Abstract
Background
The US Food and Drug Administration issued an Emergency Use Authorization for remdesivir use in patients with severe COVID-19.
Methods
We utilized data from 2 quaternary acute care hospitals. The outcomes of interest were the impact of remdesivir on in-hospital death by day 28 and time to recovery, clinical improvement, and discharge. We utilized Cox proportional hazards models and stratified log-rank tests.
Results
Two hundred twenty-four patients were included in the study. The median age was 59 years; 67.0% were male; 17/125 patients (13.6%) who received supportive care and 7/99 patients (7.1%) who received remdesivir died. The unadjusted risk for 28-day in-hospital death was lower for patients who received remdesivir compared with patients who received supportive care (hazard ratio [HR], 0.42; 95% CI, 0.16–1.08). Although this trend remained the same after adjusting for age, sex, race, and oxygen requirements on admission (adjusted HR [aHR], 0.49; 95% CI, 0.19–1.28), as well as chronic comorbidities and use of corticosteroids (aHR, 0.44; 95% CI, 0.16–1.23), it did not reach statistical significance. The use of remdesivir was not associated with an increased risk of acute kidney injury (AKI) or liver test abnormalities. Although not statistically significant, the rate ratios for time to recovery, clinical improvement, and discharge were higher in women and black or African American patients.
Conclusions
Patients on remdesivir had lower, albeit not significant, all-cause in-hospital mortality, and the use of remdesivir did not increase the risk for AKI. Promising signals from this study need to be confirmed by future placebo-controlled randomized clinical trials.
Keywords: COVID-19, efficacy, remdesivir, safety, SARS-CoV-2
This single-center analysis adds to our understanding of the potential benefits from remdesivir in patients with severe COVID-19. Patients on remdesivir had a strong signal suggesting lower in-hospital mortality and the use of remdesivir did not increase acute kidney injury.
The development of safe and effective therapeutic agents against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a top priority in the battle against the COVID-19 pandemic. Remdesivir, also known as GS-5734, is an intracellularly metabolized nucleotide prodrug that inhibits viral RNA polymerases and has a broad spectrum of antiviral activity, which includes corona- and flaviviruses [1]. Animal studies have shown remdesivir to be efficacious against SARS-CoV-1 and Middle East respiratory syndrome (MERS)–CoV [2, 3]. During the early days of the COVID-19 pandemic, it was among the first drugs to show in vitro activity against SARS-CoV-2 [4].
Despite high expectations for remdesivir in treating COVID-19 [5, 6], the results from the first randomized placebo-controlled trial from China were inconclusive [7]. However, the results of a larger-scale multicenter trial conducted by the National Institute of Allergy and Infectious Diseases showed a significant clinical benefit in terms of time to recovery [8]. Subsequently, the US Food and Drug Administration (FDA) issued an Emergency Use Authorization for remdesivir use in patients with severe COVID-19 [9].
In March 2020, our institution became part of a multicenter open-label, phase 3 clinical trial that studied the use of remdesivir in patients with severe COVID-19. For this study, we analyzed data from patients who participated in the aforementioned trial and compared them with patients with severe COVID-19 who received supportive care at our institution to investigate the efficacy and safety of remdesivir in patients with severe COVID-19.
METHODS
Study Setting and Design
We utilized data from 2 quaternary, acute care hospitals, Rhode Island Hospital (RIH) and The Miriam Hospital (TMH), located in Providence, Rhode Island, which function as a single academic medical center. All consecutive hospitalized patients from February 27 to May 11, 2020, who had a positive polymerase chain reaction (PCR) nasopharyngeal or oropharyngeal SARS-CoV-2 swab were screened for potential study inclusion. The institutional review board of RIH and TMH approved this observational study.
