Abstract
Background
Kidney graft recipients receiving immunosuppressive therapy may be at heightened risk for coronavirus disease 2019 (Covid-19) and adverse outcomes. It is therefore important to characterize the clinical course and outcome of Covid-19 in this population and identify safe therapeutic strategies.
Methods
We performed a retrospective chart review of 73 adult kidney graft recipients evaluated for Covid-19 from 13 March to 20 April 2020. Primary outcomes included recovery from symptoms, acute kidney injury, graft failure and case fatality rate.
Results
Of the 73 patients screened, 54 tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—39 with moderate to severe symptoms requiring hospital admission and 15 with mild symptoms managed in the ambulatory setting. Hospitalized patients were more likely to be male, of Hispanic ethnicity and to have cardiovascular disease. In the hospitalized group, tacrolimus dosage was reduced in 46% of patients and mycophenolate mofetil (MMF) therapy was stopped in 61% of patients. None of the ambulatory patients had tacrolimus reduction or discontinuation of MMF. Azithromycin or doxycycline was prescribed at a similar rate among hospitalized and ambulatory patients (38% versus 40%). Hydroxychloroquine was prescribed in 79% of hospitalized patients. Graft failure requiring hemodialysis occurred in 3 of 39 hospitalized patients (8%) and 7 patients died, resulting in a case fatality rate of 13% among Covid-19-positive patients and 18% among hospitalized Covid-19-positive patients.
Conclusions
Data from our study suggest that a strategy of systematic triage to outpatient or inpatient care, early management of concurrent bacterial infections and judicious adjustment of immunosuppressive drugs rather than cessation is feasible in kidney transplant recipients with Covid-19.
Keywords: immunosuppression, kidney transplantation, SARS-CoV-2
KEY LEARNING POINTS
What is already known about this subject?
to date, there have been limited case series describing only small numbers of kidney transplant recipients with coronavirus disease 2019 (Covid-19) with incomplete follow-up;
our transplant center, located in New York City, the epicenter of Covid-19, represents the largest US community affected as of April 20, 2020;
data are lacking on graft and patient outcomes in kidney transplant recipients as well as strategies for immunosuppression management in the setting of Covid-19 illness.
What this study adds?
this study suggests that a structured outpatient evaluation can differentiate kidney transplant recipients who can be successfully managed as outpatients versus those that require hospitalization; and
although immunosuppressive medications may require adjustment, complete cessation of immunosuppression is not necessary for all kidney transplant recipients with Covid-19 infection.
What impact this may have on practice or policy?
ambulatory evaluation and monitoring of kidney transplant recipients with Covid-19 are feasible for those without hypoxia and without concurrent bacterial infection;
immunosuppressive therapies can be continued in kidney transplant recipients with Covid-19 diagnosis; and
all kidney transplant recipients with Covid-19 should be evaluated for concurrent bacterial infections and acute kidney injury.
INTRODUCTION
With the emergence of coronavirus disease 2019 (Covid-19) as a global pandemic, there are justifiable concerns regarding immunosuppressed organ graft recipients being at an increased risk for both contracting the virus and for adverse outcomes including death following the infection. Yet as we learn more about the virus and its mechanisms of injury, many questions remain about immunosuppressed solid organ transplant recipients, including whether a reflex reduction in immunosuppressive therapy is an appropriate management strategy. This is in part because a large study screening existing US Food and Drug Administration approved drugs for repurposing as antiviral therapeutics reported three different immunosuppressive agents widely used in transplant recipients—tacrolimus, mycophenolate and sirolimus—as potential therapies for Covid-19 [1]. Moreover, in vitro studies have demonstrated that mycophenolic acid has activity against other coronaviruses, namely Middle East respiratory syndrome (MERS)-CoV [2]. It is possible therefore that immunosuppressive agents may actually confer protective effects against this particular virus, yet the general early management strategy within the transplant community has been to reduce or withhold immunosuppression, particularly mycophenolate mofetil (MMF) in transplant recipients testing positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3–6]. It is also unclear whether the cytokine release syndrome, occuring in response to SARS-CoV-2 infection and contribute to acute respiratory distress syndrome, is more likely or less likely to occur in patients who are on immunosuppressive therapy known to block transcriptional elements related to cytokine release. It is therefore critical to understand the clinical course and outcomes in SARS-CoV-2-infected transplant recipients as compared with the general population so that treatment strategies can be optimized.
New York City is currently the Covid-19 epicenter of the world, with the prevalence rates - ranging from 27% to 78% in some of the most affected areas [7]. Since the first report of Covid-19 in five kidney graft recipients by Zhang et al. [3], several case reports of Covid-19 in kidney transplant recipients have been reported [5, 6, 8–13].
At our institution, New York Presbyterian Hospital–Weill Cornell Medicine (NYP-WCM), we developed a systematic approach for the evaluation of patients suspected to be infected with SARS-CoV-2 and a set of criteria for admission. In this report we describe our center’s approach and the characteristics of our first 54 consecutive kidney allograft recipients confirmed to have Covid-19.
