Abstract
Background
The course of disease in mild and moderate COVID-19 has many implications for mobile patients, such as the risk of spread of the infection, precautions taken, and investigations targeted at preventing transmission.
Methods
Three hundred thirty-one adults were hospitalized from January 21 to February 22, 2020, and classified as severe (10%) or critical (4.8%) cases; 1.5% died. Two hundred eighty-two (85.2%) mild or moderate cases were admitted to regular wards. Epidemiological, demographic, clinical, chest computed tomography (CT) scan, laboratory, treatment, and outcome data from patient records were analyzed retrospectively.
Results
Patients were symptomatic for 9.82±5.75 (1–37) days. Pulmonary involvement was demonstrated on a chest CT scan in 97.9% of cases. It took 16.81±8.54 (3–49) days from the appearance of the first symptom until 274 patients tested virus-negative in naso- and oropharyngeal (NP) swabs, blood, urine, and stool, and 234 (83%) patients were asymptomatic for 9.09±7.82 (1–44) days. Subsequently, 131 patients were discharged. One hundred sixty-nine remained in the hospital; these patients tested virus-free and were clinically asymptomatic because of widespread persisting or increasing pulmonary infiltrates. Hospitalization took 16.24±7.57 (2–47) days; the time interval from the first symptom to discharge was 21.37±7.85 (3–52) days.
Conclusions
With an asymptomatic phase, disease courses are unexpectedly long until the stage of virus negativity. NP swabs are not reliable in the later stages of COVID-19. Pneumonia outlasts virus-positive tests if sputum is not acquired. Imminent pulmonary fibrosis in high-risk groups demands follow-up examinations. Investigation of promising antiviral agents should heed the specific needs of mild and moderate COVID-19 patients.
Keywords: Cohort study, coronavirus disease 2019 (COVID-19), lopinavir/ritonavir, Shufeng Jiedu, umifenovir
Beginning at the end of 2019, the new coronavirus pneumonia COVID-19 spread from Wuhan [1, 2] to the rest of Mainland China within 30 days [3]. By March 11, 2020, it was declared a pandemic [4], demonstrating its high infectiousness. The Coronavirus Study Group named it SARS-CoV-2 [5], but Chinese virologists recommended the name HoV-19 because it is distinct from SARS-CoV [6]. Fever, cough, sputum, and fatigue are the most prominent symptoms. Upper respiratory tract symptoms are infrequent [7, 8]. To date, the mortality rate is lower than the prior known coronavirus diseases SARS and MERS [9]. Mild or moderate COVID-19 represents the majority (81%) of cases [10]. However, the course of infection is severe in 14% and critical in 5% of cases [10]. The classification [11] is shown in Table 1.
Table 1.
Classification of Adult Cases With COVID-19 (11)
| Classification | Characteristics |
|---|---|
| Mild | Clinical symptoms mild |
| No sign of pneumonia on imaging | |
| Moderate | Radiological findings of pneumonia fever and respiratory symptoms |
| Severe | Oxygen saturation ≤93% at rest |
| Arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) | |
| ≦300 mmHg (l mmHg = 0.133 kPa) | |
| Obvious lesion progression in chest images within 24–48 h >50% | |
| Critical | Respiratory failure and requiring mechanical ventilation |
| Shock with another organ failure that requires ICU care |
Abbreviation: ICU, intensive care unit.
Pneumonia is common in COVID-19 and can occur even in patients with few symptoms. Some studies have found viral pneumonia in 100% of all symptomatic patients [12] and even in 50% of asymptomatic cases [13]. On a chest CT scan, COVID-19 is characterized by rapid evolution from focal unilateral to diffuse bilateral ground-glass opacities that progress to or coexist with consolidations within 1–3 weeks [14].
Treatments in this study population were based on recommendations released by the General Office of the Chinese National Health Commission [11]. Lopinavir/ritonavir is an HIV medication that has been effective for both in vitro and clinical studies against SARS [15]. The antimalarial substance chloroquine and the antiviral influenza medication umifenovir were found to be effective in vitro against SARS-COV-2 [16]. ShuFengJieDu (SFJD) is the first choice herbal medicine against influenza in China. It can alleviate acute lung inflammatory injury [17] and protect against it by suppressing the MAPK/NF-κB pathway [18] in rodents.