Remdesivir Study Arm
Starting on March 20, both RIH and TMH became part of a phase 3, multicenter, open-label clinical trial (NCT04292899) [10]. All patients hospitalized with PCR-confirmed COVID-19 were evaluated by Infectious Diseases specialists in both hospitals for potential trial inclusion. To be considered eligible for trial inclusion, patients had to meet the following criteria: (1) currently hospitalized, aged ≥18 years, (2) SARS-CoV-2 infection confirmed by PCR test ≤4 days before trial enrollment, (3) SpO2 ≤94% on room air or requiring supplemental oxygen at screening, (4) presence of radiographic evidence of pulmonary infiltrates. In addition, patients who met any of the following clinical exclusion criteria were not considered eligible: (1) ALT or AST >5 times of the upper normal limit (UNL), (2) creatinine clearance <50 mL/min using the Cockcroft-Gault equation, (3) pregnant or breastfeeding.
Trial participants received remdesivir intravenously (IV) as a 200-mg loading dose on day 1, followed by a daily 100-mg maintenance dose on days 2–10 or until hospital discharge, death, or meeting criteria for study drug hold or discontinuation (serious adverse event related to remdesivir [RDV], ALT >5×UNL, ALT >3×UNL and total bilirubin >2×UNL, creatinine clearance <30 mL/min). Patients who were discharged before day 28 were followed up with a postdischarge follow-up phone call on day 28. The trial protocol was approved by a centralized institutional review board and was monitored by an independent data and safety monitoring board. Each patient provided informed consent. If the patient was unable to provide consent, the patient’s legally authorized representative provided surrogate consent.
Supportive Care Study Arm Patient Selection
In the above-mentioned phase 3 open-labeled trial, all trial participants received remdesivir, and there was no placebo-controlled study arm. For the purposes of the present study, we created a control group consisting of hospitalized patients with PCR-confirmed COVID-19 who did not receive remdesivir. In order to identify controls, we screened all patients who were admitted to either RIH or TMH from February 27 to May 11, 2020, and did not receive remdesivir. After identifying those patients and in an effort to minimize selection bias, we used the following inclusion and exclusion criteria: (1) hospitalized for at least 48 hours, (2) SARS-CoV-2 infection confirmed by PCR, (3) SpO2 ≤94% on room air or requiring supplemental oxygen within the first 48 hours of admission, (4) presence of radiographic evidence of pulmonary infiltrates. In addition, patients who met any of the following clinical exclusion criteria were not considered eligible: (1) ALT or AST >5 times of the upper normal limit (UNL) and (2) creatinine clearance (CrCl) <50 mL/min using the Cockcroft-Gault equation.
Additional Exclusion Criteria
Patients from the remdesivir arm could have been enrolled in the remdesivir trial during any day of their hospital stay, as long as they met the predefined inclusion criteria. For the purposes of this study, this could have been a potential source of immortal time bias [11] because those patients should have been alive at least until the day of remdesivir trial enrollment. As a result, after defining the date of hospital admission as day 1 for both study arms, we included only patients who received remdesivir within the first 48 hours of their admission, while we excluded all patients who died within the first 48 hours from both study arms.
Data Collection
We obtained data through our institution’s electronic medical records (EMRs). For each patient, we extracted the following information: age, sex, race, ethnicity, days from onset of symptoms, imaging results, weight, vital signs and laboratory values (both on admission and during hospitalization), preexisting medical conditions, admission to the intensive care unit (ICU), use of mechanical ventilation, use of systemic corticosteroids [12], hospitalization outcome (death or discharge), and incidence of acute kidney injury (AKI) using the KDIGO criteria [13]. The first available vital signs and laboratory values within 48 hours of admission were used to assess inclusion eligibility for patients in the supportive care arm. For patients in the supportive care arm, we also calculated CrCl on admission using the Cockcroft-Gault equation.
Finally, all hospitalized patients with PCR-confirmed COVID-19 were evaluated by ID specialists for potential inclusion in the remdesivir trial, and this evaluation was documented in the EMR. For patients in the supportive care arm, we additionally extracted the initial ID evaluation and, if present, the reason/s for not enrolling in the remdesivir trial.
