Review: free radicals, antioxidants, and the immune system. Ann Clin Lab Sci. 2000;30:145–158. [PubMed] [Google Scholar]
- 88. Wu D, Meydani SN.. Vitamin E, immune function, and protection against infection In: Weber P, Birringer M, Blumberg JB, Eggersdorfer M, Frank J, eds. Vitamin E in Human Health. Humana Press; 2019:371–384. [Google Scholar]
- 89. Lewis ED, Meydani SN, Wu D.. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71:487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Marko MG, Ahmed T, Bunnell SC, et al. Age-associated decline in effective immune synapse formation of CD4+ T cells is reversed by vitamin E supplementation. J Immunol. 2007;178:1443–1449. [DOI] [PubMed] [Google Scholar]
- 91. Han SN, Pang E, Zingg J-M, et al. Differential effects of natural and synthetic vitamin E on gene transcription in murine T lymphocytes. Arch Biochem Biophys. 2010;495:49–55. [DOI] [PubMed] [Google Scholar]
- 92. Kim J, Zhang J, Cha Y, et al. Advanced bioinformatics rapidly identifies existing therapeutics for patients with coronavirus disease – 2019 (COVID-19) [published online March 27, 2020]. ChemRxiv. doi: 10.26434/chemrxiv.12037416.v1 [DOI] [PMC free article] [PubMed]
- 93. Bendich A, Machlin LJ.. Safety of oral intake of vitamin e. Am J Clin Nutr. 1988;48:612–619. [DOI] [PubMed] [Google Scholar]
- 94. Shankar AH, Prasad AS.. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68(2 suppl):447S–463S. doi: 10.1093/ajcn/68.2.447S [DOI] [PubMed] [Google Scholar]
- 95. Sherman AR. Zinc, copper, and iron nutriture and immunity. J Nutr. 1992;122(3 suppl):604–609. [DOI] [PubMed] [Google Scholar]
- 96. Keen CL, Uriu-Adams JY, Ensunsa JL, et al. Trace elements/minerals and immunity In: Gershwin ME, Nestel P, Keen CL, eds. Handbook of Nutrition and Immunity. Humana Press; 2004:117–140. [Google Scholar]
- 97. Andreini C, Banci L, Bertini I, et al. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006;5:196–201. [DOI] [PubMed] [Google Scholar]
- 98. Keen CL, Gershwin ME.. Zinc deficiency and immune function. Annu Rev Nutr. 1990;10:415–431. [DOI] [PubMed] [Google Scholar]
- 99. Dardenne M. Zinc and immune function. Eur J Clin Nutr. 2002;56(suppl 3):S20–S23. [DOI] [PubMed] [Google Scholar]
- 100. Lesourd BM. Nutrition and immunity in the elderly: modification of immune responses with nutritional treatments. Am J Clin Nutr. 1997;66:478S–484S. doi: 10.1093/ajcn/66.2.478S [DOI] [PubMed] [Google Scholar]
- 101. Tuerk MJ, Fazel N.. Zinc deficiency. Curr Opin Gastroenterol. 2009;25:136–143. [DOI] [PubMed] [Google Scholar]
- 102. Haase H, Rink L.. Multiple impacts of zinc on immune function. Metallomics. 2014;6:1175–1180. [DOI] [PubMed] [Google Scholar]
- 103. Awotiwon AA, Oduwole O, Sinha A, et al. Zinc supplementation for the treatment of measles in children. Cochrane Database Syst Rev. 2017;(6):CD0011177.doi: 10.1002/14651858.CD011177.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lassi ZS, Moin A, Bhutta ZA.. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2016;(12):CD005978.doi: 10.1002/14651858.CD005978.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Singh M, Das RR.. Zinc for the common cold. Cochrane Database Syst Rev. 2013;(6):CD001364.doi: 10.1002/14651858.CD001364.pub4 [DOI] [PubMed] [Google Scholar]
- 106. Hemilä H. Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open. 2017;8:205427041769429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Brown MA, Thom JV, Orth GL, et al. Food poisoning involving zinc contamination. Arch Environ Health. 1964;8:657–660. [DOI] [PubMed] [Google Scholar]
- 108. Fosmire GJ. Zinc toxicity. Am J Clin Nutr. 1990;51:225–227. [DOI] [PubMed] [Google Scholar]
- 109. te Velthuis AJW, van den Worml SHE, Sims AC, et al. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6:E1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liu W, Zhang S, Nekhai S, et al. Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival [published online April 20, 2020]. Curr Clin Microbiol Rep. doi: 10.1007/s40588-020-00140-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Weiss G, Ganz T, Goodnough LT.. Anemia of inflammation. Blood. 2019;133:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mullick S, Rusia U, Sikka M, et al. Impact of iron deficiency anaemia on T lymphocytes & their subsets in children. Indian J Med Res. 2006;124:647–654. PMID: 17287552 [PubMed] [Google Scholar]
- 113. Jayaweera J, Reyes M, Joseph A.. Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci Rep. 2019;9:12637. doi: 10.1038/s41598-019-49122-z [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114. de Pontual L. Fer et prédisposition aux infections [Iron and susceptibility to infections; in French]. Arch Pédiatrie. 2017;24(suppl):5S14–5S17. [DOI] [PubMed] [Google Scholar]