Many studies have focused on high-risk patients who have higher mortality rates [19]; they have also focused on intensive care management [20], special groups [7], or comorbidities [9]. This study focuses on the course of mild and moderate cases because these represent the majority of mobile patients who have a high risk of transmission of the disease. The analyzed data set of this study is unique because it was acquired in an early phase of the epidemic where there was the capacity to hospitalize patients with low-grade symptoms. With the worldwide spread of the disease, these patients are now candidates for home quarantine. Hence, defining disease stages and the identifying stages’ determining factors are instructive for the definition of standards for home quarantine. The necessary clinical and laboratory observations are required, and, for personal safety, adequate protective measures must be implemented to prevent further spread of the disease. So far, less attention has been focused on the follow-up of clinically recovered patients.
METHODS
We collected data from 331 adults with confirmed COVID-19 who were hospitalized at the Shanghai Public Health Clinic Center from January 21 to February 22, 2020. The cohort study protocol was approved by the Shanghai Public Health Clinic Center Ethics Committee (YJ-2020-S015–01), and written informed consent was obtained. Patients were classified as mild, moderate, severe, or critically ill [11]. Thirty-three (10%) of these patients were of the severe type. Sixteen patients (4.8%), of whom 5 patients (1.5%) died, were critically ill and had to be treated in the intensive care unit. The data of 282 (85.2%) patients submitted to regular care inpatient wards with mild (n = 6, 1.8%) or moderate (n = 276, 83.1%) symptoms on admission were evaluated.
Data Collection
We obtained epidemiological, demographic, clinical, laboratory, chest CT scan, and outcome data from patients’ medical records (Centers for Disease Control and Prevention [CDC] reporting forms). If the epidemiological data were not clear enough, a telephone or face-to-face interview with patients or contacts was conducted. Patients were followed up for clinical outcomes through March 24, 2020. Laboratory confirmation of COVID-19 was done by the Shanghai CDC. Clinical symptoms were monitored daily by the attending physicians. Blood tests, including white blood cell count (WBC), platelets, hemoglobin, alanine aminotransferase (ALT), asperatate aminotransferase (AST), total bilirubin, direct bilirubin, blood urinary nitrogen, creatinine, CD4, CD8, CD4/CD8 ratio, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin (PCT), were taken at admission and before discharge.
COVID-19 Detection
Nasopharyngeal swabs, oropharyngeal swabs, urine, stool (once every 3 days), serum (2 samples on admission and 2–4 weeks after the acute phase) of all patients were collected to do viral nucleic acid analysis by real-time reverse transcriptase polymerase chain reaction (rRT-PCR) until 2 successive (>24 hours) negative results were obtained in all tests. Procedures were performed in accordance with World Health Organization guidance [21]. Negative rRT-PCR results from at least 2 consecutive sets of nasopharyngeal and throat swabs (collected at least 24 hours apart from a patient with COVID-19) were needed before discontinuing transmission-based precautions. Four negative specimens are needed to meet this requirement [21]. COVID-19 was confirmed by real-time rT-PCR using a protocol based on the recommendation given by the National Institute for Viral Disease Control and Prevention (China).
Chest CT Scans
All CT scans were performed with a 64-section scanner (SCENARIA, Hitachi Medical, Hitachi, Tokyo, Japan) without using contrast material. The parameters were as follows: tube voltage, 120 kV; automatic tube current, 180 mA–400 mA; iterative reconstruction technique; detector, 64 mm; rotation time, 0.35 seconds; section thickness, 5 mm; collimation, 0.625 mm; pitch, 1.5; matrix, 512 × 512; mediastinal window with a window width of 350 HU and a window level of 40 HU; lung window with a width of 1200 HU and a level of −600 HU. Patients were in the supine position—at full inhalation breath hold—consistent with prior studies on COVID-19 [22]. The chest CT scan pictures were evaluated by 3 radiologists with ~6–32 years’ experience in thoracic imaging evaluation, especially in the diagnosis of viral pneumonias, such as H1N1 and H7N9 pneumonia. CT scans were performed at admission and before discharge.