Outcomes of Interest
The primary outcome of interest was the impact of remdesivir on all-cause in-hospital death by day 28. As secondary outcomes, we assessed the impact of remdesivir on time to clinical recovery, time to clinical improvement, and time to discharge. All outcomes were censored at day 28 for patients hospitalized >28 days. Recovery was achieved when a patient satisfied categories 1 or 2 on the following 6-point ordinal scale: 1 = not hospitalized; 2 = hospitalized without requiring further supplemental oxygen; 3 = hospitalized requiring supplemental oxygen; 4 = hospitalized requiring noninvasive positive pressure ventilation (NIPPV) or high-flow oxygen devices (HFODs); 5 = hospitalized requiring invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); 6 = death. Similarly, clinical improvement was achieved when a patient had a 2-point improvement on the aforementioned 6-point ordinal scale. Finally, using the KDIGO criteria, we assessed the incidence of AKI in patients receiving remdesivir compared with patients receiving supportive care. KDIGO defines AKI as any of the following: increase in serum creatinine by ≥0.3 mg/dL within 48 hours or increase in serum creatinine to ≥1.5 times baseline within the last 7 days or urine output <0.5 mL/kg/h for 6 hours [13].
Statistical Analysis
For patients’ baseline characteristics, we represented continuous measurements as means (SDs) or medians (IQRs), and we compared them using the Student t test and the Mann-Whitney-Wilcoxon test, respectively. For categorical data, we used Pearson’s chi-square test.
For the primary study outcome, that is, the impact of remdesivir use on in-hospital death, we utilized both univariate and multivariate Cox proportional hazards models. In the multivariate model, we accounted for potential confounders associated with COVID-19 mortality such as age, sex, race, chronic comorbidities (heart disease, diabetes, hypertension, chronic pulmonary disease, obesity), use of systemic corticosteroids, and patients’ oxygen requirements on admission. Similar models were implemented to assess the risk of developing AKI during the 28-day follow-up period. For all models, we assessed the proportional hazards assumption using weighted Schoenfeld residuals.
To evaluate the secondary outcomes of time to clinical recovery, time to clinical improvement, and time to discharge (measured from the time of admission), we utilized a stratified log-rank test and calculated the Mantel-Cox rate ratios. We stratified based on oxygen requirements on admission (ie, room air with spO2 <94%, low-flow oxygen, NIPPV or HFOD, mechanical ventilation), age group (18–49, 50–64, ≥65 years), race, sex, and date of symptom onset (<7 days, ≥7 days). If a patient died before day 28, they were right-censored at day 28. Outcomes of patients who were discharged to a hospice before day 28 were censored at day of discharge.
For our analyses, 95% confidence intervals and P values are shown. The statistical significance threshold was set at .05. All analyses were performed using Stata, version 16.1 (Stata Corporation, College Station, TX, USA).
RESULTS
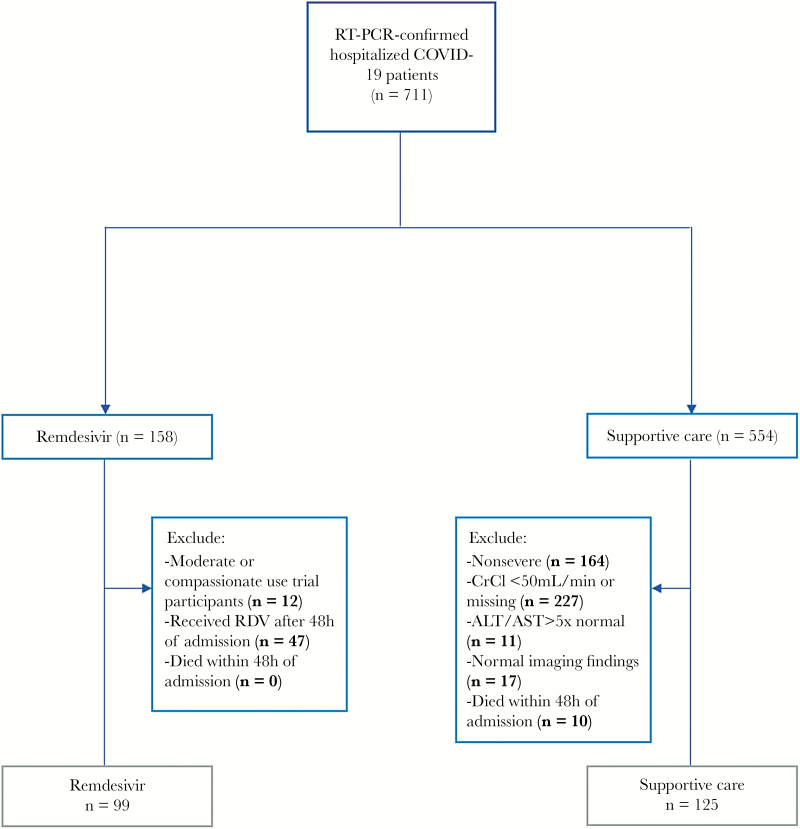
