The outbreak of coronavirus disease 2019 (COVID-19) has rapidly evolved into a global pandemic. Acute kidney injury (AKI) is common among critically ill patients with COVID-19, affecting ~20% according to experience in Europe [1]. Studies have described outcomes of patients with AKI secondary to COVID-19 [2], but information characterizing patients with subsequent AKI is limited.
The cause of kidney involvement in COVID-19 is likely to be multifactorial, with cardiovascular comorbidity and predisposing factors (e.g. sepsis and nephrotoxins) as important contributors. However, tubular damage is universal and has been linked to the cytopathic effects of kidney-resident cells and cytokine storm syndrome [3, 4]. Increased proteinuria upon admission has been reported in >40% of COVID-19 cases [2], but proteinuria has been measured semiquantitatively using urine dipsticks and quantitative assessment of proteinuria has not been reported.
Urinary tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7) have been implicated in G1-phase arrest in renal tubules [5] and may serve as early indicators of acute kidney stress, but their value in COVID-19 is untested. Accordingly, we sought to evaluate the incidence of AKI and its association with urinary biomarkers in hospitalized COVID-19 patients.
This is an initial report of an ongoing prospective, observational, single-centre study started on 21 April 2020 (Supplementary data). Adults admitted to the University Hospital of Giessen and Marburg, Giessen, Germany diagnosed with COVID-19 according to World Health Organization criteria were eligible. Patients were excluded if they had Stage 5 chronic kidney disease [6], if they received maintenance dialysis or if they were recipients of a solid organ transplant. The study protocol was approved by the local ethics committee (AZ 58/20) and complied with the Declaration of Helsinki. Participants provided written informed consent but, if incapable, legally authorized representatives did so. The study was registered at clinicaltrials.gov (NCT04353583).
The primary outcome was AKI incidence during hospitalization. AKI was diagnosed using full Kidney Disease: Improving Global Outcomes criteria [7], by incorporating baseline serum creatinine (SCr) levels and by correction of SCr levels for fluid balance (if available) (Supplementary data, Methods). AKI reversal was defined as the absence of any stage of AKI based on SCr or urine output within 7 days after admission [8]. Spot urine samples were collected upon hospital admission and 12, 24 and 48 h after admission. Values (in mg/g creatinine) >150, ≥30 and ≥20 were considered as increased for proteinuria, albuminuria and tubular proteinuria (α1-microglobulin), respectively [6]. Urinary [TIMP-2]•[IGFBP7] >0.3 and >2 (ng/mL)2/1000 were taken for AKI risk stratification [5]. The renal resistive index (RRI) was measured upon admission. Assessments (including laboratory methods and statistical analyses) are described in detail in the Supplementary data.
Twenty-three patients (median age 60.0 years; 82.6% male) with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection were included (Supplementary data, Figure S1). Eleven (47.8%) were admitted to isolation wards and 12 (52.2%) were transferred to the intensive care unit (ICU) due to respiratory failure secondary to acute respiratory distress syndrome, with 3 requiring non-invasive ventilation and 9 mechanical ventilation.
Twelve (52.2%) patients developed Stage 1 AKI at a median of 4 (range 2–6) days post-admission (Supplementary data, Table S1). Ten of 12 cases with AKI were treated in the ICU. AKI was diagnosed and staged according to positive SCr criteria for AKI and correcting the SCr level for fluid balance in ICU patients did not impact AKI staging. Seven (58.3%) patients experienced AKI reversal <7 days after admission. Among five patients with AKI non-reversal, one progressed from Stage 1 to Stage 2 and four progressed from Stage 1 to Stage 3 at a median of 10.0 (range 8–11) days post-admission as a sequel to septic shock; three required renal replacement therapy (RRT) but died.
Comorbidity was more common among patients who subsequently developed AKI compared with those who did not. Higher white blood cell count and levels of C-reactive protein, interleukin-6, ferritin and D-dimer were noted upon admission for AKI versus non-AKI patients. Patients with subsequent AKI had lower kidney function compared with those who did not develop AKI, with a marked difference when using the creatinine–cystatin C equation for estimating glomerular filtration rate {37.5 [95% confidence interval (CI) 20.0–95.0] versus 84.0 [59.0–128.0] mL/min/1.73 m2}. Nineteen patients (82.6%; all male) exhibited increased proteinuria independent of subsequent AKI, whereas the remaining four patients with physiologic proteinuria (<120 mg/g creatinine) were female. Proteinuria was higher in AKI versus non-AKI patients [442.0 (95% CI 150.0–1230.0) versus 142.0 (66.4–460.0) mg/g creatinine] and its differentiation showed a predominant tubular (α1-microglobulin) pattern. All 23 patients had increased RRI upon admission [0.79 (95% CI 0.72–0.87)]. Twelve (52.2%) patients had [TIMP-2]•[IGFBP7] >0.3 (ng/mL)2/1000 upon admission with no detectable difference with respect to subsequent AKI development.