MATERIALS AND METHODS
Patient screening and triage
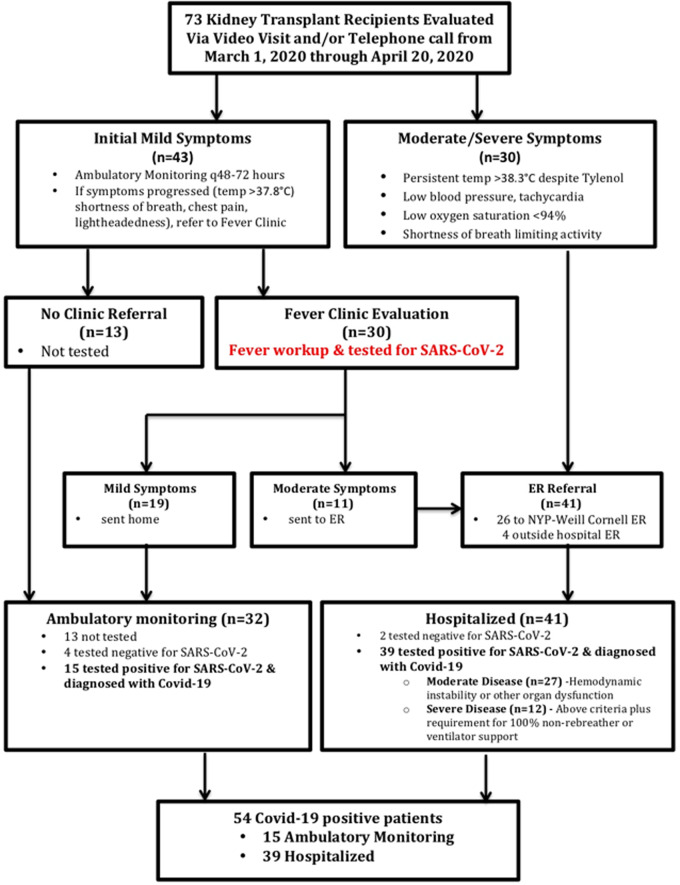
We retrospectively studied adult (age >18 years) recipients of kidney allografts screened for mild, moderate or severe symptoms compatible with Covid-19 diagnosis from 13 March to 20 April 2020. All patients were followed for a minimum of 19 days, with a median follow-up of 37 days, and the data presented here are inclusive of patient follow-up as of 15 May 2020. A total of 73 outpatients reported symptoms suspicious for Covid-19. Each patient was initially evaluated by telemedicine using telephone encounters or video visits. Figure 1 illustrates the stepwise approach that was used to triage the patients. Patients with mild symptoms consisting of low-grade fever (<37.8°C), cough and/or myalgias were followed with sequential telephone encounters every 48–72 h. Patients were advised to self-isolate; monitor their temperature and vitals, including oxygen saturation if monitoring was available to them; wear a face mask; perform hand sanitization frequently and call their transplant care provider if their symptoms worsened. Patients were referred to either the WCM Fever Clinic or the NYP-WCM Emergency Department (ED) based on symptomology. The WCM Fever Clinic is a program initiated by the primary care physicians at NYP-WCM to provide outpatient in-person medical care to patients with symptoms of Covid-19 in a safe, efficient and coordinated manner, while preventing transmission of infection to healthcare workers and other patients. In the WCM Fever Clinic, patients (wearing facemasks) were evaluated in dedicated exam rooms by a physician wearing both an N95 mask and full personal protective equipment and rooms were disinfected after each patient visit. At the WCM Fever Clinic, resources were available to complete a comprehensive evaluation, including onsite phlebotomy, X-ray andelectrocardiogram equipment. Of the 73 patients, 13 patients early in the course of the outbreak had mild symptoms and were not evaluated at the WCM Fever Clinic while 30 were referred to the WCM Fever Clinic and 30 were referred directly to the NYP-WCM ED. The indication for referral to the WCM Fever Clinic included temperature >37.8°C, shortness of breath, productive cough, chest pain and lightheadedness or if symptoms did not improve during the first week. Patients who were referred to the NYP-WCM ED met the criteria for moderate symptoms as defined by persistent temperature ≥38.3°C, hemodynamic disturbance with BP lower than baseline or tachycardia and low oxygen saturation of <94% or shortness of breath that interfered with normal activities (Supplementary data, Figure S1).
FIGURE 1.
Flow chart of outpatientmanagement and referral of patients for ambulatory monitoring, WCM Fever Clinic and/or NYP-WCM ED. Mild symptoms were defined as temperature >37.8°C/100°F, shortness of breath, productive cough, chest pain and/or lightheadedness. Moderate symptoms were defined as persistent temperature ≥38.3°C/101°F, hemodynamic disturbance with systolic blood pressure lower than baseline or tachycardia, oxygen saturation <94% and/or shortness of breath interfering with normal activities. Patients referred to the WCM Fever Clinic who on presentation were found to have moderate symptoms were then sent to the NYP-WCM ED for hospital admission.
All patients presenting to the WCM Fever Clinic had a nasopharyngeal swab specimen collected by trained personnel and the sample was tested for the presence of SARS-CoV-2 via polymerase chain reaction using one of the following tests: cobasSARS-CoV-2 (Roche Diagnostics, Rotkreuz, Switzerland), XpertXpress SARS-CoV-2 (Cepheid, Sunnyvale, CA, USA) or Panther Fusion SARS-CoV-2 (Hologic, Marlborough, MA, USA) assay. Of the 30 patients evaluated in the WCM Fever Clinic, 19 had mild symptoms and were sent home for ambulatory monitoring while 11 patients had moderate symptoms (as defined above) and were sent to the NYP-WCM ED. Of the 19 patients with mild symptoms, 15 tested positive for SARS-CoV-2 and 4 tested negative. Of the 41 patients sent to the ED, 39 tested positive for SARS-CoV-2.
During the initial phase from 8 to 28 March 2020, patients were encouraged to stay at home and continue ambulatory monitoring and there was a high threshold for referring patients for testing and evaluation given the overcrowding in the NYP-WCM ED. After 28 March 2020, our threshold for referral to the WCM Fever Clinic decreased due to increased availability of appointments in the WCM Fever Clinic resulting from increased staffing and availability of outpatient testing for SARS-CoV-2 on our premises. Prior to 28 March 2020, 33% of patients were evaluated in the WCM Fever Clinic, while after 28 March 2020, 53% of patients were evaluated in the WCM Fever Clinic.
Antiviral and antibacterial therapy
Admitted patients were evaluated by both a transplant physician and a transplant infectious disease specialist who guided antibacterial and/or antiviral therapies [including azithromycin, doxycycline and/or hydroxychloroquine (HCQ)]. The choice of additional antibiotics for bacterial pneumonia and/or sepsis was at the discretion of the treating physicians. Our standard protocol for HCQ dosing included 600 mg twice a day × 2 doses, then 400 mg daily for 4 additional days. Patients were evaluated for inclusion in the remdesivir clinic trials and for other experimental therapies [interleukin (IL)-6 receptor antagonist, convalescent plasma].
Data collection
Baseline demographics, comorbidities, transplant details, immunosuppressive therapies, concomitant infections, treatment approaches and clinical course were collected for all patients. Data collected were extracted from the electronic medical records system. Our review was covered by our WCM Institutional Review Board approved protocol #1207012637, Utilizing a Transplant Database for Quality Assessment and Performance Improvement and Clinical Outcomes, that collects data for our kidney transplant recipients for the purposes of quality improvement and clinical research. Procedures followed were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013. Early findings of 12 of the 54 patients included in this series were included in a study by Pereira et al. [13].
Data analysis
We performed a detailed analysis of the 54 patients who tested positive for SARS-CoV-2 and compared the baseline and clinical parameters, including patient and graft outcomes, between those that required hospitalization (n = 39) and those that did not (n = 15). Of those who were hospitalized, we classified those requiring ventilator support or 100% nonrebreather mask as severe (n = 13) and those that remained on room air or required oxygen by nasal cannula as moderate (n = 26) and compared the admission presenting symptoms, laboratory test results and management.
RESULTS
Characteristics of kidney transplant recipients with Covid–19
The first case of Covid-19 in a kidney transplant recipient was diagnosed at our center on 13 March 2020. Figure 2 demonstrates the time course from 8 to 20 April 2020 over which the 54 SARS-CoV-2-positive cases occurred and the cumulative cases over time. The peak of infections occurred during the week of 5–11 April 2020, mirroring the peak of infections seen in New York City.
FIGURE 2.