From February 27 to May 1, we identified 711 consecutive patients hospitalized with PCR-confirmed COVID-19. Among those patients, 554 received supportive care and 158 received remdesivir. Among patients who received supportive care, 125 met the inclusion criteria of our study and comprised the control group. More specifically, 227 patients were excluded due to CrCl <50 L/min or because CrCl could not be calculated due to missing weight measurement, 164 patients did not meet severity inclusion criteria, 11 were excluded due to ALT or AST >5 times the UNL, 17 patients were excluded due to normal chest imaging findings, and 10 patients were excluded due to death within the first 48 hours after admission. Among the 158 patients who received RDV, we excluded 12 patients who received remdesivir for the purposes of other clinical trials (a moderate severity trial or compassionate use) and 47 patients who received RDV after the first 48 hours (Supplementary Table 1). In Figure 1, we present the detailed patient selection flowchart. As of June 7, 2020, all patients had completed the 28-day observational period, were discharged, or died. Of note, none of the patients included in this analysis met the criteria for remdesivir discontinuation.
Figure 1.
Patient selection flowchart. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CrCl, creatinine clearance; RDV, remdesivir; RT-PCR, reverse transcription polymerase chain reaction.
Baseline Characteristics
In total, 224 patients were included in the study, and their baseline characteristics are depicted in Table 1. The median age (interquartile range [IQR]) was 59 (49–67) years, and 67.0% were male. Moreover, 82 (36.6%) were Non-Hispanic white, 98 (43.8%) were Hispanic, 30 (13.4%) were black or African American, and 2 (0.9%) were Asians. No differences in terms of age, race, and sex distribution were noted among the 2 study arms. In terms of preexisting chronic conditions, patients on the supportive care arm had higher prevalence of chronic heart disease (28.0%), compared with patients who received remdesivir (14.1%,). The median time from symptom onset assessed at the time of admission (IQR) was 6 (3–8) days, while there were no significant differences in terms of oxygen requirements at the time of admission between the 2 study arms.
Table 1.
Baseline Patients Characteristics on Admission
| Total | Supportive Care | Remdesivir | ||
|---|---|---|---|---|
| n = 224 | n = 125 | n = 99 | P Value | |
| Age | 59.00 (50.00–68.00) | 60.00 (50.00–68.00) | 58.00 (50.00–68.00) | .52 |
| Gender | .44 | |||
| Female | 74 (33.0) | 44 (35.2) | 30 (30.3) | |
| Male | 150 (67.0) | 81 (64.8) | 69 (69.7) | |
| Race | .65 | |||
| Asian | 2 (0.9) | 1 (0.8) | 1 (1.0) | |
| Black or African American | 30 (13.4) | 20 (16.0) | 10 (10.1) | |
| Hispanic or Latino | 98 (43.8) | 50 (40.0) | 48 (48.5) | |
| Other/unknown | 12 (5.4) | 7 (5.6) | 5 (5.1) | |
| White or Caucasian | 82 (36.6) | 47 (37.6) | 35 (35.4) | |
| Hypertension | 87 (38.8) | 55 (44.0) | 32 (32.3) | .075 |
| Heart disease | 49 (21.9) | 35 (28.0) | 14 (14.1) | .013 |
| Chronic pulmonary disease | 26 (11.6) | 15 (12.0) | 11 (11.1) | .84 |
| Diabetes | 60 (26.8) | 32 (25.6) | 28 (28.3) | .65 |
| Renal failure | 10 (4.5) | 7 (5.6) | 3 (3.0) | .36 |
| Liver disease | 9 (4.0) | 5 (4.0) | 4 (4.0) | .99 |
| Obesity | 103 (46.0) | 60 (48.0) | 43 (43.4) | .50 |
| Days from symptoms onset | 6.00 (3.00–8.00) | 6.00 (2.00–7.00) | 6.00 (3.00–8.00) | .59 |
