The rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and the resulting coronavirus disease 2019 (COVID-19) poses unprecedented challenges for healthcare all around the world. Also for nephrology, the present COVID-19 epidemic marks difficult times. Previous studies showed that the most important risk factors for mortality in COVID-19 are an advanced age (older than 70 years) and male sex [1]. Furthermore, comorbidities such as obesity, hypertension, diabetes mellitus, cardiovascular disease, chronic lung disease, chronic kidney disease and cancer are known to be associated with increased mortality [1–4].
Because patients on kidney replacement therapy (KRT; including dialysis and kidney transplantation) are generally older and often have one or more of the aforementioned comorbidities, they may have an increased risk of death when they acquire a SARS-CoV-2 infection [5]. In addition, kidney transplant recipients are treated with immunosuppressive drugs, which may impede viral clearance and increase their risk of developing severe symptoms [6]. However, KRT patients are well aware of their elevated risk and may have adjusted their behaviour, regarding hygiene and social distancing, accordingly. Another factor is that hyperinflammation is observed in patients with severe COVID-19, which may play a role in disease pathology [7, 8]. It is unclear if and how the use of immunosuppressive medication can affect this hyperinflammatory response. Moreover, some immunosuppressive drugs (including cyclosporine) have been demonstrated to inhibit the replication of coronaviruses in vitro, while it is unknown if this translates into similar effects in vivo in humans [9].
Given this uncertainty, it is important to collect reliable information on the incidence, clinical course and outcomes of COVID-19 infection in patients with end-stage kidney disease who are treated with KRT. This information is essential to guide clinicians in the management of these patients. To accelerate knowledge on the clinical course and outcomes of COVID-19-positive patients on KRT, standardized and coordinated data collection is pivotal.
EUROPEAN RENAL ASSOCIATION COVID-19 DATABASE
There are national initiatives for clinical data collection on KRT patients with COVID-19 in several countries. However, a pan-European initiative makes it possible to reach a larger patient sample in a shorter time frame than would be possible on an individual country level and would allow statistically well-founded conclusions on outcomes and risk factors to be drawn in this vulnerable patient population in a crucial phase of the epidemic.
For this reason, the European Renal Association COVID-19 Database (ERACODA) was established on 21 March 2020. The mission of this dedicated COVID-19 KRT database is to gain as soon as possible insight into what the COVID-19 epidemic means for patients on KRT across Europe, in the hope of improving the prognosis in affected patients by intervening on modifiable risk factors.
The primary purpose of ERACODA is to investigate the clinical course and outcomes—including hospital admission, intensive care unit (ICU) admission and mortality—of KRT patients with COVID-19. A secondary aim is to gain information on risk factors for mortality. Such information may guide clinical treatment decisions and support triage strategies for admission to critical care units. Moreover, knowledge on modifiable patient and treatment characteristics associated with outcome may lead to interventions or changes in transplantation strategies that can improve prognosis. This knowledge may, for example, influence the decision on whether a dialysis patient on the waiting list should undergo transplantation during the COVID-19 epidemic if a donor kidney becomes available.
ERACODA is endorsed by the ERA-EDTA and includes data from countries in Europe and bordering the Mediterranean Sea. A Working Group was formed that designed and runs ERACODA. It consists of Luuk Hilbrands (chair of Transplantation Subdatabase), Casper Franssen (chair of Dialysis Subdatabase), Kitty Jager (director of the ERA-EDTA Registry), Marc Hemmelder (chair of the Dutch Renal Registry) and Ron Gansevoort (ERA-EDTA Council Member). This Working Group is assisted by a team of epidemiologists and database managers, and by an international advisory board.
DATA COLLECTION
Data are collected from all adult patients (aged ≥18 years) with a functioning kidney allograft or on maintenance dialysis treatment who are diagnosed with COVID-19. The COVID-19 diagnosis needs to be based on a positive result on a real-time polymerase chain reaction assay of nasal or pharyngeal swab specimens, and/or compatible findings on a computed tomography scan or chest X-ray of the lungs. Data from both outpatient and hospitalized patients are collected. The primary outcome of the study is a vital status at Day 28 after diagnosis.
Unlike many registries, ERACODA contains granular data on a large set of patient characteristics (including demographics, primary kidney disease, comorbidities and medication use) and COVID-19 characteristics (including symptoms, vital signs and laboratory test results). For all included patients, also the score on the Clinical Frailty Scale—ranging from very fit to terminally ill—is recorded, since this information can influence the decision to admit a patient to the hospital or the ICU [10]. Furthermore, ERACODA takes the unique opportunity to study the impact of the current epidemic on quality of life-related outcome measures. Physicians are asked whether their patients have reached their pre-COVID-19 functional and mental status 3 months after the initial presentation. If this is not the case, further questions address the expected recovery and potential factors limiting recovery. All data are collected for both kidney transplant recipients and dialysis patients, which will allow the risks of transplantation versus the continuation of dialysis to be weighed.
Besides detailed patient information, ERACODA also gathers information on centre-related factors that may be associated with risk of transmission, for instance on what type of screening and preventive strategies were followed in dialysis units. In a second phase of ERACODA, it is expected that biosamples will be collected in conjunction with clinical data. Analysis of these samples could answer important questions. For example, whether patients on KRT with a suppressed immune response develop sufficient antibodies against COVID-19 to prevent them from getting re-infected. Furthermore, it may well be that high-risk groups like KRT patients will be excluded from vaccination trials because of their vulnerability, like they are often excluded from clinical trials [11]. In that case, it may be necessary to analyse the effectiveness of vaccination against SARS‐CoV‐2 in KRT patients in investigator-driven studies. ERACODA would provide an excellent platform of highly motivated collaborators who are likely willing to participate in such research endeavours.