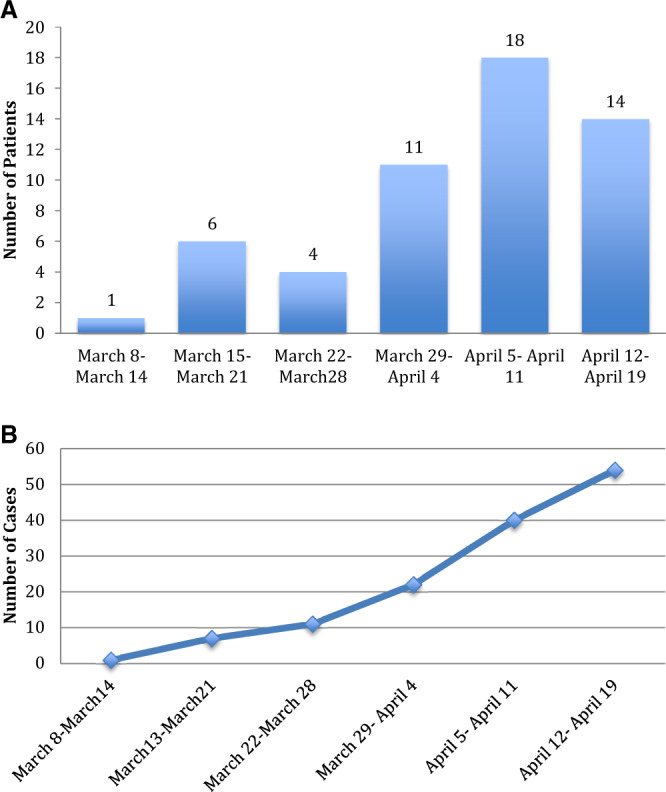
(A) Weekly cases of kidney transplant recipients with Covid-19. The graph displays the number of kidney transplant recipients from our transplant center who received a diagnosis of Covid-19 for each week during the study period. (B) Cumulative cases of kidney transplant recipients with Covid-19. The graph displays the cumulative number of cases for each week during the study period.
Characteristics of all 54 SARS-CoV-2-positive kidney transplant recipients are listed in Table 1 and are separated into a hospitalized cohort (n = 39) and an ambulatory cohort (n = 15). The median age was 57 years in the entire cohort, 59 years in the hospitalized group and 55 years in the ambulatory group. The proportions of male (79% versus 47%) and Hispanic recipients (36% versus 20%) were higher in the hospitalized cohort, while the percentage of Caucasians (40% versus 28%)was higher in the ambulatory group. The ABO blood group type, body mass index (BMI) and smoking history were similar between those hospitalized and those in the ambulatory setting. There was a greater prevalence of cardiovascular disease (41% versus 20%) and pulmonary disease (18% versus 7%) in the hospitalized patients compared with the ambulatory patients. The prevalence of diabetes was similar between the two groups and the cause of end-stage renal disease (ESRD) was also similar between the two groups.
Table 1.
Characteristics of kidney transplant recipients with Covid-19
| Characteristics | Total | Hospitalized | Ambulatory |
|---|---|---|---|
| (N = 54) | (n = 39) | (n = 15) | |
| Age (years), median (range) | 57 (29–83) | 59 (29–83) | 55 (31–73) |
| Sex (male), n (%) | 38 (70) | 31 (79) | 7 (47) |
| Race/ethnicity, n (%) | |||
| Caucasian/White | 17 (31) | 11 (28) | 6 (40) |
| Hispanic | 17(31) | 14(36) | 3 (20) |
| Black/African American | 13 (24) | 26(10) | 3 (20) |
| Asian | 6 (11) | 4 (10) | 2 (13) |
| Middle Eastern | 1 (2) | 0 (0) | 1 (7) |
| ABO blood group type, n (%) | |||
| Type A | 15 (28) | 11 (28) | 4 (27) |
| Type B | 8 (15) | 6 (15) | 2 (13) |
| Type AB | 2 (3) | 2 (5) | 0 (0) |
| Type O | 29 (54) | 20 (51) | 9 (60) |
| Smoking history, n (%) | 12 (22) | 8 (21) | 4 (27) |
| BMI, median (IQR) | 28 (18–43) | 27 (18–43) | 29 (18–34) |
| Comorbidities, n (%) | |||
| Diabetes | 16 (30) | 12 (31) | 4 (27) |
| Cardiovascular disease | 19 (35) | 16 (41) | 3 (20) |
| Stroke | 4 (7) | 3 (8) | 1 (7) |
| Pulmonary disease | 8 (15) | 7 (18) | 1 (7) |
| Medications for hypertension | 50 (93) | 37 (95) | 13 (93) |
| ACE inhibitor or ARB | 19 (37) | 12 (32) | 7 (47) |
| Cause of ESRD, n (%) | |||
| Hypertension | 11 (20) | 8 (21) | 3 (20) |
| Diabetes | 14 (26) | 11(28) | 3 (20) |
| Glomerulonephritis | 13 (24) | 11 (28) | 2 (13) |
| Lupus | 2 (4) | 1 (3) | 1 (7) |
| Polycystic kidney disease | 3 (6) | 1 (3) | 2 (13) |
| Other | 11 (20) | 7 (18) | 4 (27) |
| Kidney transplant variables, n (%) | |||
| Transplant type, living donor | 37 (69) | 26 (67) | 11 (73) |
| HLA A, B, DR mismatch, median (range) | 4 (0–6) | 5 (0–6) | 4 (2–6) |
| Donor-specific antibodies at transplant | 15 (28) | 11 (28) | 4 (27) |
| T cell–depleting induction | 39 (72) | 30 (77) | 9 (60) |
| Steroid maintenance, n (%) | 20 (37) | 14(35) | 6 (40) |
| Prior acute rejection episode, n (%) | 4 (7) | 4 (10) | 0(0) |
| Baseline serum creatinine (mg/dL), mean ± SD | 1.52 ± 0.67 | 1.58 ± 0.74 | 1.34 ± 0.41 |
| Viral infections within the past 3 months, n (%) | 12 (22) | 9 (23) | 3 (20) |
| Influenza | 2 | 2 | |
| Coronavirus | 1 | 1 | |
| RSV | 2 | 0 | |
| CMV | 2 | 0 | |
| BKV | 1 | 1 | |
| Other | 2 | 0 |
ARB, angiotensin receptor blocker; HLA, human leukocyte antigen; RSV, respiratory syncytial virus; CMV, cytomegalovirus; BKV, polyomavirus BK.
Kidney transplant specific characteristics
The type of organ donor (living versus deceased donor), the number of human leukocyte antigen mismatches between the recipient and the donor and the presence of preformed circulating donor-specific antibodies were similar in both groups. Induction therapy with T cell–depleting antibodies and the proportion of patients on steroid-free maintenance immunosuppressive regimen (our standard-of-care protocol) were similar between both groups. The baseline mean ± standard deviation (SD) serum creatinine was 1.58 ± 0.74 mg/dL in the hospitalized cohort versus 1.34 ± 0.41 mg/dL in the ambulatory cohort. Interestingly, 22% of our patients had laboratory evidence of other viral infections within 3 months of the diagnosis of SARS-CoV-2, with a similar incidence in the hospitalized versus ambulatory group (Table 1).