| Baseline oxygen | .75 | |||
| Invasive mechanical ventilation | 43 (19.5) | 24 (19.7) | 19 (19.2) | |
| Low-flow supplemental oxygen | 108 (48.9) | 60 (49.2) | 48 (48.5) | |
| NIPPV or HFOD | 55 (24.9) | 28 (23.0) | 27 (27.3) | |
| Room air | 15 (6.8) | 10 (8.2) | 5 (5.1) | |
| Corticosteroidsa | 61 (27.2) | 34 (27.2) | 27 (27.3) | .99 |
| Hydroxychloroquine | 25 (11.2) | 20 (16.0) | 5 (5.1) | .010 |
| Convalescent plasma | 19 (8.5) | 16 (12.8) | 3 (3.0) | .009 |
| Weighted Elixhauser index (van Walraven) | .009 | |||
| <0 | 53 (23.7) | 24 (19.2) | 29 (29.3) | |
| 0 | 61 (27.2) | 27 (21.6) | 34 (34.3) | |
| 1–4 | 30 (13.4) | 21 (16.8) | 9 (9.1) | |
| ≥5 | 80 (35.7) | 53 (42.4) | 27 (27.3) |
Bold values: P < 0.05. Data are presented as No. (%) or median (interquartile range). Comorbidities were estimated using ICD-10 codes according to the Elixhauser Comorbidity Index.
Abbreviations: HFOD, high-flow oxygen devices; ICD-10, International Classification of Diseases, 10th Revision; NIPPV, noninvasive positive pressure ventilation.
aAt least 1 dose of dexamethasone, fludrocortisone, hydrocortisone, methylprednisolone, or prednisone.
Among the control group, reasons for not enrolling in the remdesivir trial included the following: 45 patients (36.0%) were deemed eligible for enrollment by the trial investigators, but they were not enrolled for undocumented reasons; 33 patients (26.4%) refused participation; 19 patients (15.2%) were not eligible given that their SARS-COV-2 PCR was older than 96 hours (trial exclusion criterion) but met the rest of the inclusion/exclusion criteria; 25 patients (20.0%) were eligible, but the trial was paused to enrollment at the time of assessment; and 3 patients (2.4%) were eligible but were unable to provide consent, and a legally authorized representative was not available.
Primary Outcome
In total, 24/224 patients (10.7%) died within 28 days of their hospital admission. More specifically, 17/125 patients (13.6%) who received supportive care and 7/99 patients (7.1%) who received remdesivir died. In our primary unadjusted analysis, the risk of 28-day all-cause in-hospital death was lower for patients who received remdesivir compared with patients who received supportive care (hazard ratio [HR], 0.42; 95% CI, 0.16–1.08), albeit it did not reach statistical significance. These estimates remained after adjusting for age, sex, race, oxygen requirements on admission (adjusted HR [aHR], 0.49; 95% CI, 0.19–1.28), use of systemic corticosteroids, and chronic comorbidities such as hypertension, heart disease, chronic pulmonary disease, obesity, and diabetes (aHR, 0.44; 95% CI, 0.16–1.23) (Table 2; Supplementary Figure 1).
Table 2.
Cox Proportional Hazards Models for 28-Day Outcomes
| Remdesivir vs Supportive Care Hazard Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| Outcome | Unadjusted | Adjusted for Age, Sex, Race, and O2 Requirements on Admission | Fully Adjusteda |
| All cause in-hospital death | 0.42 (0.16–1.08) | 0.49 (0.19–1.28) | 0.44 (0.16–1.23) |
| Development of AKI | 0.98 (0.58–1.63) | 0.97 (0.57–1.64) | 1.10 (0.64–1.90) |
Abbreviation: AKI, acute kidney injury.
aAge, sex, race, O2 requirements on admission, use of systemic corticosteroids, heart disease, hypertension, chronic pulmonary disease, diabetes, obesity.