Treatment
Antiviral therapy during the intervention period was as follows: Umifenovir tablets (Arbidol, Ouyi Pharmaceutical Co., Ltd. of Shiyao group, 0.2 g, 3 times daily) or lopinavir/ritonavir tablets (Abbott, Germany, 2 tablets, twice a day), hydroxychloroquine (Shanghai Zhongxi Sanwei Pharmaceutical Co., Ltd., 4 tablets, once daily) or SFJD capsules (Anhui Jiren Pharmaceutical Co., Ltd., 2.08 g, 3 times daily), darunavir and cobicistat tablets (Janssen Ortho, LLC, 1 tablet, once daily). Supportive medications included vitamin C (Tianjin Lisheng Pharmaceutical Co., Ltd, 2 tablets, 3 times daily).
Data Analysis and Statistics
Statistical analysis was performed using OriginPro 9.0 (OriginLab Corporation, Northampton, USA). Data were presented as mean ± SD. Significant differences between the means were determined using the Student t test and Wilcoxon test. P values <.05 were considered significant.
RESULTS
Baseline Characteristics
The study consisted of 141 female patients (50%) and 141 males (50%). The mean age was 48.23±15.55 (15–85) years, which was younger in comparison with the severe and critical cases: 59.78±15.42 (25–88) years (t = 4.8335; P < .001). In addition, 109 (39.7%) patients were suffering from chronic disease: cardiovascular disease (n = 62), endocrine disease (n = 28), chronic respiratory disease (n = 5), cancer (n = 2), chronic abdominal disease (n = 9), and chronic hepatitis B (n = 3). Their course of COVID-19 did not differ statistically from patients without chronic disease in terms of duration of hospitalization or duration of symptoms, nor did patients have a more severe form of pneumonia.
Clinical Symptoms
Patients had a fever (n = 244, 86.5 %) with a mean duration of 7.51±3.08 (1–17) days. The mean maximum temperature was 38.23°C±0.57°C (37.4°C–40°C). Pulmonary symptoms like cough, shortness of breath, and tightness in the chest (n = 171, 60.6%) had a mean duration of 7.24±4.10 (1–20) days. Expectoration of sputum was present in 40.1% of the cases (n = 113), with a mean duration of 6.18±4.04 (1–17) days. Fatigue or dizziness (n = 106, 36.3%) persisted for 5.93±3.39 (1–14) days. Minor symptoms were headache or muscle pains (n = 61, 21.6%), upper respiratory tract symptoms like rhinorrhea and pharyngalgia (n = 33, 17%), and abdominal symptoms like diarrhea, constipation, and poor appetite (n = 50, 17.7%). In summary, the 282 patients suffered from at least 1 of the above-mentioned symptoms for 9.82±5.75 (1–37) symptomatic days.
Laboratory Results
The mean CRP was 16.91±21.40 (0.5–112) mg/L (normal value, <3 mg/L) with a value >50 mg/L in 25 cases on admission. CRP decreased in all cases to 1.39±2.63 (0.5–30.74) mg/L at discharge (t = 4.4867; P < .001). The ESR (normal level ≥15 male, ≥20 female) was pathological in 238 (84.4%) patients on admission (62.37±37.68; 4–140) and in 197 (68.8%) patients on discharge (50.23±35.11; 2–140). All other blood parameters (WBC, platelets, hemoglobin, PCT, ALT, AST, total bilirubin, direct bilirubin, blood urinary nitrogen, creatinine, CD4, CD8, CD4/CD8 ratio) showed no significant mean difference from admission to discharge.
Chest CT Scan
On a chest CT scan, 275 patients (97.5%) showed COVID-19-associated pneumonia; 57 patients (20.2%) had infiltrates in 1 lobe of 1 lung, 9 patients (3.2%) had infiltrates in 2 lobes of 1 lung, 25 patients (8.9%) had infiltrates in 2 lobes in both lungs, and 30 patients (10.6%) had infiltrates in 3 or more lobes in 2 lungs; 154 (54.6%) patients had diffuse infiltrations of both lungs. Seven patients (2.5%) showed no infiltrates.
The lesions were characterized as bullae (n = 1), patch shadow (n = 8, 2.8%), or nodules (n = 9, 3.2%). Typical ground-glass opacities were seen in 256 patients (90.8%); in 215 (76.2%) as the only characteristic lesion; in 3 (1.1%) cases with beginning consolidation fibrosis; in 2 (0.7%) patients in combination with pleural effusion; and in 36 (12.8%) in combination with consolidation fibrosis and pleural effusion. Two (0.7%) patients had pleural effusion alone. Six (2.1%) patients had no lesions.