The median time from kidney transplantation to Covid-19 diagnosis was 4.7 years (range 0.3–35) (Table 2). Among the patients who received T cell–depleting therapy, the median time from transplantation to Covid-19 diagnosis was 4.9 years (range 0.3–14.5) for hospitalized patients and 5.4 years (range 0.6–12.7) for ambulatory patients. Both hospitalized and ambulatory cohorts included patients within 1 year of transplantation at a similar rate (18% versus 13%). Tacrolimus is the calcineurin inhibitor of choice in our kidney transplant recipients and the tacrolimus dose was higher in the hospitalized recipients compared with ambulatory recipients (4.8 ± 2.67 versus 4.12 ± 2.48 mg/day), whereas the total dose per day of MMF was lower in the hospitalized group compared with ambulatory patients (1.18 ± 0.48 versus 1.30 ± 0.52 g/day). Thirty-seven of 54 patients were on a steroid-free maintenance protocol. Two of the 54 patients were on a calcineurin inhibitor–free protocol, one on everolimus and one on belatacept. Two of the 54 kidney transplant recipients diagnosed with Covid-19 did not receive MMF as maintenance therapy and were instead on the mammalian target of rapamycin inhibitor rapamycin (n = 2).
Table 2.
Kidney transplant recipients with Covid-19: presenting symptoms
| Symptoms | Total | Hospitalized | Ambulatory |
|---|---|---|---|
| (N = 54) | (n = 39) | (n = 15) | |
| Covid-19 diagnosis | |||
| Post-transplant years, median (range) | 4.7 (0.3–35) | 4.7 (0.3–14.4) | 4.6 (0.5–35.3) |
| Days from symptoms to diagnosis, mean ± SD | 8.2 ± 6.0 | 8.5 ± 6.6 | 7.6 ± 4.5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
36/42 (86) |
|
|
Based on 52 patients on tacrolimus, 1 on everolimus and 1 on belatacept.
Data available for 38 in the hospitalized group and 4 in the not hospitalized group.
Data available for 37 in the hospitalized group and 5 in the not hospitalized group.
Covid-19 illness presentation
The average time from initial phone call to WCM Fever Clinic evaluation was 6 ± 5 days and of the 30 patients evaluated in the WCM Fever Clinic, 11 (37%) were referred to the NYP-WCM ED. Among the 30 patients referred to the WCM Fever Clinic, 18 were seen within 24–48 h and the remaining patients were seen 5–22 days after the initial telephone encounter, based on symptom progression. The average time from initial telephone encounter to NYP-WCM ED evaluation was 3 ± 3 days. Of the 30 patients initially referred to the NYP-WCM ED, 18 were asked to go to the NYP-WCM ED on the first telephone encounter and the remainder were referred to the NYP-WCM ED after the second telephone encounter.
The most common presenting symptom was fever (74%), followed by cough (59%), shortness of breath (52%), myalgias/fatigue (43%) and gastrointestinal symptoms of diarrhea (39%) and nausea/vomiting (9%) (Table 2). Of the entire cohort of 54 patients, 42 (78%) underwent a chest X-ray as part of their evaluation and the predominant finding was bilateral patchy airspace opacities in the lower lung fields in both groups.
Management of Covid-19 in kidney transplant recipients
We elected to continue immunosuppressive drug therapy, but at a reduced level, in many of our patients. Tacrolimus dosage was adjusted downwards so that the tacrolimus trough levels were 4–6 ng/mL, resulting in a reduction from baseline in 46% of hospitalized patients. No tacrolimus adjustments were made in patients managed at home. Of note, three of the six patients who were hospitalized had a tacrolimus level >8 ng/mL, requiring dosage reduction on admission, and had diarrhea as the chief complaint. MMF was continued at the baseline dosage in 9 of 14 ambulatory patients and was reduced by 50% in the remaining 5 patients (Table 3). In hospitalized patients, a more aggressive reduction in MMF dosage was undertaken, with MMF being withheld in 24 patients (61%), a 50% dose reduction in 10 patients (26%) and no reduction in the remaining 4 patients (11%). Two patients maintained on rapamycin continued to receive the same therapy with monitoring of drug levels and one patient maintained on belatacept continued to receive the same maintenance dose. Prednisone was continued in the 22 patients on a steroid maintenance regimen and the 29 of 32 patients on a steroid-free protocol remained steroid free. Five patients received additional steroids during hospitalization. Prednisone was not withheld in any patient who was receiving steroids on admission.
Table 3.
Medical management of Covid-19 in kidney transplant recipients
| Management | Total | Hospitalized | Ambulatory |
|---|---|---|---|
| (N = 54) | (n = 39) | (n = 15) | |
| Adjustment intacrolimus dose,an (%) | |||
| Reduction from baseline dose | 17 (33) | 17 (46) | 0 (0) |
| No adjustment from baseline | 35 (67) | 20 (54) | 15 (100) |
| Held | 0 (0) | 0 (0) | 0 (0) |
| Adjustment in MMF dose,bn (%) | |||
| No reduction/<50% reduction | 13 (28) | 4 (11) | 9 (64) |
| 50% reduction | 15 (28) | 10 (26) | 5 (33) |
| Discontinued drug | 24 (44) | 24 (61) | 0 (0) |
| Adjustment in steroid dose, n (%) | |||
| Continued maintenance steroidc | 22 (41) | 16 (41) | 6 (40) |
| Remained steroid freed | 29 (54) | 20 (51) | 9 (60) |
| Additional steroid therapy | 5 (9) | 5 (13) | 0 (0) |
| ACE inhibitor/ARB discontinued,en (%) | 11 (58) | 10 (83) | 1 (14) |
| Antibiotics, n (%) | 21 (39) | 15 (38) | 6 (40) |
| Azithromycin | 12 (22) | 7 (18) | 5 (33) |
| Doxycycline | 8 (17) | 8 (21) | 1 (7) |
| Experimental therapies, n (%) | |||
| HCQ | 32 (62) | 31 (79) | 1 (7) |
| Remdesivir | 2 (4) | 2 (5) | 0 (0) |
| IL-6 receptor inhibitor | 2 (4) | 2 (5) | 0 (0) |
| Convalescent plasma | 1 (2) | 1 (3) | 0 (0) |
| Management of bacterial infection at diagnosis,fn (%) | 25 (48) | 23 (62) | 2 (13) |
| Treated for urinary tract infection | 6 (11) | 4 (10) | 2 (13) |
| Treated for presumed bacterial pneumonia | 15 (28) | 15 (38) | 0 (0) |
| Treated for SIRS/sepsis | 4 (7) | 4 (10) | 0 (0) |
Based on 52 patients on tacrolimus; 2 calcineurin inhibitor free, 1 on everolimus, 1 on belatacept.
Based on 52 patients on MMF, 2 were on sirolimus.
Based on 22 patients on maintenance steroids.
Based on 32 patients on steroid-free immunosuppression.
Based on 19 patients on ACE/ARB.
Based on data from 52 patients, 37 hospitalized.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; SIRS, systemic inflammatory response syndrome.