- 115. Chandra RK. Trace elements and immune responses. Immunol Today. 1983;4:322–325. [DOI] [PubMed] [Google Scholar]
- 116. Wessling-Resnick M. Crossing the iron gate: why and how transferrin receptors mediate viral entry. Annu Rev Nutr. 2018;38:431–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Fan BE, Ong KH, Chan SSW, et al. Blood and blood product use during COVID-19 infection [published online April 22, 2020]. Am J Hematol. 2020;95:E158–E160. doi: 10.1002/ajh.25823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Weinkove R, McQuilten Z, Adler J, et al.. Managing haematology and oncology patients during the COVID-19 pandemic: interim consensus guidance [ preprint published March 20, 2020]. Med J Aust. 2020;212:481–489. [10.5694/mja2.50607] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Arthur JR, McKenzie RC, Beckett GJ.. Selenium in the immune system. J Nutr. 2003;133:1457S–1459S. [DOI] [PubMed] [Google Scholar]
- 120. Marciel MP, Hoffmann PR.. Molecular mechanisms by which selenoprotein K regulates immunity and cancer. Biol Trace Elem Res. 2019;192:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Turner RJ, Finch JM.. Selenium and the immune response. Proc Nutr Soc. 1991;50:275–285. [DOI] [PubMed] [Google Scholar]
- 122. Kiremidjian-Schumacher L, Roy M.. Selenium and immune function. Z Ernahrungswiss. 1998;37(suppl 1):50–56. PMID: 9558729 [PubMed] [Google Scholar]
- 123. Beck MA, Shi Q, Morris VC, et al. Rapid genomic evolution of a non-virulent Coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med. 1995;1:433–436. [DOI] [PubMed] [Google Scholar]
- 124. Beck MA, Nelson HK, Shi Q, et al. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001;15:1481–1483. [PubMed] [Google Scholar]
- 125. Harthill M. Review: micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol Trace Elem Res. 2011;143:1325–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ma X, Bi S, Wang Y, et al. Combined adjuvant effect of ginseng stem-leaf saponins and selenium on immune responses to a live bivalent vaccine of Newcastle disease virus and infectious bronchitis virus in chickens. Poult Sci. 2019;98:3548–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhang J, Will ET, Bennett K, et al. Association between regional selenium status and reported outcome of COVID-19 cases in China [published online April 28, 2020]. Am J Clin Nutr. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095/5826147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gutiérrez S, Svahn SL, Johansson ME.. Effects of omega-3 fatty acids on immune cells. Int J Mol Sci. 2019;20:5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fat Acids. 2006;75:197–202. [DOI] [PubMed] [Google Scholar]
- 130. Cai C, Koch B, Morikawa K, et al. Macrophage-derived extracellular vesicles induce long-lasting immunity against hepatitis C virus which is blunted by polyunsaturated fatty acids. Front Immunol. 2018;9:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Morita M, Kuba K, Ichikawa A, et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. [DOI] [PubMed] [Google Scholar]
- 132. Dugan HL, Henry C, Wilson PC.. Aging and influenza vaccine-induced immunity. Cell Immunol. 2020;348:103998. doi: 10.1016/j.cellimm.2019.103998 [DOI] [PubMed] [Google Scholar]
- 133. Kumar R, Burns EA.. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines. 2008;7:467–479. [DOI] [PubMed] [Google Scholar]
- 134.United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2019: Highlights United Nations. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf. Published 2019. Accessed May 18, 2020.
- 135. Chhetri JK, Chan P, Arai H, et al. Prevention of COVID-19 in older adults: a brief guidance from the International Association for Gerontology and Geriatrics (IAGG) Asia/Oceania region [published online April 14, 2020]. J Nutr Health Aging. 2020;24:471–472.doi: 10.1007/s12603-020-1359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Morley JE, Vellas B.. Editorial: COVID-19 and older adults. J Nutr Health Aging. 2020;24:364–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Barazzoni R, Bischoff SC, Breda J, et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:P1631–P1638. doi: 10.1016/j.clnu.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Li T, Zhang Y, Gong C, et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China [published online April 22, 2020]. Eur J Clin Nutr. 2020;74:871–875. doi: 10.1038/s41430-020-0642-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kinney JM. Nutritional frailty, sarcopenia and falls in the elderly. Curr Opin Clin Nutr Metab Care. 2004;7:15–20. [DOI] [PubMed] [Google Scholar]