In 162 patients, a prolonged hospitalization (7.38±3.42; 3–26) was needed; 143 patients still had signs of widespread pneumonia with only minimal signs of absorption on chest CT scan, and 20 patients had increasing pulmonary infiltrates. On discharge, 272 patients still had detectable lesions on chest CT scans, though the absorption of pulmonary lesions had started. Table 2 summarizes the chest CT scan findings. Supplementary Figures 1 and 2 show chest CT scans in the course of the disease in 2 cases.
Table 2.
Chest CT Scan Findings of 282 COVID-19 Patients With Mild and Moderate Course
| Chest CT Scan Findings | No. | % |
|---|---|---|
| Location of infiltrates | ||
| No infiltrates | 7 | 2.5 |
| One lobe in 1 lung | 57 | 20.2 |
| Two lobes in 1 lung | 9 | 3.2 |
| Two lobes in both lungs | 25 | 8.9 |
| Three lobes in both lungs | 30 | 10.6 |
| Diffuse infiltrations in both lungs | 154 | 54.6 |
| Characteristics of lesions | ||
| None | 6 | 2.1 |
| Bullae | 1 | 0.4 |
| Patch shadow | 8 | 2.8 |
| Nodules | 9 | 3.2 |
| Ground-glass opacity | 215 | 76.2 |
| Ground-glass opacity, and consolidation fibrosis | 3 | 1.1 |
| Ground-glass opacity, and pleural effusion | 2 | 0.7 |
| Ground-glass opacity, pleural effusion, and consolidation fibrosis | 36 | 12.8 |
| Pleural effusion | 2 | 0.7 |
| Outcome | ||
| No pneumonia during the whole course of infection | 6 | 2.1 |
| Completely cured on discharge | 4 | 1.4 |
| Residual infiltrates without fibrosis | 233 | 82.6 |
| Residual infiltrates and, consolidation fibrosis | 39 | 13.8 |
Abbreviation: CT, computed tomography.
Stages of the Disease
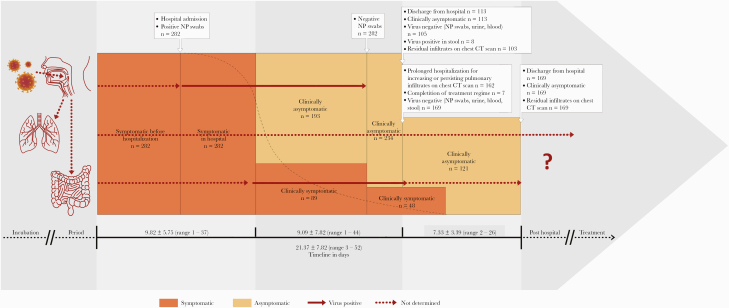
All patients had symptoms for a mean time of 5.13±3.66 (1–23) days before hospital admission. In the hospital, their clinical symptoms persisted for another 4.69±4.15 (0–28) days. Together, the patients had clinical symptoms for 9.82±5.75 (1–37) days. The time of admission to negative results in nasoand oropharyngeal (NP) swabs was 8.18±6.86 (2–35) days. The time of first symptom to negative NP swaps was 13.14±7.45 (2–41) days. When the NP swabs were negative, 193 patients (68.4%) had been asymptomatic for a mean time of 7.84±6.96 (1–32) days; 89 (31.6%) patients had been symptomatic for 2.15±2.92 (1–17) days. Despite negative NP swabs, the virus was still present in stool, blood, or urine for another 3.40±4.85 (0–40) days. It took a mean time of 11.58±7.99 (2–47) days from admission before 274 (97.2%) patients showed negative results in all virus tests, which was 16.81±8.54 (3–49) days from the time of the first symptoms. When all tests were negative, 234 patients (83%) had been asymptomatic for a mean time of 9.09±7.82 (1–44) days. When negative test results were confirmed, 113 (40.1%) patients were discharged from the hospital. Eight patients, however, still showed positive results for the virus in stool, and 169 (59.9%) patients, though virus-free, remained in the hospital for another 7.33±3.39 (3–26) days. This decision was made for pulmonary reasons in 162 cases and was based on the evaluation of chest CT scans. At this point, 48 (17%) patients were still symptomatic for another 1.81±2.46 (1–11) days. Seven patients, whose treatment regime had been modified, stayed in the hospital to complete the course of treatment.
