Abstract
Background
The outbreak of coronavirus disease 2019 (COVID-19) has aroused global public health concerns. Multiple clinical features relating to host profile but not for virus have been identified as the risk factors for illness severity and/or the outcomes in COVID-19.
Methods
The clinical features obtained from a cohort of 195 laboratory-confirmed, nasopharynx-sampled patients with COVID-19 in Guangdong, China from January 13 to February 29, 2020 were enrolled to this study. The differences in clinical features among 4 groups (mild, moderate, severe, and critical) and between 2 groups (severe vs nonsevere) were compared using one-way analysis of variance and Student’s t test, respectively. Principal component analysis and correlation analysis were performed to identify the major factors that account for illness severity.
Results
In addition to the previously described clinical illness severity-related factors, including older age, underlying diseases, higher level of C-reactive protein, D-dimer and aspartate aminotransferase, longer fever days and higher maximum body temperature, larger number of white blood cells and neutrophils but relative less lymphocytes, and higher ratio of neutrophil to lymphocytes, we found that the initial viral load is an independent factor that accounts for illness severity in COVID-19 patients.
Conclusions
The initial viral load of severe acute respiratory syndrome coronavirus 2 is a novel virological predictor for illness severity of COVID-19.
Keywords: COVID-19, SARS-CoV-2, viral load
The ongoing outbreak of coronavirus disease 2019 (COVID-19), caused by a novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) infection [1], has aroused global public health concerns and was officially characterized as a pandemic by the World Health Organization (WHO) on March 11, 2020. Globally, there were more than 1 000 000 confirmed cases with over 50 000 deaths by April 4, 2020 (https://www.who.int/), and the number of cases and deaths is still growing. The epidemiological surveys have indicated that most patients only have mild symptoms; however, more than a few cases develop severe symptoms [2–5]. In contrast to the patients with mild symptoms with good prognosis, the severe patients are more likely to have organ failure and even death [6]. Given the lack of therapeutics of proven effectiveness against SARS-CoV-2 infection, it is especially important to develop an appropriate management approach for treating the disease according to the severity of symptoms. It is thought that the identification of risk factors or predictors that account for illness severity may provide valuable insight into the control of disease progression.
Several clinical parameters, including high Sequential Organ Failure Assessment (SOFA) score, older age, and low levels of lymphocytes and proinflammatory cytokines, have been reported as risk factors associated with illness severity or poor outcomes in recent retrospective studies [7, 8]. It is interesting to note that all of the reported factors are related to patients, namely, the host; however, virological risk features such as viral load have not yet been discovered. As a viral infectious disease, in which the interplay between the virus and the host defense determines the pathogenesis and clinical progress of the illness [9], we speculated that, apart from the clinical parameters from host, the virological profile, such as initial viral load, may also contribute to the severity of symptoms. In this study, we investigated the correlation between SARS-CoV-2 viral loads at hospital admission (in short initial viral load) and illness severity in a cohort of 195 patients in Guangdong, China.
METHODS
Patients
A cohort of 195 nasopharynx-sampled, laboratory-confirmed COVID-19 patients who were hospitalized at Guangzhou Eighth People’s Hospital, the Third Affiliated Hospital of Sun Yat-sen University, Yuedong Hospital, and the First People’s Hospital of Foshan in Guangdong Province, China from January 13, 2020 to February 2020 were enrolled in this study. All patients did not receive any therapy before hospitalization, besides antipyretics. The diagnosis of COVID-19 and clinical classification were performed according to the New Coronavirus Pneumonia Diagnosis and Treatment Plan (trial version 3) released by the National Health Committee of the People’s Republic of China ([China NHC] http://www.nhc.gov.cn/). The study was approved by The Third Affiliated Hospital of Sun Yat-Sen University Yuedong Hospital Ethics Committee, and written informed consent was obtained from patients before enrollment when data were collected retrospectively.
Baseline Data Collection
A nasopharynx swab sample was taken from all patients with suspected SARS-CoV-2 infection at admission, and the samples were stored in virus transport medium, which were transported to the Guangdong Center for Disease Control and Prevention (CDC) for laboratory diagnosis. Epidemiological history, comorbidity, vital signs, symptoms, and signs were recorded in detail in addition to laboratory tests including biochemical indicators, blood routine, C-reactive protein, chest radiograph, or computed tomography scan.