Physicians responsible for the care of these patients can register voluntarily and are asked to enter data of all consecutive kidney transplant and maintenance dialysis patients from their centre with COVID-19 into a central database. The ERACODA database is hosted at the University Medical Center Groningen, the Netherlands and uses REDCap software (Research Electronic Data Capture, Vanderbilt University Medical Center, Nashville, TN, USA), which is a secure, web-based software platform designed to support data capture for research. All patient identifiable information is stripped from each record and data are stored pseudonymized. The study was assessed by the Institutional Review Board of the University Medical Center Groningen, the Netherlands, and the database is compliant with European Union General Data Protection Regulation (GDPR, 2016/679).
WE NEED YOUR HELP
By 11 June 2020, over 1430 unique patient records have been entered by 197 physicians from 98 centres in 26 countries, predominantly from Europe. Based on these data, the first papers are currently being prepared, among others on the 28-day case fatality rate of COVID-19. Agreements have been reached with several national societies, such as those of Finland, Spain, Switzerland and Turkey. They will add their COVID-19 KRT patient registries to ERACODA. These registries, in general, have less detailed patient, disease and treatment data, but offer the advantage of increasing power to detect risk factors for worse outcome.
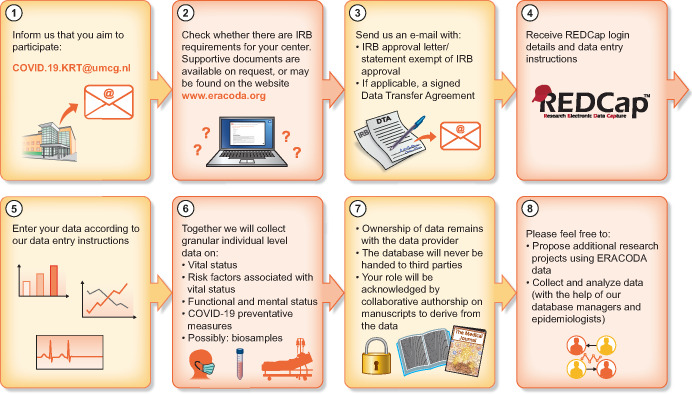
We are extremely grateful to all physicians who have already helped us in the data collection and would like to encourage all clinicians working with KRT patients with COVID-19 to join ERACODA as well. If one (or more) of your patients is diagnosed with COVID-19, please contact us by e-mail at: COVID.19.KRT@umcg.nl. Henceforth, you will be added as a user to the data platform REDCap within one working day. When your account is ready, you will receive an automatically generated e-mail, including your login details and data entry instructions (Figure 1). If there is a similar, national initiative for data collection in your country, we would like to ask you also to join ERACODA.
FIGURE 1.
ERACODA infographic
Please note that you will remain the owner of the data that you submit. When you submit data, you will be a partner in the project and will be acknowledged appropriately as a collaborating author. ERACODA is meant to be an open database. In case people have research questions that potentially could be answered with the collected data, they are cordially invited to submit study proposals.
Contact
If you want to collaborate, or if you have any questions about ERACODA or suggestions for future research, please contact us at COVID.19.KRT@umcg.nl.
ACKNOWLEDGEMENTS
We thank all clinicians who have already entered information in the ERACODA database for their participation and support, and especially all healthcare workers who have taken care of the included COVID-19 patients.
CONFLICT OF INTEREST STATEMENT
None declared.
Footnotes
The members of the ERACODA Working Group (including Casper F. M. Franssen, Marc H. Hemmelder, Luuk B. Hilbrands, Kitty J. Jager and Ron T. Gansevoort) and the members of the ERACODA Advisory Board (including Daniel Abramowicz, Carlo Basile, Adrian Covic, Marta Crespo, Ziad A. Massy, Sandip Mitra and J. Emilio Sanchez).
Contributor Information
ERACODA Working Group:
Casper F M Franssen, Marc H Hemmelder, Luuk B Hilbrands, Kitty J Jager, Ron T Gansevoort, Daniel Abramowicz, Carlo Basile, Adrian Covic, Marta Crespo, Ziad A Massy, Sandip Mitra, and J Emilio Sanchez
REFERENCES
- 1. Docherty AB, Harrison EM, Green CA; ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020; 369: m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062 (erratum in Lancet 2020; 395: 1038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng Y, Luo R, Wang K. et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Liang WH, Zhao Y. et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020; 55: 2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akalin E, Azzi Y, Bartash R. et al. Covid-19 and kidney transplantation. N Engl J Med 2020; 382: 2475–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vabret N, Britton GJ, Gruber C. et al. Immunology of COVID-19: current state of the science. Immunity 2020; 52: 910–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blanco-Melo D, Nilsson-Payant BE, Liu W-C. et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181: 1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Russell B, Moss C, George G. et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. Ecancer 2020; 14: 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rockwood K, Song X, MacKnight C. et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005; 173: 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zoccali C, Blankestijn PJ, Bruchfeld A. et al. Children of a lesser god: exclusion of chronic kidney disease patients from clinical trials. Nephrol Dial Transplant 2019; 34: 1112–1114 [DOI] [PubMed] [Google Scholar]