Secondary Outcomes
The median time to clinical recovery was 10 days for the remdesivir arm (95% CI, 8–12) and 10 days for the supportive care arm (95% CI, 8–13). Using Mantel-Cox rate ratios, we found no significant difference in time to recovery, regardless of the stratification variable. Similarly, we found no significant differences between the 2 study arms in terms of time to clinical improvement and time to discharge. Although not reaching statistical significance, the rate ratios for time to recovery, clinical improvement, and discharge were higher in women and black or African American patients who received remdesivir. Finally, the rate ratio for time to recovery and time to discharge was higher among patients who received remdesivir during the first 7 days after symptom onset, albeit without reaching statistical significance (rate ratio [RR], 1.26; 95% CI, 0.83–1.92; and RR, 1.24; 95% CI, 0.81–1.90; respectively). Results are presented in Table 3.
Table 3.
Mantel-Cox Rate Ratios for Time Clinical Recovery, Improvement, or Discharge
| Remdesivir vs Supportive Care Rate Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| Stratification Subgroup | Time to Recovery | Time to Clinical Improvement | Time to Discharge |
| Sex | |||
| Male | 0.83 (0.56–1.22) | 0.82 (0.56–1.21) | 0.85 (0.58–1.26) |
| Female | 1.48 (0.83–2.63) | 1.50 (0.85–2.64) | 1.38 (0.76–2.52) |
| Age group | |||
| 18–49 y | 1.17 (0.61–2.21) | 1.02 (0.54–1.93) | 1.13 (0.59–2.13) |
| 50–64 y | 0.95 (0.57–1.56) | 0.94 (0.57–1.54) | 0.93 (0.56–1.54) |
| ≥65 y | 0.96 (0.55–1.69) | 1.06 (0.60–1.87) | 1.01 (0.55–1.83) |
| Race | |||
| Black or African American | 2.12 (0.85–5.28) | 1.67 (0.68–4.08) | 2.29 (0.94–5.57) |
| Hispanic or Latino | 0.82 (0.51–1.33) | 0.76 (0.47–1.24) | 0.72 (0.44–1.18) |
| White or Caucasian | 1.14 (0.66–1.97) | 1.19 (0.68–2.07) | 1.27 (0.73–2.24) |
| Other | 0.37 (0.08–1.63) | 0.18 (0.03–1.06) | 0.36 (0.07–1.69) |
| Oxygen requirementsa | |||
| IMV | 0.93 (0.40–2.14) | 0.93 (0.42–2.07) | 1.07 (0.44–2.61) |
| NIPPV or HFOD | 0.87 (0.44–1.70) | 0.97 (0.50–1.87) | 0.86 (0.44–1.69) |
| Low-flow oxygen | 1.06 (0.67–1.68) | 1.04 (0.67–1.63) | 1.03 (0.66–1.69) |
| Room air | 1.30 (0.30–5.67) | 1.18 (0.32–4.36) | 1.18 (0.32–4.36) |
| Days from symptoms onseta | |||
| <7 d | 1.26 (0.83–1.92) | 1.18 (0.77–1.79) | 1.24 (0.81–1.90) |
| ≥7 d | 0.76 (0.46–1.26) | 0.82 (0.50–1.34) | 0.71 (0.42–1.18) |
Abbreviations: HFNC, high-flow nasal cannula; HFOD, high-flow oxygen devices; IMV, invasive mechanical ventilation; NIPPV, noninvasive positive pressure ventilation.
aOn admission, IMV, NIPPV, HFNC.
Adverse Events and Risk of AKI Development
Utilizing the KDIGO criteria for AKI and the Common Terminology Criteria for Adverse Events (CTCAE; version 5.0), we studied the incidence of AKI, transaminitis, and hyperbilirubinemia and found no statistically significant difference among the 2 groups (Table 4). The use of remdesivir was not associated with an increased risk of AKI in both univariate (HR, 0.98; 95% CI, 0.58–1.63) and multivariate Cox proportional hazards models, which were adjusted for age, sex, race, and oxygen requirements on admission (aHR, 0.97; 95% CI, 0.57–1.64), as well as use of systemic corticosteroids and chronic comorbidities (aHR, 1.10; 95% CI, 0.64–1.90) (Table 2).
Table 4.