For Covid-19, those requiring supplemental oxygen were initiated on HCQ therapy, with 79% of hospitalized patients receiving HCQ. Among those who did not require hospital admission, only one received HCQ for Covid-19 and another one continued on HCQ for systemic lupus erythematosus. In addition, an equal number of hospitalized and ambulatory patients received empiric treatment with azithromycin or doxycycline (38% and 40%) for symptoms of respiratory illness (Table 3). Forty-eight percent of our patients overall and 62% of those hospitalized were diagnosed with a concurrent bacterial infection and were treated with additional antibiotic therapy.
Among those with severe illness, nine received additional therapies: five received additional corticosteroids, two received treatment with remdesivir as part of a study protocol, one received treatment with IL-6 receptor antagonist and one received both IL-6 receptor antagonist and convalescent plasma (Table 3).
Hospital course and outcomes
Among those hospitalized, 13 were classified as having severe illness based on the need for intubation or 100% nonrebreather mask. Those classified as having severe disease had increased levels of C-reactive protein, procalcitonin, d-dimer and IL-6.
Detailed information including vitals and laboratory test results are shown in Table 4 stratified by the severity of illness. Among the 39 hospitalized patients, 27 (69%) required supplemental oxygen, 14 (36%) required nasal cannula, 2 (5%) required nonrebreather mask and 11 (28%) required intubation and mechanical ventilation. Among the 32 patients with evidence of pneumonia on admission to the hospital, 14 required oxygen therapy with nasal cannula, 2 required oxygen via a nonrebreather mask and 11 required intubation. No patients in this study were placed on other types noninvasive positive pressure airway support. Among the patients requiring oxygen therapy, the median time from symptom onset (as outpatients) to needing oxygen therapy was 9 days (interquartile range 5–11). Among the 10 patients requiring ventilator support, 4 required ventilator support at the time of admission. Most patients were not hypotensive or febrile upon admission and only five patients (14%), all in the severe illness category, required pressor support during their hospital course. Our patients were relatively lymphopenic upon admission. Although not all data were available on admission to the hospital, patients who had more severe illness were more likely to have elevated d-dimer, C-reactive protein and procalcitonin levels (Table 4).
Table 4.
Kidney transplant recipients with Covid-19: admission vitals and laboratory values in hospitalized patients
| Characteristics | All hospitalized (N = 39) | Severe illness (n = 13) | Moderate illness (n = 26) |
|---|---|---|---|
| Admission vitals | |||
| Temperature (°C) | 37.7 (37.1–38.2) | 37.7 (37.2–37.8) | 37.7 (37.1–38.3) |
| Oxygen saturation | 93 (89–96) | 90 (85–92) | 95 (92–98) |
| Respiratory rate | 20 (18–23) | 21 (18–26) | 18 (18–20) |
| Heart rate | 94 (81–107) | 97 (83–105) | 94 (83–107) |
| Systolic blood pressure (mmHg) | 128 (114–141) | 120 (110–144) | 131 (120–138) |
| Respiratory support, n (%) | |||
| Nasal cannula | 14 (36) | 0 (0) | 14 (54) |
| 100% nonrebreather mask | 2 (5) | 2 (17) | 0 (0) |
| Ventilation | 11 (28) | 11 (85) | 0 (0) |
| Laboratory values | |||
| Creatinine (mg/dL), mean ± SD | 2.6 ± 2.3 | 2.9 ± 3.1 | 2.5 ± 1.8 |
| AKI,an (%) | 20 (51) | 7 (54) | 13 (50) |
| White blood cell countb (×103/μL) | 5.7 (3.6–8) | 5.6 (4.3–9.6) | 6.1(3.3–7.5) |
| % lymphoctyesb | 11.4 (6.6–17.1) | 10.8 (5–14.4) | 11.9 (8–17.1) |
| Absolute lymphocyte countb (×103/μL) | 0.6 (0.3–1.0) | 0.5 (0.3–0.9) | 0.7 (0.4–1.0) |
| Albumin, g/dLb | 3.1 (2.7–3.6) | 2.8 (2.7–3.6) | 3.2 (3.0–3.5) |
| C-reactive protein, mg/dLb | 11.4 (5.3–30.2) | 16.5 (13.2–18.6) | 10.1 (5.3–32.5) |
| Procalcitonin, ng/mLb | 0.3 (0.1––0.6) | 0.6 (0.3–0.9) | 0.2 (0.1–0.4) |
| D-dimer, ng/mLb | 394 (278–589) | 506 (456–705) | 350 (259–405) |
| Ferritin, ng/mLb | 1498 (383–2646) | 1152 (644–2041) | 1844 (316–2597) |
| IL-6, pg/mLb | 8 (4.5–92) | 170 (92–219) | 4.5 (4–7.3) |
Values presented as median (IQR) unless stated otherwise.
AKI as defined by absolute creatinine increase of ≥0.5 mg/dL or 30% increase from baseline creatinine.
Data based on the following numbers of patients: creatinine, white blood cell and lymphocyte count, n = 37; procalcitonin, n = 35; albumin, n = 34; C-reactive protein, n = 29; ferritin, n = 28; d-dimer, n = 26; IL-6, n = 9.
Patients were followed for a median of 37 days from diagnosis of SARS-CoV-2, with a median follow-up of 29 days (range 5–53) in the hospitalized cohort and 37 days (range 21–40) in the ambulatory cohort. We classified the patients as having Covid-19 symptoms ‘resolved’ if they were at home and no longer symptomatic, ‘improved’ if some of their symptoms were still present but overall feeling better and ‘not improved’ if they remained symptomatic or hospitalized. Figure 3A shows the percentage of patients with the outcome of Covid-19 symptoms in those hospitalized as well as the ambulatory cohort as of 15 May 2020.
FIGURE 3.
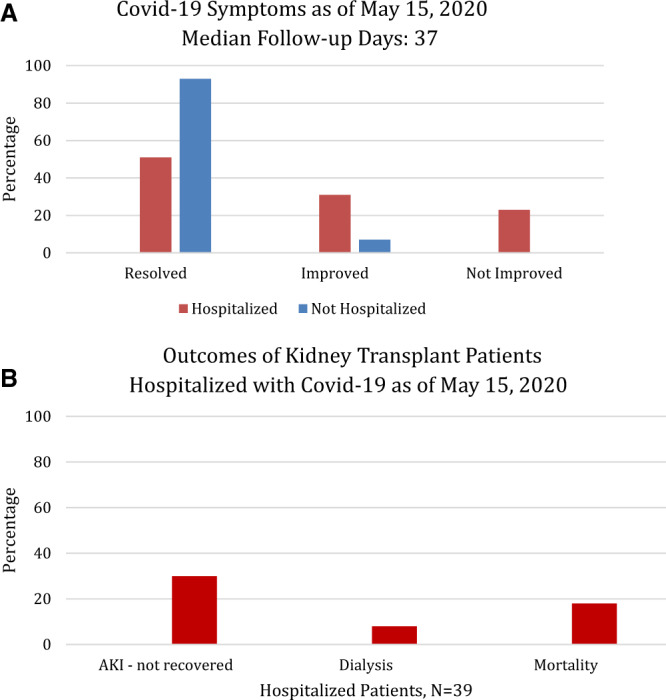
(A) The graph displays the percentage of patients who self-reported on a telephone interview that their symptoms associated with Covid-19 diagnosis were completely resolved, improved or not improved among those hospitalized (n = 39) and not hospitalized (n = 15) for Covid-19. Those who were still hospitalized or died were counted as ‘not improved’. (B) The clinical outcomes of patients hospitalized for Covid-19 are shown as a percentage of the total hospitalized cohort. AKI was defined by a 30% increase in serum creatinine from baseline or an absolute increase of ≥0.5 mg/dL. Recovery in AKI was defined by a return of serum creatinine to a value within 15% of the baseline serum creatinine value. The eGFR was calculated using the Modification of Diet in Renal Disease formula.