Laboratory Confirmation by Real-Time Reverse-Transcription Polymerase Chain Reaction
The nasopharyngeal specimens from suspected patients were collected at admission and transported to Guangdong CDC for laboratory diagnosis. The ribonucleic acid (RNA) was extracted and tested by real-time reverse-transcription polymerase chain reaction (RT-PCR) with SARS-CoV-2-specific primers and probes targeting the N and Orf1b genes in accordance with the diagnosis protocol for COVID-19 established by the WHO. The negative or positive for SARS-CoV-2 of samples was determined by the cycle threshold (Ct) values of real-time RT-PCR: samples were considered to be negative if the Ct value exceeded 40 cycles or positive if the Ct value was ≤40. The viral RNA copy number, converted from Ct value, is based on a standard curve of copy number versus Ct values of viral plasmid deoxyribonucleic acid (DNA). Each dilution of plasmid DNA was tested in duplicate to produce the standard curve.
Statistical Analysis
According to the classification criterion (see detailed classification criteria in Supplementary Method) of illness severity established by China NHC, all of the confirmed COVID-19 patients were classified as mild, moderate, severe, and critical groups. For dichotomous comparison, the mild and moderate groups were combined as the nonsevere group, whereas the remaining severe and critical groups were combined as the severe group.
Continuous data were presented with mean ± standard deviation, whereas categorical data were presented with number and percentage. The statistical difference in continuous data between the 2 groups (severe vs nonsevere) were compared using the Student’s t test, whereas the comparison in continuous data among the 4 groups (mild, moderate, severe, and critical) were performed with one-way analysis of variance, after a Tukey’s post hoc test. Categorical data were tested using a χ 2 test or Fisher’s exact text (if the expected value was ≤5). Principal component analysis (PCA) was performed to identify the major contributing factors for illness severity. The rotation method for PCA plot was varimax with Kaiser normalization and the minimal component’s initial eigenvalue was set at 1. P < .05 was considered to be statistically significant for each 2-tailed test. All analyses were performed using IBM SPSS version 25 (SPSS Statistics V25; IBM Corporation, Somers, NY).
RESULTS
Comparison of Clinical and Virological Characteristics in 195 Nasopharynx-Sampled Patients
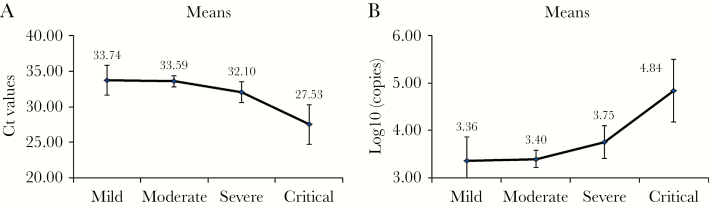
The clinical and virological characteristics of 195 nasopharynx-sampled patients (94 males and 101 females), comprising 6 mild, 132 moderate, 41 severe and 6 critical patients, are summarized in Table 1. Their average age was 49.24 ± 15.99 years old. The average time from illness onset (1) to hospitalization, (2) to be diagnosed, (3) to be sampled, and (4) to turning negative were 3.65 ± 3.24 days, 4.88 ± 3.96 days, 6.69 ± 4.66 days, and 15.17 ± 6.06 days, respectively. More severe patients seem to have the following characteristics: older age; higher frequency with underlying diseases; higher maximum body temperature within 24 hours after hospitalization; longer time for virus clearance (from illness onset to turning negative) and duration of fever (days); higher plasma C-reactive protein (CRP), D-dimer, procalcitonin (PCT), and aspartate aminotransferase (AST); larger count of white blood cells (WBC) and neutrophil (NE), but relatively reduced lymphocyte count (marginally significant; P = .095); higher NE to lymphocyte ratio (NLR); and higher initial viral load (Ct and log10 [copies/mL]) (except lymphocyte [P = .095], all P < .05) (Table 1). Figure 1 shows a significant increasing trend of initial viral load versus illness severity.
Table 1.