In summary, it took a mean time of 16.81±8.43 (3–49) days from detection of the first symptom(s) to virus-negative results in all fluids. The mean length of hospitalization was 16.24±7.57 (2–47) days, and the mean time from first symptom to discharge was 21.37±7.85 (3–52) days. Figure 1 describes the course of COVID-19 infection, and Table 3 compares the duration of each period.
Figure 1.
Timeline of the course of mild and moderate COVID-19 cases. Abbreviations: CT, computed tomography; NP, naso- and oropharyngeal swab.
Table 3.
Time Course of Coronavirus Infection in Mild and Moderate Cases
| Period | No. | Days | ± | Range |
|---|---|---|---|---|
| Incubation period | Unknown | |||
| Symptomatic period | 282 | 9.82 | 5.75 | 1–37 |
| Asymptomatic period before virus-negative in NP swabs | 192 | 7.84 | 6.96 | 1–33 |
| Asymptomatic period before virus-negative in NP swabs, blood, urine, and stool | 234 | 9.09 | 7.82 | 1–44 |
| From first symptom to virus-negative NP swabs | 282 | 13.14 | 7.45 | 2–41 |
| From first symptom to virus-negative in NP swabs, blood, urine, and stool | 274 | 16.81 | 8.43 | 3–49 |
| Virus still positive in stool after dismission day | 8 | Unknown | ||
| Hospitalization | 282 | 16.24 | 7.57 | 2–47 |
| Prolonged hospitalization after virus was negative | 169 | 7.33 | 3.39 | 3–26 |
| From first symptom to hospital dismission | 282 | 21.37 | 7.85 | 9–52 |
| Treatment necessity after hospital discharge | Unknown |
Abbreviation: NP, naso- and oropharyngeal swab.
Treatment
One hundred forty-eight patients received antiviral monotherapy (umifenovir n = 50, lopinavir/ritonavir tablets n = 63, hydroychloroquine n = 16, darunavir n = 13 SFJD n = 6). In 48 cases, 2 therapies were combined (umifenovir plus SFJD n = 17, lopinavir/ritonavir tablets plus umifenovir n = 18, umifenovir plus hydroxychloroquine n = 7, lopinavir/ritonavir plus SFJD n = 5, darunavir plus lopinavir/ritonavir n = 1). In 20 cases, 3 drugs were combined (lopinavir/ritonavir plus SFJD plus umifenovir n = 19, umifenovir plus hydroxychloroquine plus SFJD n = 1). Combinations of medications were not used from the beginning, but depending on a patient’s condition, an escalation of therapy could result in a very heterogeneous picture. No statistical difference was found by comparison of the antiviral therapies; 66 patients received no specific antiviral medication. Supportive therapy with vitamin C was used in 59 cases.
DISCUSSION
The results of this study confirm prior reports in relation to symptomatology [8] and case severity as related to age [7]. However, patients had a lower frequency of severe infection (9.9%), and less deadly cases (1.5%) occurred in comparison with prior reports [10]. In contrast to mild and moderate symptomatology, 276 of 282 (97.7%) patients had typical ground-glass opacification with or without consolidative abnormalities on chest CT scans, representing viral pneumonia [12, 14, 23]. However, pleural effusions were common (13.5%), which had been previously described as rare (4%) [11, 24].
The course of COVID-19 is determined by the following characteristics: the incubation period, the symptomatic phase, an asymptomatic phase until virus detection becomes negative, and by the features of disease activity on chest CT scans. Despite the low severity of the described cases, the course of the disease was remarkably long.
The incubation period was not determined, but other studies estimated it to be from 2 to 24 days [25–27].
The symptomatic phase was 9.82±5.75 (1–37) days. Most cases showed mild symptoms, so COVID-19 may be misdiagnosed as a common cold. Furthermore, virus transmission by asymptomatic [13, 25] or presymptomatic persons is possible [28]. Hence, the threshold for suspicious cases should be low, and virus testing should become extensive.
