Abstract
Background
Data pertaining to COVID-19 in pregnancy are limited; to better inform clinicians, we collated data from COVID-19 cases during pregnancy and summarized clinical trials enrolling this population.
Methods
We performed a systematic literature review of PubMed/MEDLINE to identify cases of COVID-19 in pregnancy or the postpartum period and associated outcomes. We then evaluated the proportion of COVID-19 clinical trials (from ClinicalTrials.gov) excluding pregnant or breastfeeding persons (both through June 29, 2020).
Results
We identified 11 308 published cases of COVID-19 during pregnancy. Of those reporting disease severity, 21% (416/1999) were severe/critical. Maternal and neonatal survival were reassuring (98% [10 437/10 597] and 99% [1155/1163], respectively). Neonatal disease was rare, with only 41 possible cases of infection reported in the literature. Of 2351 ongoing COVID-19 therapeutic clinical trials, 1282 were enrolling persons of reproductive age and 65% (829/1282) excluded pregnant persons. Pregnancy was an exclusion criterion for 69% (75/109) of chloroquine/hydroxychloroquine, 80% (28/35) of lopinavir/ritonavir, and 48% (44/91) of convalescent plasma studies. We identified 48 actively recruiting or completed drug trials reporting inclusion of this population.
Conclusions
There are limited published reports of COVID-19 in pregnancy despite more than 14 million cases worldwide. To date, clinical outcomes appear reassuring, but data related to important long-term outcomes are missing or not yet reported. The large number of clinical trials excluding pregnant persons, despite interventions with safety data in pregnancy, is concerning. In addition to observational cohort studies, pregnancy-specific adaptive clinical trials could be designed to identify safe and effective treatments.
Keywords: breastfeeding, coronavirus, COVID-19, pregnancy, SARS-CoV-2
Worldwide, it is estimated that there are nearly 2 billion women of reproductive age, and women comprise upwards of 70% of the total health care workforce [1, 2]. Pregnant women and women of reproductive potential are therefore at a high risk of contracting COVID-19. In a recent Centers for Disease Control and Prevention (CDC) report of 7162 patients with COVID-19 in the United States, as many as 2% of cases occurred in pregnant women [3]. In prior work examining influenza, severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS), pregnant women had increased morbidity and mortality when compared with nonpregnant women [4–6]. Currently, there is insufficient evidence to determine whether the same is true for pregnant women with COVID-19. It is hypothesized that the natural physiologic state of immune tolerance associated with pregnancy may increase the risk of infection and severe complications [7].
There is a need to identify safe and effective treatment options for pregnant persons with COVID-19, as there is for all populations, but there are multiple barriers preventing their inclusion in clinical trials. Due to safety concerns for the mother and the potential for teratogenicity, both pregnant and breastfeeding people are frequently excluded from clinical research studies, often in spite of established pharmacological safety data in pregnancy. However, drug metabolism (and therefore efficacy) in pregnancy differs from the general population, arguing for their inclusion in trials; optimal dosing requires special attention to physiologic changes (eg, increased glomerular filtration rate or decreased plasma albumin concentrations), which can alter pharmacokinetics and pharmacodynamics of drug concentrations. Fetal organogenesis also needs to be factored in when possible. Finally, as pregnancy is a state of immunologic change to accommodate the fetus, it is unclear how therapeutic immune-modulators used in COVID-19 (eg, tocilizumab and sarilumab) may impact maternal and fetal outcomes.
In this systematic review, we sought to highlight the heterogeneity of treatment and outcome data for pregnant persons with COVID-19 and summarize the literature related to COVID-19 in pregnancy. We provide an overview of the proportion of completed and actively recruiting interventional clinical trials permitting the inclusion of pregnant or breastfeeding individuals to underscore how frequently this population is ineligible for trial participation and to discuss the potential implications.
METHODS
Systematic Literature Review
A systematic literature review was conducted to identify cases of COVID-19 in pregnancy that were reported as of June 29, 2020, following the PRISMA checklist. The first author searched PubMed/MEDLINE for all language publications and preprints using a combination of the following MeSH keywords: “pregnancy,” “postpartum,” “COVID-19,” and “SARS-CoV-2.” The first and third authors screened abstracts and reviewed the potential publications and references for relevance to identify any missing articles that were not identified using the above-listed keywords. Details as they pertained to presenting symptoms, any treatments received, and maternal, fetal/neonatal outcomes were recorded. Studies unrelated to cases of COVID-19 in pregnancy or the peripartum period were excluded.