Clinical and Virological Characteristics in 195 Nasopharynx-Sampled Patients With COVID-19
| Parameters | Mild (n = 16) | Moderate (n = 132) | Severe (n = 41) | Critical (n = 6) | All (n = 195) | P |
|---|---|---|---|---|---|---|
| Sex | .629 | |||||
| Male | 6 (37.50%) | 63 (47.73%) | 21 (51.22%) | 4 (66.67%) | 94 (48.21%) | |
| Female | 10 (62.50%) | 69 (52.27%) | 20 (48.78%) | 2 (33.33%) | 101 (51.79%) | |
| Age, year | 36.63 ± 16.25 | 47.32 ± 15.32 | 58.39 ± 12.49 | 62.67 ± 15.06 | 49.24 ± 15.99 | <.001 |
| Age Group | <.001 | |||||
| <50 | 12 (75.00%) | 73 (55.30%) | 7 (17.07%) | 1 (16.67%) | 93 (47.69%) | |
| ≥50 | 4 (25.00%) | 59 (44.70%) | 34 (82.93%) | 5 (83.33%) | 102 (52.31%) | |
| BMI | 23.15 ± 3.00 | 23.91 ± 4.07 | 24.60 ± 4.33 | 24.02 ± 4.39 | 24.00 ± 4.05 | .663 |
| Basic Diseases | ||||||
| Hypertension | 5 (33.33%) | 16 (14.55%) | 8 (32.00%) | 0 (0.00%) | 29 (19.21%) | .109 |
| Diabetes mellitus | 2 (13.33%) | 2 (1.82%) | 1 (4.00%) | 0 (0.00%) | 5 (3.31%) | .291 |
| Cardiovascular disease | 0 (0.00%) | 7 (6.36%) | 3 (12.00%) | 0 (0.00%) | 10 (6.62%) | .367 |
| Cerebrovascular disease | 0 (0.00%) | 2 (1.82%) | 2 (8.00%) | 0 (0.00%) | 4 (2.65%) | .390 |
| Chronic kidney disease | 0 (0.00%) | 1 (0.91%) | 2 (8.00%) | 0 (0.00%) | 3 (1.99%) | .249 |
| All above diseases | 6 (40.00%) | 22 (20.00%) | 11 (44.00%) | 0 (0.00%) | 39 (25.83%) | .047 |
| Onset to hospitalization (days) | 4.19 ± 4.42 | 3.24 ± 2.79 | 4.39 ± 3.51 | 6.00 ± 5.44 | 3.65 ± 3.24 | .048 |
| Onset to diagnosed (days) | 5.57 ± 5.02 | 4.75 ± 3.98 | 4.90 ± 3.56 | 5.83 ± 4.07 | 4.88 ± 3.96 | .822 |
| Onset to be sampled (days) | 5.75 ± 4.20 | 6.58 ± 4.55 | 7.38 ± 5.18 | 7.00 ± 4.98 | 6.69 ± 4.66 | .659 |
| Onset to turning negative (days) | 12.44 ± 4.98 | 13.69 ± 4.90 | 19.71 ± 6.39 | 24.17 ± 6.88 | 15.17 ± 6.06 | <.001 |
| Fever days | 5.38 ± 4.60 | 6.93 ± 5.59 | 9.26 ± 5.75 | 6.20 ± 2.05 | 7.32 ± 5.56 | .141 |
| Maximum body temperature in 24hrs | 37.80 ± 0.89 | 37.80 ± 0.78 | 38.53 ± 0.74 | 38.02 ± 0.54 | 38.00 ± 0.82 | .002 |
| Fever | .014 | |||||
| No | 7 (43.75%) | 36 (27.27%) | 5 (12.20%) | 0 (0.00%) | 48 (24.62%) | |
| Yes | 9 (56.25%) | 96 (72.73%) | 36 (87.80%) | 6 (100.00%) | 147 (75.38%) | |
| Ct | 33.74 ± 3.92 | 33.59 ± 4.45 | 32.10 ± 4.64 | 27.53 ± 2.63 | 33.10 ± 4.54 | .004 |
| Log10 (copies/mL) | 3.36 ± 0.93 | 3.40 ± 1.06 | 3.75 ± 1.10 | 4.84 ± 0.62 | 3.51 ± 1.08 | .004 |
| CRP (mg/dL) | 14.93 ± 18.93 | 16.29 ± 14.78 | 30.11 ± 32.19 | 39.59 ± 16.64 | 19.82 ± 20.92 | <.001 |
| WBC (109/L) | 5.80 ± 1.11 | 4.96 ± 1.82 | 5.92 ± 2.71 | 7.74 ± 6.76 | 5.35 ± 2.41 | .009 |
| Neutrophil (109/L) | 3.08 ± 1.29 | 3.06 ± 1.45 | 4.27 ± 2.79 | 6.21 ± 6.00 | 3.47 ± 2.23 | <.001 |
| Lymphocyte (109/L) | 1.89 ± 0.75 | 1.46 ± 0.93 | 1.24 ± 0.56 | 1.08 ± 0.82 | 1.42 ± 0.85 | .095 |
| NLR | 2.16 ± 1.99 | 2.53 ± 1.61 | 5.07 ± 5.25 | 6.70 ± 5.16 | 3.26 ± 3.28 | <.001 |
| AST (U/L) | 22.51 ± 10.48 | 23.89 ± 15.79 | 30.90 ± 17.60 | 48.48 ± 28.52 | 26.35 ± 17.20 | .001 |
| ALT (U/L) | 25.93 ± 10.84 | 28.80 ± 24.48 | 34.55 ± 20.71 | 35.87 ± 16.82 | 30.21 ± 22.75 | .469 |
| D-Dimer (mg/L) | 934.00 ± 340.27 | 1495.14 ± 3637.77 | 3358.85 ± 6227.96 | 10 230.00 ± 0.00 | 1900.67 ± 4258.42 | .046 |
| PCT (μg/L) | 17.92 ± 26.72 | 41.28 ± 61.11 | 141.88 ± 271.22 | 1068.00 ± 0.00 | 68.46 ± 165.89 | <.001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; Ct, cycle threshold value; NLR, neutrophil/lymphocyte ratio; PCT, procalcitonin; WBC, white blood cell.