When NP swabs were reported as virus-negative, 68.4% of the patients were already asymptomatic for 7.84±6.96 (1–32) days. So far, this observation has only been reported in a few cases [29]. This raises the question of whether patients can still transmit the disease in this long asymptomatic phase. Transmission mainly occurs via respiratory droplets when a person with infection coughs or sneezes [30]. Hence, the risk of transmission should be lower in the asymptomatic phase. However, as long as NP swabs are still positive, patients’ secretions from mouth and nose are still potentially infectious and a patient can by touching contaminate their immediate surroundings. Similar to primary asymptomatic transmission [25], secondary asymptomatic patients whose NP swabs are virus-positive can transmit the virus from their breath while talking [30]. The virus is stable in aerosols for many hours [31], but most likely can be impeded by simple surgical masks, as shown for other viruses [32].
In contrast, 31.6% of the patients still showed symptoms despite negative tests, which might be explained by nondetected viral activity in the lungs.
Generally, the virus is first detected in NP swabs. Later it passes to the lungs, where virus concentration is generally higher [28]. None of the classic laboratory tests reliably determine the actual state of the disease. CRP was mildly elevated and compatible with a virus infection on admission, which decreased until discharge as expected. ESR, an unspecific marker of inflammation, was high on admission and persisted until discharge in 68.8% patients, despite virus negativity as a possible sign of ongoing inflammation. However, typical chest CT scan features can be found even before positive NP swabs [33]. Furthermore, 162 patients showed persisting signs of widespread pneumonia in chest CT scans after 16.84±8.43 days, in spite of negative virus tests. In 20 (12%) virus-negative patients, their infiltrates even increased at this point, which was observed in only 3.5% previously [34]. These features can be signs of postinfectious lung damage. However, these infiltrates can also represent signs of persisting active viral pneumonia, because some NP-negative patients can still have positive sputum findings even after inducing expectoration [35]. Hence, patients should wear masks, especially when expectorants are prescribed. Thus, negative NP swabs do not mark the end of the disease.
A shift from a positive NP swab to positivity in feces during late infection was observed. Hence, NP swabs become unreliable during the course of the disease; 274 patients were asymptomatic for 9.98±7.68 (1–30) days until discharge, before the virus became negative in stool. However, 8 patients had positive virus tests in stool on discharge. Viral loads in feces are generally lower compared with respiratory samples [36]. However, the level of infection in feces is not clear [37]; furthermore, live viruses have been detected in the feces of asymptomatic patients [38]. Hence, it is important to base the decision of ending hospitalization only on negative NP swabs, because patients may still shed the virus by the oral–fecal route [38]. Asymptomatic virus-positive patients should wear medical masks. In addition, the practice of rigorous hygiene [39] is mandatory during home quarantine until all virus tests are negative to protect household members.
Consolidation fibrosis was seen in 13.8% of patients on discharge, with an unknown risk of chronic lung disease. COVID-19 imaging features overlap with SARS and MERS [40]. Transient reticular opacities were observed in SARS and MERS lasting for months [40]. In some cases, recovery and consolidation of lung fibrosis took up to 2 years in SARS [41], and fibrosis often became a chronic problem in MERS [42]. Hence, follow-up chest CT scans in patients recovering from COVID-19 should be considered, especially in patients who remain symptomatic or had severe disease, in order to evaluate disease activity and long-term or even permanent pulmonary damage, including fibrosis, as seen in SARS and MERS.
The development and approval of new agents can take time, so it is essential to assess the effectiveness of approved existing drugs. The therapies used in this study [11] were grounded on basic research data and experience with drugs in other infectious diseases. Physicians chose polypragmatic treatments, partly combining drugs for clinical reasons. Hence, the collected data do not allow therapeutic recommendations. Meanwhile, the drugs used have to be evaluated. Lopinavir/ritonavir and umifenovir did not show clinical effectiveness [43, 44]. Clinical pilot studies showed that SFJD might, on its first appearance, positively influence symptoms and time to virus negativity [45, 46], and 1 ingredient of SFJD taxifolin [47] showed high positive binding at the main protease of the virus. The role of hydroxychloroquine remains a matter of debate. A single study showed positive results [48], while large observational studies showed no effectiveness [49], indicating the need for further confirmation. Furthermore, the drug has potential detrimental effects [50].
While moderate and mild cases represent the absolute majority of cases, studies should also focus on the development of well-tolerated drugs that influence symptoms and the disease course of mild and moderate patients in order to avoid long-term pulmonary damage.