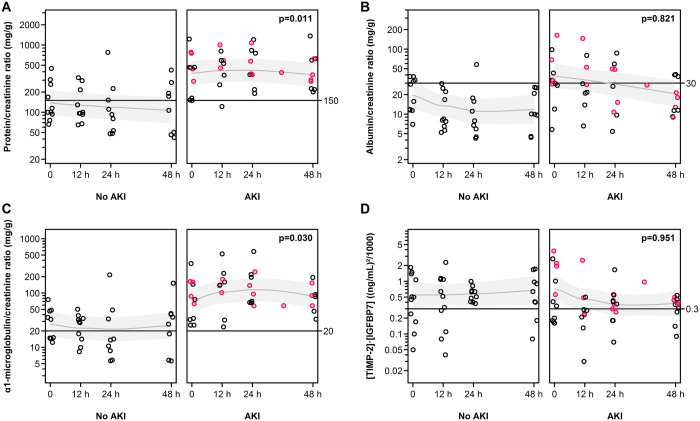
Figure 1 shows the time course of urinary biomarkers categorized by AKI. Proteinuria (P = 0.011) and α1-microglobulin excretion (P = 0.030) were higher in patients who subsequently developed AKI compared with those who did not. There were no clear differences between trends in [TIMP-2]•[IGFBP7] (concentration measured or normalized to urinary creatinine excretion or urine osmolality) of AKI versus non-AKI. However, among the AKI patients, those who progressed from Stage 1 to Stage 2 and Stage 3 AKI [5/12 (41.7%)] had higher [TIMP-2]•[IGFBP7] levels compared with those who did not [1.95 (95% CI 0.51–3.78) versus 0.20 (0.15–1.68); P < 0.001]. Furthermore, all AKI patients with [TIMP-2]•[IGFBP7] levels >2 (ng/mL)2/1000 upon admission [2/12 (16.7%)] progressed to Stage 3 AKI and required RRT but eventually died. In contrast, none of the AKI patients with [TIMP-2]•[IGFBP7] levels ≤0.3 ng/mL)2/1000 [6/12 (50.0%)] experienced progression of their AKI stage. The median α1-microglobulin excretion was higher among AKI patients who experienced progression of their AKI stage compared with those who did not [87.2 (95% CI 12.3–163.7) versus 34.1 (25.6–350.3) mg/g creatinine; P = 0.04]. In contrast, median proteinuria levels did not differ between AKI progressors and AKI non-progressors [442.3 (95% CI 100.0–781.4) versus 459.9 (122.1–1226.7) mg/g creatinine; P = 0.84]. A similar finding was observed when median admission levels of α1-microglobulin excretion and proteinuria were compared between patients who died during the observational period [3/23 (13.0%)] and those who did not [α1-microglobulin excretion 87.2 (95% CI 58.5–159.7) versus 34.7 (14.6–350.3) mg/g creatinine; P = 0.04; proteinuria 390.4 (95% CI 289.5–781.4) versus 214.8 (66.4–1226.7) mg/g creatinine; P = 0.36].
FIGURE 1.
Time course of proteinuria, albuminuria, urinary α1-microglobulin excretion and urinary [TIMP-2]•[IGFBP7] categorized by AKI. Horizontal lines indicate the levels of non-physiologic concentrations [5, 6]. Biomarker values highlighted in pink indicate those patients who progressed from Stage 1 to Stage 2–3 AKI. P-values show the empirical significance of the mean difference between groups adjusted for time trends, from generalized linear mixed models (Supplementary data, Methods). Fitted models are indicated by a line showing the conditional means and grey areas represent the approximate 95% confidence intervals of conditional mean values.
Correlation analyses of urinary biomarkers with key clinical and laboratory data are shown in Supplementary Figure S2. Briefly, the clearest relationships were seen between urinary biomarkers and the Sequential Organ Failure Assessment score, D-dimer, ferritin, procalcitonin and driving pressure. Data were inconclusive regarding [TIMP-2]•[IGFBP7] with the tested variables and with the other urinary biomarkers (data not shown).