Figure 1.
The curve graph illustrating the differences in initial viral load among illness severity groups in 195 nasopharynx-sampled patients. The viral load was represented as (A) Ct value or (B) log10 (copies/mL). Y axis indicates the mean of Ct values (A) or logarithm value of viral RNA (B). The vertical lines on the curve represent standard deviation (SD).
To simplify the comparison, we classified these 195 nasopharynx-sampled patients into 2 groups (nonsevere, n = 47 vs severe, n = 148), namely, a combination of the mild and moderate groups as the nonsevere group while merging the critical patients into the severe group. As listed in Supplementary Table S1, there were more older patients in the severe group; onset to hospitalization days, onset to turning negative days, fever rate, and highest body temperature in 24 hours were bigger and/or longer and/or higher than the nonsevere group; significantly lower Ct and higher log10 (copies/mL) indicated higher viral load in the severe group; and higher levels of CRP, WBC, NE, NLR, AST, D-Dimer, and PCT were observed in the severe group (all P < .05) (Supplementary Table S1). A comparatively lower lymphocyte level in the severe group was also observed (marginally significant, P = .059).
Predictive Factors for Illness Severity
Principal component analysis was performed to identify major contributing factors for illness severity. To avoid bias estimation, only the variables whose missing rate lower was than 30% was used in this analysis. The overall Kaiser-Meyer-Olkin (KMO) value was 0.582, and the explanatory ratio of variance reached 79.19% with 7 components comprising 1 and 5: (1) the immune-related features, (2) the time about illness onset to medical intervention, (3) age, (4) the indicator for liver failure (AST), (5) the indicator for liver failure (alanine aminotransferase), (6) other (the turning negative time, basic diseases, and sex), and (7) viral load as a single-variable component (Supplementary Table S2).
Pearson’s correlation coefficients between independent variables and severity or initial viral load were calculated to illustrate their relationships. As presented in Table 2, age, fever, peak body temperature in 24 hours after hospitalization, CRP, WBC, NE, NLR, AST, D-Dimer, and PCT are positively correlated with severity, regardless of whether they are classified as 4 or 2 groups (all P < .05). The time from illness onset to hospitalization (days) was only found to be positively correlated to dichotomous severity (P < .05), whereas lymphocyte was only found to be significantly negative correlated to 4-grouped severity (P < .05) (Table 2). Because the 2 conversely correlated indexes for initial viral load, log10 (copies/mL) and Ct value, were found to be significantly positive and negative, respectively, and correlated to severity (both in 4- and 2-grouped levels), we can conclude that the initial viral load is positively correlated to illness severity (Table 2).
Table 2.
Pearson’s Correlation Coefficients Between Variables and Illness Severity
| Parameters | Severity (4 Levels) | Severity (2 Levels) |
|---|---|---|
| Ct | −0.22a | –0.20a |
| Log10 (copies/mL) | 0.22a | 0.20a |
| Onset to hospitalization | 0.13 | 0.17a |
| Onset to be sampled | 0.08 | 0.08 |
| Onset to turning negative | 0.46a | 0.48a |
| Age | 0.39a | 0.34a |
| Age group (≥50 or not) | 0.34a | 0.35a |
| Sex | –0.09 | –0.06 |
| BMI | 0.10 | 0.10 |
| Fever | 0.21a | 0.18a |
| Fever days | 0.14 | 0.16 |
| Peak body temperature in 24 hours | 0.28a | 0.37a |
| CRP | 0.30a | 0.31a |
| WBC | 0.19a | 0.21a |
| NE | 0.30a | 0.29a |
| Lymphocyte | –0.19a | –0.15 |
| NLR | 0.37a | 0.38a |
| AST | 0.28a | 0.25a |
| ALT | 0.12 | 0.12 |
| D-Dimer | 0.22a | 0.21a |
| PCT | 0.40a | 0.35a |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CRP, C-reactive protein; Ct, cycle threshold value; NE, neutrophil; NLR, neutrophil to lymphocyte ratio; PCT, procalcitonin; WBC, white blood cell.
a P < .05.