This study had certain limitations. Data analysis was retrospective and did not fulfill the criteria of a randomized controlled study. However, all patients had similar severity of the disease, so examination of real-life situations provided useful information. Furthermore, we did not get results from bronchoalveolar fluids. Testing was usually limited to patients with severe illness or those undergoing mechanical ventilation [51].
In conclusion, the 21.37±7.85 (3–52)-day-long course from first symptom(s) to hospital dismission of mild and moderate cases of COVID-19 is unexpectedly long and has potential pitfalls. The asymptomatic phase until virus negativity is problematic, with an associated unclear level of infection. While the virus moves quickly from the NP region to the lungs and intestines, detection in NP swabs is not reliable in later stages of the disease, and potential infectiousness from stool or expectoration has to be further evaluated. Relatively mild symptoms obscure the fact that these patients mostly suffer from viral pneumonia. Increased pulmonary infiltrates in 12.3% of asymptomatic patients detected in chest CT scans after virus negativity can be signs of either postinfectious lung damage or a nondetected continuation of pulmonary virus activity. This underlines the relevance of chest CT scan follow-up examinations, because disease activity can otherwise only be determined by invasive sample collections of sputum. In times of an exploding pandemic, mild and moderate cases of COVID may be sent home to quarantine. However, evaluation and close monitoring, the definition of parameters for hospitalization, hygienic measures, and testing frequencies are also essential. Furthermore, possible long-term pulmonary damage like fibrosis demands follow-up examinations. Promising antiviral agents should be investigated in clinical trials as soon as possible. These trials should consider the concerns of patients affected by mild and moderate COVID-19.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Lenja Schröder for figure editing; Elisabeth Buhlmann, MD, Gesa Meyer-Hamme, MD, Ulrike Baumann-Schröder, MD, Joana Schröder, cand. med., for the data analysis discussion; Nils Muiznieks, PhD, for language editing. They were not compensated for their contributions.
Financial support. “Novel Coronavirus Pneumonia Innovative Treatment Regimen Research of Shanghai Science and Technology Commission Second Batch of Emergency Science and Technology Tackling Key Project” (20411950200); “Chinese Medicine Administration of Shanghai Health Protection Committee, Chinese Medicine Prevention and Treatment of Novel Coronavirus Pneumonia Emergency Special Subject, Chinese Medicine Treatment New Coronavirus Pneumonitis Real World Clinical Observation Research” (2020NCP001); “Shanghai Major Projects on Infectious Diseases” (shslczdzk01102); “Shanghai ‘Rising Stars of Medical Talent’ Youth Development Program, Specialist Program” (No. 2019–72); “HanseMerkur Insurance Group (HMZTCM-C19-2020-1).”
Role of the funder/sponsor. The funders had no role in the design or conduct of the study; in the collection, management, analysis, or interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision(s) to submit the manuscript for publication.
Potential conflicts of interest. None reported. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. Lu Xia and Jun Chen had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Hongzhou Lu and Sven Schröder are co–senior authors, Lu Xia and Jun Chen are co–first authors. Concept and design: Hongzhou Lu, Sven Schröder. Acquisition, analysis, or interpretation of data: Lu Xia, Jun Chen, Thomas Friedemann, Zongguo Yang, Yun Ling, Xuhui Liu, Shuihua Lu, Tao Li, Zhigang Song, Wei Huang, Yunfei Lu, Sven Schröder, Hongzhou Lu. Drafting of the manuscript: Sven Schröder, Thomas Friedemann, Lu Xia. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Friedemann. Obtained funding: Hongzhou Lu, Sven Schröder. Administrative, technical, or material support: Hongzhou Lu, Sven Schröder. Supervision: Hongzhou Lu and Sven Schröder.
Ethical approval. The study protocol was approved by the SPHCC Ethics Committee (YJ-2020-S015–01).
References
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020; 382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu H, Stratton CW, Tang Y. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol 2020; 92:401–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tian S, Hu N, Lou J, et al. Characteristics of COVID-19 infection in Beijing. J Infect 2020; 80:P401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020.2020 Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed 15 March 2020.
- 5. Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020; 5:536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang S, Shi Z, Shu Y, et al. A distinct name is needed for the new coronavirus. Lancet 2020; 395:949–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Luca D. Managing neonates with respiratory failure due to SARS-CoV-2. Lancet Child Adolesc Heal 2020; 4:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang T, Du Z, Zhu F, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 2020; 395:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 11. General Office of Chinese National Health Commission. Diagnosis and treatment protocol for novel coronavirus pneumonia trial version 7. Chinese, English translation organized by WHO China Office.2020 Available at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf. Accessed 13 March 2020.