The kidney graft and patient outcomes are listed in Table 5 and Figure 3B. Twenty patients (51%) who were admitted to the hospital developed acute kidney injury (AKI) during the course of their illness. As of the most recent follow-up, 45% had resolution of their AKI, 25% had partial resolution and the remaining 30% did not have resolution of their AKI (Figure 3B). Of the four hospitalized patients (10%) who required renal replacement therapy during hospitalization, three remain dialysis dependent. At the most recent follow-up, 8 of 39 (20%) have an estimated GFR <20 mL/min/1.73 m2 (Figure 3B). Of the eight patients with eGFR <20 mL/min/1.73 m2, all had baseline chronic kidney disease (CKD); six had Stage 3 CKD, one had Stage 4 CKD and one had Stage 5 CKD at baseline. This group of eight patients with eGFR <20 mL/min/1.73 m2 includes three patients with graft loss and baseline CKD Stage 3.
Table 5.
Outcomes in kidney transplant recipients with Covid-19
| Outcomes | Total | Hospitalized | Ambulatory |
|---|---|---|---|
| (N = 54) | (n = 39) | (n = 15) | |
| AKI,an (%) | 21 (39) | 20 (51) | 1 (7) |
| Outcomesof AKI, n (%) | |||
| Resolvedb | 9 (43) | 9 (45) | 0 (0) |
| Partially resolvedb | 6 (29) | 5 (25) | 1 (100) |
| Not resolved | 6 (29) | 6 (30) | 0 (0) |
| Graft outcome | |||
| eGFR <20 mL/min/1.73 m2 | 8 (15) | 8 (21) | 0 (0) |
| Remains hospitalized, n (%) | 2 (4) | 2 (5) | 0 (0) |
| Patient death, n (%) | 7 (13) | 7 (18) | 0 (0) |
| Follow-up SARS-CoV-2 testing at median 29 days | |||
| Number tested | 20 | 13 | 7 |
| Number tested negative | 12 | 8 | 4 |
AKI as defined by absolute creatinine increase of ≥0.5 mg/dL or 30% increase from baseline creatinine.
Resolved AKI defined as a return to within 15% of baseline creatinine at the last follow-up and partially resolved defined as an improvement of serum creatinine that is not within 15% of baseline.
As of 15 May 2020, seven patients had died and the case fatality rate was 13% for all 54 patients and 18% among the 39 hospitalized patients. All seven deaths occurred in the hospitalized patients, and among 32 patients alive, 30 (77%) improved and were discharged either to home (n = 29) or to a rehabilitation center (n = 1) and 2 (5%) remain hospitalized (both are out of the ICU). Of the 11 patients who required intubation, 7 died and 4 have been extubated successfully. Among the 15 patients managed as outpatients, 14 (93%) have had complete resolution of symptoms and the remaining patient (7%) stated that his/her symptoms have improved. None of the 13 patients who were suspected of having Covid-19 but not tested were hospitalized at the last follow-up; however, 1 of the 13 did die of an unknown cause. Thirteen of 39 hospitalized patients and 7 of 15 ambulatory patients were retested for SARS-CoV-2 at a median of 32 days (range 22–45) after the initial diagnosis, and repeat testing was negative in 8 of the 13 (62%) hospitalized patients and 4 of the 7 (57%) ambulatory patients (Table 5).
DISCUSSION
Despite initial fears that transplant patients may be among those at the highest risk of adverse outcomes during the Covid-19 pandemic, our initial results from 54 kidney allograft recipients diagnosed with Covid-19 demonstrate that a coordinated ambulatory and inpatient effort can effectively manage kidney transplant recipients with Covid-19. With a median follow-up of 37 days, our total overall case fatality rate was 13% and hospitalized case fatality rate was 18%. Unique aspects of our management include careful adjustment of immunosuppressive therapies, aggressive evaluation and management of secondary bacterial infections and judicious and monitored use of unproven therapies such as HCQ. By systematically evaluating patients via telemedicine applications and coordinating our outpatient care, we were able to manage many patients in the ambulatory setting. Patients with more severe symptoms were sent directly to the Covid-19 triage area of the NYP-WCM ED or to the WCM Fever Clinic, thus helping to minimize the risk of infecting other transplant patients who were coming to the transplant clinic for routine follow-ups. To our knowledge, this is the first study to provide granular data on the time course of symptoms from initial contact with the provider to referral for evaluation in a specifically designated Covid-19 Fever Clinic and/or referral to the ED and data on follow-up testing for SARS-CoV-2. In addition, our study presents complete follow-up of patients diagnosed with Covid-19 during the initial stage of the outbreak in New York City. As of 15 May 2020, 30 of 39 hospitalized patients were discharged alive, 7 had died and 2 remained hospitalized, both of which are out of the ICU. All patients in the ambulatory setting have reported symptom resolution or significant improvement.
Risk factors for more severe disease in our transplant population mirror those reported in the general Covid-19 population [14]. In our cohort, patients who were hospitalized had more comorbidities, specifically cardiovascular disease, and were more likely to have more severe disease and require admission to the hospital. Patients with more severe disease were also found to have elevations of ferritin, d-dimer, procalcitonin, IL-6 and C-reactive protein, which is similar to what has been reported in other cohorts [15].
One of the most pressing questions currently is how to manage immunosuppression in transplant recipients diagnosed with Covid-19. The general consensus in the transplant community thus far appears to be to decrease or withhold antimetabolites like mycophenolic acid [3, 6, 10, 12, 16]. There is less agreement, however, about the optimal management strategy for calcineurin inhibitors.