Adverse Events and Lab Abnormalities
| Total | Supportive Care | Remdesivir | P Value | |
|---|---|---|---|---|
| n = 224 | n = 125 | n = 99 | ||
| Acute kidney injury | .12 | |||
| Stage 1 | 31 (52) | 17 (50) | 14 (54) | |
| Stage 2 | 5 (8) | 5 (15) | 0 (0) | |
| Stage 3 | 24 (40) | 12 (35) | 12 (46) | |
| Aspartate aminotransferase increase | .20 | |||
| Grade 1 | 66 (96) | 31 (91) | 35 (100) | |
| Grade 2 | 1 (1) | 1 (3) | 0 (0) | |
| Grade 3 | 2 (3) | 2 (6) | 0 (0) | |
| Alanine aminotransferase increase | .25 | |||
| Grade 1 | 63 (95) | 32 (91) | 31 (100) | |
| Grade 2 | 0 (0) | 0 (0) | 0 (0) | |
| Grade 3 | 2 (3) | 2 (6) | 0 (0) | |
| Grade 4 | 1 (2) | 1 (3) | 0 (0) | |
| Serum total bilirubin increase | .94 | |||
| Grade 1 | 16 (47) | 8 (44) | 8 (50) | |
| Grade 2 | 11 (32) | 6 (33) | 5 (31) | |
| Grade 3 | 7 (21) | 4 (22) | 3 (19) |
Data are presented as No. (%). ALT, AST, and bilirubin were graded using CTCAE, version 5.0. AKI was graded according to the KDIGO criteria.
Abbreviations: AKI, acute kidney injury; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events.
DISCUSSION
In order to provide a cohesive assessment of the efficacy of remdesivir, we compared the clinical outcomes of patients who were hospitalized with severe COVID-19 (requiring supplemental oxygen and having abnormal imaging findings) and received either remdesivir or supportive care. After using similar inclusion and exclusion criteria for both study arms, we found that patients on remdesivir had lower all-cause in-hospital mortality, but this did not reach statistical significance. In addition, we observed a shortened time to recovery, time to clinical improvement, and time to discharge among women and Black or African American patients, again without reaching statistical significance. Importantly, the use of remdesivir did not increase liver test abnormalities or the risk for AKI.
To date, there are limited studies describing the safety and efficacy of remdesivir in patients with COVID-19. In the study by Grein et al. [5], the authors described the compassionate use of remdesivir in patients with severe COVID-19. In this multicenter study, 53 patients received remdesivir for 10 days (200 mg on day 1 followed by 100 mg for 9 days) and had a median follow-up of 18 days. The authors reported oxygen need improvement in 68% of patients, while 7 out of 53 patients (13%) died. However, the interpretation of those findings was limited by the lack of a control arm and the small sample size. In April 2020, Wang et al. reported the first randomized, double-blind, placebo-controlled trial comparing patients with severe COVID-19 who received either remdesivir or placebo [7]. The study included 237 patients from China and was terminated early due to lack of eligible study participants (initial sample goal was 453 patients). The authors found that remdesivir use did not significantly improve the time to clinical improvement (HR, 1.23) or 28-day mortality.
The first study to yield a clear clinical benefit from remdesivir use in patients with COVID-19 was the preliminary report from the ongoing double-blind, randomized, placebo-controlled trial by the National Institute of Allergy and Infectious Diseases (NIAID) [8]. In this study, the authors found that patients who received 10 days of remdesivir had a significantly faster time to recovery compared with the placebo group. Our study did not yield the same clinical benefit in terms of time to recovery, possibly due to a smaller sample size, differences in patient characteristics and baseline oxygen needs, and lack of randomization. However, our mortality estimates were similar. Specifically, Beigel et al. reported a 7.1% and 11.9% 14-day mortality for the remdesivir and placebo groups, respectively, while they reported an HR for death of 0.70 (95% CI, 0.47–1.04). Similarly, we found a 7.1% and 13.6% 28-day in-hospital mortality for the remdesivir and supportive care groups, respectively, while our adjusted HR estimate for death was 0.44 (95% CI, 0.16–1.23).