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci 2020; 63:706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu CM. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax 2004; 59:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther 2020; 14(1):58–60. [DOI] [PubMed] [Google Scholar]
- 17. Yuan Y, Liao Q, Xue M, et al. Shufeng Jiedu capsules alleviate lipopolysaccharide-induced acute lung inflammatory injury via activation of GPR18 by verbenalin. Cell Physiol Biochem 2018; 50:629–39. [DOI] [PubMed] [Google Scholar]
- 18. Tao Z, Gao J, Zhang G, et al. Shufeng Jiedu capsule protect against acute lung injury by suppressing the MAPK/NF-κB pathway. Biosci Trends 2014; 8:45–51. [DOI] [PubMed] [Google Scholar]
- 19. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020; 8:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization. Laboratory testing of human suspected cases of novel coronavirus (NCoV) infection.2020. Available at: https://apps.who.int/iris/bitstream/handle/10665/330374/WHO-2019-nCoV-laboratory-2020.1-eng.pdf?sequence=1&isAllowed=y. Accessed 19 April 2020.
- 22. Song F, Shi N, Shan F, et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020; 295:210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am J Roentgenol 2020; 214:1072–7. [DOI] [PubMed] [Google Scholar]
- 24. Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology 2020; 296:E46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bai Y, Yao L, Wei T, et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020; 323:1406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Situation report-7. Available at: https://www.who.int/publications-detail/global-surveillance-for-. Accessed 7 March 2020.
- 27. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. New Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 2020; 20:411–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang D, Mo G, Yuan X, et al. Time kinetics of viral clearance and resolution of symptoms in novel coronavirus infection. Am J Respir Crit Care Med 2020; 201:1150–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020; 382:1564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 2020; 26:676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology 2020; 296:E41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; 296:E32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Han H, Luo Q, Mo F, et al. SARS-CoV-2 RNA more readily detected in induced sputum than in throat swabs of convalescent COVID-19 patients. Lancet Infect Dis 2020; 20:655–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ 2020; 369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020; 5:434–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 2020; 9:386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. World Health Organization. Home care for patients with suspected novel coronavirus (nCoV) infection presenting with mild symptoms and management of contacts.2020 Available at: https://www.who.int/publications-detail/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts. Accessed 14 March 2020.
- 40. Hosseiny M, Kooraki S, Gholamrezanezhad A, Reddy S, Myers L. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. Am J Roentgenol 2020; 214(5):1078–82. [DOI] [PubMed] [Google Scholar]
- 41. Zhang P, Li J, Liu H, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res 2020; 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Das KM, Lee EY, Singh R, et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging 2017; 27:342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen J, Ling Y, Xi X, et al. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Chinese J Infect Dis 2020; 38:E008. [Google Scholar]
- 44. Cao B, Zhang D, Wang C. A trial of lopinavir-ritonavir in COVID-19. Reply. N Engl J Med 2020; 382:e68. [DOI] [PubMed] [Google Scholar]
- 45. Qi X, Wu J, Jiang Y, et al. Analysis of the value of Shufeng Jiedu capsules combined with Abidol in the treatment of mild COVID-2019 (Chinese). 2020. Available at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&filename=ZYJZ202005002&dbname=CJFDAUTO. Accessed 19 April 2020.
- 46. Qu X, Hao S, Ma J, et al. Observation on the clinical effect of Shufeng Jiedu capsule combined with arbidol hydrochloride capsules in the treatment of COVID-19. 2020. Available at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFQ&dbname=CJFDLAST2020&filename=ZCYO202005012&v=MDAwNDFyQ1VSN3FmWWVSdUZpdmhWTC9PUHk3U1liRzRITkhNcW85RVpvUjhlWDFMdXhZUzdEaDFUM3FUcldNMUY=. Accessed 19 April 2020.
- 47. Fischer A, Sellner M, Neranjan S, Lill MA, Smieško M. Inhibitors for novel coronavirus protease identified by virtual screening of 687 million compounds. Int J Mol Sci 2020; 21:3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents 2020; 55:105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med 2020; 382:2411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Touret F, de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res 2020; 177:104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323:1843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.