Treatment strategies have varied across centers and countries. In a case series comprised of 10 cases in Wuhan, China by Zhu et al. [17], 9 of 10 kidney transplant recipients were treated successfully with withdrawal of calcineurin inhibitors and antimetabolites and treatment with high-dose steroids. In the New York City series of 36 patients by Akalin et al. [5] (8 in the ambulatory setting and 28 hospitalized), antimetabolite was withheld in 24 patients (86%) and calcineurin inhibitor was withheld in 6 severe cases (21%). Our study of 54 kidney transplant recipients with Covid-19 utilized a more measured approach where there was a minimal reduction of calcineurin inhibitors in both the hospitalized and ambulatory care setting. The decision to withhold MMF for hospitalized patients was based on the severity of illness and was not withheld for outpatients. Calcineurin inhibitor adjustments were made in most cases by targeting a lower tacrolimus trough for inpatients. Despite lowering and in some cases withholding MMF, there were no confirmed cases of acute rejection in our study cohort. However, due to a lack of kidney transplant biopsies in the setting of AKI, the true incidence of acute rejection in our study is not known. Upon discharge from the hospital, immunosuppressive medications that were withheld were reinitiated and doses were slowly increased to baseline levels, and this has not resulted in new admissions or readmissions from this cohort of 54 kidney allograft recipients.
As of 15 May 2020, only 2 of the 39 hospitalized patients remain hospitalized and both of these patients are out of the ICU. The 18% hospitalized case fatality rate observed in our study is less than the 28% mortality rate reported at a median of 21 days from a New York City transplant center but greater than 10.2% mortality rate of nontransplant hospitalized patients with Covid-19 at our center [7, 14]. It is worth noting that 12 patients of the consecutive 36 kidney allograft recipients (43%) were still hospitalized in the study by Akalin et al. [6] and just under half of the patients were still hospitalized in the study by Periera et al. [13] when those series were reported; it is not clear therefore what the final mortality rate will be for those patients. Unlike the aforementioned studies with short follow-up time and with many patients still hospitalized at the time of publication, in our series the outcomes are known for all patients in the study. In addition, we also learned that at a median of 37 days, overall 60% of kidney transplant recipients on immunosuppression have a negative polymerase chain reaction test for SARS-CoV-2. As of now, the timing and durability of the antibody response remain unknown.
Despite the lack of clear evidence regarding its efficacy, the majority of our hospitalized patients received HCQ. HCQ, in combination with azithromycin, has been associated with QT prolongation and potential adverse effects, such as cardiac arrhythmias [18, 19]. In our cohort, the decision to treat with azithromycin or doxycycline was not linked to the use of HCQ but is related to respiratory symptoms. Among our patients, only one patient developed prolongedQTc, causing the duration of therapy to be shortened, and two patients experienced new-onset atrial fibrillation. Further complicating the use of HCQ is that results regarding efficacy in treating SARS-CoV-2 has been conflicting. A nonrandomized 20 patient study showed that HCQ reduced the viral load, but it failed to correlate results with clinical outcomes, providing the basis for many centers’ adoption of this experimental therapy [20]. A subsequent, larger nonrandomized study failed to show a reduction in risk of mechanical ventilation and demonstrated increased mortality with treatment [21]. It remains to be seen whether HCQ use in large, randomized, placebo-controlled trials will yield beneficial results. No definite conclusions can be drawn from our study regarding HCQ efficacy in transplant patients, although the majority of our outpatients did not receive HCQ and 86% had improvement or resolution of symptoms. In light of the excellent outcome observed in our patients managed in the ambulatory setting, we would recommend against routine use of HCQ in kidney transplant recipients, especially in the outpatient setting, where fatal cardiac rhythm abnormalities may go undetected.
Only two of our patients were enrolled in remdesivir studies, one of which did not require intubation and was discharged from the hospital and the other who remains an inpatient but is no longer in the ICU. While initial data on the compassionate use of remdesivir seems promising, follow-up data and specific outcomes in solid organ transplant patients enrolled in ongoing trials are yet to be reported [22]. Two of our patients received IL-6 receptor antagonists. Both are still hospitalized and it is unclear whether this strategy is useful in kidney transplant recipients.
There are emerging data that immunosuppressive agents used in kidney transplant recipients may provide an as yet unappreciated benefit against SARS-CoV-2. In vitro studies have demonstrated a potential role for immunosuppressive agents, including tacrolimus, sirolimus and MMF as antiviral agents [1, 2, 23, 24]. In vivo effects of these agents in humans on SARS-CoV-2 replication are lacking. It is of interest that MMF was identified as a potential therapeutic agent for another coronavirus, MERS-CoV, and was studied in an animal model. In this model, MMF treatment was associated with more severe disease, higher viral loads and increased mortality. Whether or not these findings can be translated to humans remains unknown [25]. In our cohort of kidney transplant recipients, continuing MMF was not associated with a worse prognosis, as 13 of 14 hospitalized patients continued on MMF and were successfully discharged from the hospital. Additionally, one of the most serious complications of SARS-CoV-2 infection is the cytokine storm contributing to acute respiratory distress syndrome. Some immunomodulatory agents such as IL-6 and IL-1 antagonists, intravenous immunoglobulin and steroids have been suggested to have a role in combatting this inflammatory response [26]. Calcineurin inhibitors may be potentially beneficial in this setting because of their ability to reduce cytokine production via inhibition of nuclear localization of the nuclear factor of activated T-cells [27]. It is worth noting that all patients in our study were continued on tacrolimus, albeit at a reduced dosage and with due consideration of tacrolimus trough levels. Altogether our data suggest immunosuppression reduction rather than cessation may be a reasonable approach, especially when viewed through the lens of their ability to inhibit cytokine production and their potential antiviral activities.
In our series, we observed a higher rate of AKI than that reported in the general Covid-19 population, which has ranged from 3 to 9% in early studies to 15% in more recent studies [28]. While 16% of all Covid-19-related hospitalizations at our center had AKI, 51% of our hospitalized transplant patients experienced AKI. AKI in Covid-19 is thought to be one of the sequelae from sepsis and cytokine storm syndrome; however, SARS-CoV-2 has been found in the kidneys and urine of Covid-19 patients, suggesting there may be a direct mechanism of renal injury as well [7, 28, 29]. It is hypothesized that the virus can directly infect renal tubules, resulting in acute tubular damage, and induces CD68+ macrophage– and complement C5b-9–mediated damage [29]. Zhang et al. [3] proposed a genetic mechanism via the expression of human angiotensin-converting enzyme 2 in the kidney and kidney-specific expression quantitative train loci of potential direct viral targets in the kidney. There are several potential reasons why the transplant population may be more predisposed to AKI during Covid-19 infection. First, many kidney transplant recipients have baseline Stage 2 or 3 CKD, which may have contributed to AKI, especially in the setting of an acute viral illness. Elevated tacrolimus trough levels were observed in several of our patients at admission, which may have further exacerbated AKI. It has been reported that the bioavailability of tacrolimus is increased due to reduced gut transit time with diarrhea [30]. The high trough levels we observed may be a consequence of diarrhea and other gastrointestinal disturbances in our study cohort. Despite the high rates of AKI in our cohort, most patients (70%) either had full or partial renal recovery at the most recent follow-up. Thus, while Covid-19 primarily manifests as a respiratory disease, there is clearly an important kidney component, and the long-term prognosis with regards to kidney function warrants further study.