DISCUSSION
Consistent with previous studies, our data also support that several clinical features such as the patients age and the patients with and/or without underlying diseases are correlated to illness severity of COVID-19 [4, 10]. More important, we first identified the upper respiratory tract viral RNA load of SARS-CoV-2 at hospital admission as an independent predictive factor for illness severity. It suggests that patients with higher upper respiratory tract viral load at admission are more likely to develop severe symptoms and they may need more aggressive treatment.
Researchers believe that the interplay between virus and host immune response, rather than the single factor, determines the pathogenesis and disease progression of COVID-19 [10–13]. The replication of SARS-CoV-2 does not directly lyse the host cells [14], but the virus-specific immune response may kill these cells [15]. It is possible that more virus (higher viral load) can infect more cells, and more alveolar cells, upon infected, may be killed by host immune system [16]. The acute, large quantity of cell death may contribute to the disease severity. Meanwhile, higher viral load may invoke stronger immune response, leading to release larger quantity of cytokines by the activated immune cells. There is evidence that a variety of proinflammatory cytokines such as interleukin (IL)2, IL6, IL7, IL10, interferon (IFN)-γ-inducible protein-10, IFNγ, and tumor necrosis factor-α at significantly higher levels can promote disease severity [17].
Our study has some limitations. First, we only investigated the upper respiratory tract viral load but lacked the lower respiratory tract virological data. Second, because the detected samples may have mixed viral RNAs from dead virus, the viral RNA loads do not exactly equal the titers or amount of live infectious virus. Third, there is a difference, although it did not achieve statistical significance (P > .05), in the duration of viral RNA detection posthospitalization between different groups with varying degrees of symptoms. We noticed that the use of therapeutics (eg, antiviral drugs, convalescent plasma, corticosteroids, and immunomodulators) may visibly affect viral loads; however, this factor is ignorable in this study, and it had little effect on initial viral load, because the patients did not receive drug treatment until they were diagnosed or their samples were tested.
CONCLUSIONS
In summary, we identified that the upper respiratory tract viral RNA load of SARS-CoV-2 at the time of hospital admission is an independent prognostic factor of COVID-19.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We appreciate all of the volunteer patients who enrolled in this study.
Financial support. This work was funded by the the National Science and Technology Major Project (Grant number 2018ZX10302204-002), the National Natural Science Foundation of China (Grant Number 81700531), the 5010 Project of Clinical Research in Sun Yat-sen University (Grant number 2016009), Open project of Key Laboratory of Tropical Disease Control (Sun Yat-sen University), Ministry of Education (Grant number 2020KFKT05), and the Tackling of key scientific and emergency special program of Sun Yat-sen University, China (SYSU-TKSESP to B. L.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Zhu N, Zhang D, Wang W, et al. ; China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munster VJ, Koopmans M, van Doremalen N, et al. A novel coronavirus emerging in China–key questions for impact assessment. N Engl J Med 2020; 382:692–4. [DOI] [PubMed] [Google Scholar]
- 7. Berhane M, Melku M, Amsalu A, et al. The role of neutrophil to lymphocyte count ratio in the differential diagnosis of pulmonary tuberculosis and bacterial community-acquired pneumonia: a cross-sectional study at Ayder and Mekelle hospitals, Ethiopia. Clin Lab 2019; 65. [DOI] [PubMed] [Google Scholar]
- 8. Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 286:1754–8. [DOI] [PubMed] [Google Scholar]
- 9. Peiris JS, Chu CM, Cheng VC, et al. ; HKU/UCH SARS Study Group Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003; 361:1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chu CM, Poon LL, Cheng VC, et al. Initial viral load and the outcomes of SARS. CMAJ 2004; 171:1349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chu CM, Cheng VC, Hung IF, et al. Viral load distribution in SARS outbreak. Emerg Infect Dis 2005; 11:1882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016; 14:523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oh MD, Park WB, Choe PG, et al. Viral load kinetics of MERS Coronavirus infection. N Engl J Med 2016; 375:1303–5. [DOI] [PubMed] [Google Scholar]
- 14. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol 2020; 92:424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016; 19:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Guo Q, Yan Z, et al. ; CAP-China Network Factors associated with prolonged viral shedding in patients with avian influenza A(H7N9) virus infection. J Infect Dis 2018; 217:1708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.