In our cohort, [TIMP-2]•[IGFBP7] did not have much utility for detecting Stage 1 AKI, which is not surprising because much of Stage 1 AKI may represent low risk and is associated with renal function decline without kidney damage. In patients with progression of AKI, those with increased [TIMP-2]•[IGFBP7] levels seemed to have worse outcomes as recently described in critically ill patients [9]. On the other hand, the identification of increased urinary biomarkers of kidney stress/damage in patients without subsequent AKI suggests that subclinical kidney injury may be common in COVID-19 and warrants further investigation [10, 11].
This is the first study evaluating proteinuria by quantitative measures and [TIMP-2]•[IGFBP7] in COVID-19. Study strengths are its prospective design and multiple variable assessments. Study limitations are the single-centre design and, at the moment, the small sample size and short duration of follow-up. Biomarkers were collected for a short time period, therefore only limited informational value can be drawn regarding AKI development, AKI progression and mortality. α1-microglobulin excretion and [TIMP-2]•[IGFBP7] upon admission appeared to improve risk stratification for severe outcomes (Stage 3 AKI, RRT and death) in AKI patients, but the number of patients who reached that outcome was very small. Volume depletion at admission may be common in patients with COVID-19, as patients present with fever and pre-hospital fluid resuscitation is rarely performed. In our cohort, however, we did not detect a clear association between the time course of urinary biomarkers and changes in central venous pressure, B-type natriuretic peptide or cumulative fluid balance. Patients were admitted at different stages of illness, so renal disease onset and early time course of renal involvement were not elucidated.
In conclusion, AKI was common in COVID-19. The majority of patients exhibited increased proteinuria at admission, indicating tubular damage. AKI progression was mostly uniform and biphasic within 7–14 days post-ICU admission as a sequel to septic shock; patients were more likely to have higher α1-microglobulin excretion and [TIMP-2]•[IGFBP7] levels and RRT requirement and death were common. Future studies are needed to clarify the role of urinary biomarkers for risk stratification and triage of patients with COVID-19.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the nursing staff of the medical and surgical ICUs for their hard work and commitment to patient well-being. Without their support, this work would not have been possible.
DATA AVAILABILITY
The datasets used and/or analysed during the study are available from the corresponding author upon reasonable request.
AUTHORS’ CONTRIBUTIONS
The authors shared study design, data collection, data analysis and data interpretation, as well as preparation, review and approval of the manuscript. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication. F.H.-S., J.W., C.R., H.-W.B. and W.S. were responsible for the concept and design of the study. S.K., I.V., S.H., H.-D.W., J.A.K. and W.S. were responsible for the literature research and clinical advice. F.H.-S., J.W., S.K., C.R., H.-W.B., I.V., S.H., H.-D.W. and W.S. were responsible for acquisition, analyses and interpretation of data. H.-W.B., J.A.K. and C.R. were responsible for adjudication of renal function. F.H.-S., J.W., H.-W.B., J.A.K. and C.R. were responsible for manuscript drafting. F.H.-S., J.W., S.K., H.-W.B., I.V., S.H., H.-D.W., J.A.K., J.A.K., C.R. and W.S. were responsible for critical revision of the manuscript for important intellectual content. J.W. was responsible for statistical analyses. F.H., H.-W.B. and W.S. were responsible for study supervision.
CONFLICT OF INTEREST STATEMENT
W.S. received personal fees for consulting from Bayer Pharma, Liquidia Technologies and United Therapeutics outside the submitted work. The remaining authors have no competing interests. The authors declare that the results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1.Acute kidney injury in COVID-19 patients. ESICMtv Webinar. Posted April 17. https://www.esicm.org/blog/? p=2789 (29 April 2020, date last accessed)
- 2. Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Su H, Ym WC, Yi L-X. et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; 98: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Varga Z, Flammer AJ, Steiger P. et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395: 1417–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bihorac A, Chawla LS, Shaw AD. et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med 2014; 189: 932–939 [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 7. Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138 [Google Scholar]
- 8. Kellum JA, Sileanu FE, Bihorac A. et al. Recovery after acute kidney injury. Am J Respir Crit Care Med 2017; 195: 784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joannidis M, Forni LG, Haase M. et al. Use of cell cycle arrest biomarkers in conjunction with classical markers of acute kidney injury. Crit Care Med 2019; 47: e820–e826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haase M, Devarajan P, Haase-Fielitz A. et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol 2011; 57: 1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ronco C, Bellomo R, Kellum JA.. Acute kidney injury. Lancet 2019; 394: 1949–1964 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the study are available from the corresponding author upon reasonable request.