Although the current literature and our study suggest that there are potential benefits from remdesivir use, the magnitude of those benefits will also be determined by remdesivir safety, access, and cost [14]. In terms of safety, remdesivir appears to be tolerated well by most patients [7], and our findings showed that the risk of 28-day AKI development was not increased compared with the supportive care arm. Regarding the access and cost, as current supplies of the drug and its worldwide availability remain questionable [15], it is important for future studies to confirm the noninferiority of a 5-day compared with a 10-day regimen [10], as well as identify those patients who may benefit the most.
By imposing the same exclusion criteria for patients who participated in the remdesivir trial and for those who composed our study’s control arm, we minimized selection bias. This was also reflected by similar baseline characteristics and oxygen needs among the two arms. Then, by determining the reasons that patients in the control arm were not enrolled in the remdesivir trial, we confirmed that indication bias was minimized in this observational study. In addition, by including only those patients who received remdesivir within the first 48 hours after admission and excluding patients from the control arm who died within the first 48 hours after admission, we minimized immortal time bias. Finally, utilizing data from consecutive patients hospitalized in the same institution, we minimized any differences in terms of treatment protocols (eg, ventilatory strategies, concomitant medications) as well as hospitalization and discharge thresholds across the study population.
Our study has limitations that should be taken into consideration. First, the observational, nonrandomized nature of the present study introduces the possibility of bias inherent to this study design. For example, the rationale behind a patient’s decision to refuse participation in the remdesivir trial may reflect less severe disease, which cannot be measured by baseline oxygen needs alone. Although baseline oxygen needs and other related conditions were similar among the 2 study arms, there is a chance that at least some patients in the supportive arm were less sick. Furthermore, in order to minimize selection bias, we excluded patients who received remdesivir within the the first 48 hours after admission. In Supplementary Table 1, we include the characteristics and outcomes of all the patients enrolled who received remdesivir, irrespective of time of treatment initiation, including those who received remdesivir within the first 48 hours after admission and were excluded from the analysis. In addition, as in all similar trials, many patients reported an estimated and not an exact date of symptom onset. Finally, the present study might be underpowered [14].
In conclusion, our single-center analysis adds to the understanding that remdesivir may have potential clinical benefits in patients with severe COVID-19, and the use of remdesivir did not increase liver test abnormalities or the risk for AKI. Notably, there was a trend for shortened time to recovery, time to clinical improvement, and time to discharge among women and black or African American patients. These promising signals need to be confirmed by future trials. Such trials should include evaluation for women and racial groups.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
This work was facilitated by the Providence/Boston Center for AIDS Research (P30AI042853) and the NIAID AIDS Clinical Trials Unit UM1 A1069412.
Author contributions. Conceptualization: M.K., F.S., K.T.T., E.K.M., E.M.; methodology: F.S., M.K., E.K.M.; validation: M.K., K.T.T., E.K.M., F.S., E.M.; formal analysis: F.S., M.K., E.K.M.; investigation: all authors; data curation: F.S.; writing original draft: M.K., F.S.; writing review & editing: all authors; visualization: M.K., F.S.
Financial support. None.
Potential conflicts of interest. K.T.T. has received grant support from Gilead Sciences, Inc. T.P.F. and his family own stock in Gilead sciences. The rest of the authors declare no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Eastman RT, Roth JS, Brimacombe KR, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci 2020; 6:672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A 2020; 117:6771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med 2017; 9:eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID-19. N Engl J Med 2020; 382:2327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med 2020. doi: 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 9. US Food Drug and Administration. Fact sheet for health care providers: emergency use authorization (EUA) of remdesivir (GS-5734™). Available at: https://www.fda.gov/media/137565/download. Accessed 23 June 2020.
- 10. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med 2020. doi: 10.1056/NEJMoa2015301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010; 340:b5087. [DOI] [PubMed] [Google Scholar]
- 12. The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 — preliminary repor. N Engl J Med 2020. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kellum JA, Lameire N; KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care 2013; 17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Norrie JD. Remdesivir for COVID-19: challenges of underpowered studies. Lancet 2020; 395:1525–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilead Sciences Inc. Gilead sciences statement on expanding global supply of investigational antiviral remdesivir. Available at: https://www.gilead.com/news-and-press/company-statements/gilead-sciences-statement-on-remdesivir-global-supply. Accessed 23 June 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.