Limitations of this study include the observational study design, small sample size and inherent challenges associated with retrospective review. Multiple factors, not all under the control of the treating physician, such as the availability of testing, therapies, hospital/ICU beds, as well as the stage of the outbreak, may all impact management and outcomes. However, our study cohort is representative of other study populations diagnosed with Covid-19 in that there is a preponderance of males, Hispanics and individuals with multiple comorbidities. Thus our approach, a systematic evaluation and triage of kidney transplant recipients with Covid-19 symptomology, may be a useful approach to avoid unnecessary hospitalization. Early diagnosis of complications such as concurrent infections and/or AKI was an additional component of managing this complex patient cohort. It is worth noting that 14 of 15 (93%) of the kidney allograft recipients managed as outpatients had complete resolution of their symptoms and the remaining patient showed improvement as well. It is also important to note that none of the individuals selected to be managed in the ambulatory care setting required hospitalization at a median of 37 days (range 21–40) after initial diagnosis. In addition, 12 of the patients suspected of having Covid-19 (Figure 1) but not tested were also managed in the ambulatory setting and did not require admission; 1 died of an unknown cause. Regarding immunosuppressive drug treatment, tacrolimus was not discontinued in any of our kidney transplant recipients and <50% had their MMF discontinued (all in the hospitalized cohort), suggesting that immunosuppressive therapy can be continued in the setting of Covid-19 and the decision to alter the immunosuppressive drug regimen should be individualized based on the severity of illness and other clinical symptoms. Because our study was an observational study and not a randomized controlled trial, it is not possible to attribute the outcomes we observed to a specific component of our approach. However, we can deduce from our study cohort that HCQ is not required for all patients managed in the ambulatory setting since only 1 of the ambulatory patients received HCQ and none of the remaining 14 patients deteriorated and required hospitalization. Regarding additional therapeutic interventions, there were very few patients who received antiviral agents such as remdesivir or the anti-IL-6 receptor monoclonocal antibody tocilizumab to comment on their utility.
In sum, using a coordinated and multidisciplinary approach, the patients with mild symptoms were successfully managed in the ambulatory setting with close monitoring for symptom progression and with minimal reductions in immunosuppressive agents. For hospitalized patients, our treatment strategy included careful evaluation and judicious reduction in immunosuppressive drugs and prompt treatment of secondary bacterial infections. However, we did observe significant AKI in a substantial percentage of hospitalized patients. A therapeutic strategy of clinical severity–dependent reduction rather than a complete withdrawal of immunosuppressive drug therapy appears reasonable for kidney allograft recipients diagnosed with Covid-19.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all of the team members of the Division of Nephrology and Transplant Surgery for their excellent care of our transplant patients and the WCM Fever Clinic for accommodating prompt evaluation of SARS-CoV-2 infection in our patient population.
FUNDING
The study was supported by internal divisional funds.
AUTHORS’ CONTRIBUTIONS
All authors participated in the care of these patients, provided clinical management details and reviewed the manuscript for accuracy, interpretation of the data and completeness. M.L., M.A., R.S., J.M., T.S. and D.M.D. participated in data collection, analysis of the data and writing of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors do not report any competing interests related to these data and the material presented in this article. J.R.L. receives an investigator-initiated research grant from BioFire Diagnostics. M.S., D.M.D. and J.R.L. are inventors of patent 2020-0048713-A1, ‘Methods of detecting cell-free DNA in biological samples’.
REFERENCES
- 1. Gordon DE, Jang GM, Bouhaddou M. et al. A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. Nature 2020; doi: 10.1038/s41586-020-2286-9 [DOI] [PMC free article] [PubMed]
- 2. Cheng KW, Cheng SC, Chen WY. et al. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antiviral Res 2015; 115: 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang H, Chen Y, Yuan Q. et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol 2020; 77: 742–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernandez-Ruiz M, Andres A, Loinaz C. et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant 2020; doi: 10.1111/ajt.15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akalin E, Azzi Y, Bartash R. et al. Covid-19 and kidney transplantation. N Engl J Med 2020; doi: 10.1056/NEJMc2011117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Columbia University Kidney Transplant P. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol 2020; 31: 1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goyal P, Choi JJ, Pinheiro LC. et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020; 382: 2372–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michaels MG, La Hoz RM, Danziger-Isakov L. et al. Coronavirus disease 2019: implications of emerging infections for transplantation. Am J Transplant 2020; doi: 10.1111/ajt.15832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guillen E, Pineiro GJ, Revuelta I. et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant 2020; doi: 10.1111/ajt.15874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu L, Xu X, Ma K. et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant 2020; doi: 10.1111/ajt.15869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang J, Li X, Cao G. et al. COVID-19 in a kidney transplant patient. Eur Urol 2020; 77: 769–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang. JL, Wu Y, Fang Y. et al. COVID-19 in post-transplantation patients–report of two cases. Am J Transplant 2020; doi: 10.1111/ajt.15896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pereira MR, Mohan S, Cohen DJ. et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant 2020; doi: 10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richardson S, Hirsch JS, Narasimhan M. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323: 2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhong Z, Zhang Q, Xia H. et al. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant 2020; doi: 10.1111/ajt.15928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu L, Gong N, Liu B. et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol 2020; 77: 748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borba MGS, Val FdA, Sampaio VS et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study). JAMA Netw Open 2020; 3: e208857 [DOI] [PubMed]
- 19. Timothy F, Simpson M, Kovacs MD. et al. Ventricular arrhythmia risk due to hydroxychloroquine-azithromycin treatment for COVID-19. Cardiol Magaz 29 March 2020; https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19 [Google Scholar]
- 20. Gautret P, Lagier JC, Parola P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020; doi: 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magagnoli J, Narendran S, Pereira F. et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv2020; doi: 10.1101/2020.04.16.20065920 [DOI] [PMC free article] [PubMed]
- 22. Grein J, Ohmagari N, Shin D. et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 2020; 382: 2327–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carbajo-Lozoya J, Ma-Lauer Y, Malesevic M. et al. Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including Alisporivir. Virus Res 2014; 184: 44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Wilde AH, Zevenhoven-Dobbe JC, van der Meer Y. et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol 2011; 92: 2542–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Russell B, Moss C, George G. et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancer 2020; 14: 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kotch C, Barrett D, Teachey DT.. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol 2019; 15: 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Willicombe M, Thomas D, McAdoo S.. COVID-19 and calcineurin inhibitors: should they get left out in the storm? J Am Soc Nephrol 2020; 31: 1145–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naicker S, Yang CW, Hwang SJ. et al. The novel coronavirus 2019 epidemic and kidneys. Kidney Int 2020; 97: 824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lemahieu W, Maes B, Verbeke K. et al. Cytochrome P450 3A4 and P-glycoprotein activity and assimilation of tacrolimus in transplant patients with persistent diarrhea. Am J Transplant 2005; 5: 1383–1391 [DOI] [PubMed] [Google Scholar]
- 30. Diao B, Wang C, Wang R. et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv2020; doi: 10.1101/2020.03.04.20031120 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.