Inclusion of Pregnant Women in COVID-19 Clinical Trials
A second search was conducted to identify COVID-19 clinical trials as of June 29, 2020, that reported pregnancy/breastfeeding as an exclusion criterion. ClinicalTrials.gov was searched for relevant studies, using the preprogrammed search terms “COVID-19,” “SARS-CoV-2,” “2019-nCoV,” “2019 novel coronavirus,” and “severe acute respiratory syndrome coronavirus 2.” Inclusion and exclusion criteria were examined to determine whether pregnant and/or breastfeeding patients were excluded from the study. Studies listed as interventional clinical trials were then analyzed. Information related to the specific therapeutic intervention and the status of recruitment were summarized. Of the actively ongoing interventional clinical trials investigating the use of a drug (including dietary supplements and biologic agents) that did not report the exclusion of pregnant or breastfeeding women, the first author contacted study personnel for each of these studies by email to discern whether pregnant or breastfeeding women were eligible to be enrolled.
Patient Consent Statement
Patient consent is not applicable, as this article is a systematic review.
RESULTS
Literature Review
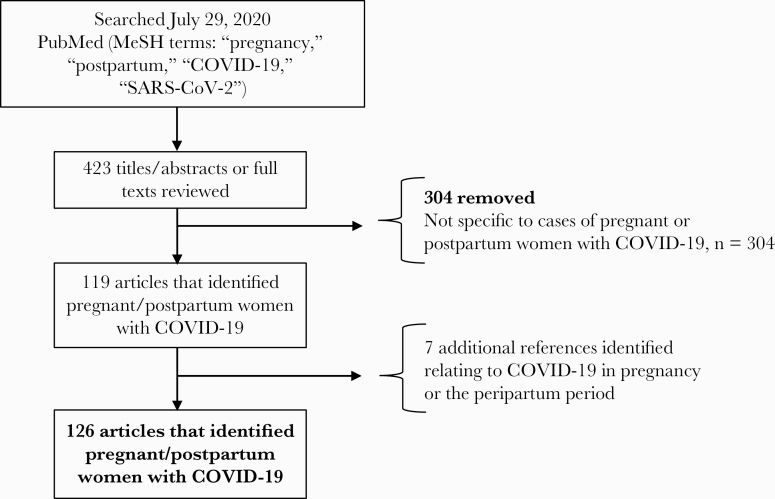
In our systematic literature review, we identified 11 308 reported cases of COVID-19 in pregnancy and the postpartum period from a total of 52 case reports, 44 case series, 25 prospective/retrospective cohort studies, 3 governmental or national reports, and 2 case–control studies (Table 1, Figure 1). Of those that reported estimated gestational age at the time of diagnosis (n = 2824), 77% (2184/2824) were in the third trimester of pregnancy (>28 weeks), with the majority in the peripartum/postpartum period; 20% (574/2824) were in the second trimester, and 2% (66/2824) were in the first trimester.
Table 1.
Systematic Review of COVID-19 in Pregnancy and the Peripartum Period
| Total No. of Cases (n = 11 308) | No. (%) |
|---|---|
| Estimated gestational age (n = 2824)a | |
| 1st trimester (1–12 wk) | 66 (2) |
| 2nd trimester (13–27 wk) | 574 (20) |
| 3rd trimester (>28 wk) | 2184 (77) |
| Symptoms (n = 11 308) | |
| Cough | 2704 (24) |
| Fever | 2051 (18) |
| Fatigue, malaise, myalgias | 1525 (13) |
| Headache | 1448 (13) |
| Shortness of breath, dyspnea | 1391 (12) |
| Otherb | 4120 (36) |
| Asymptomatic | 366 (3) |
| Severity (as defined by authors; n = 1999) | |
| Mild–moderatec | 1583 (79) |
| Severe/ICU | 416 (21) |
| Investigational treatment reported (n = 184)d | |
| Hydroxychloroquine | 106 (58) |
| Lopinavir/ritonavir | 39 (21) |
| Remdesivir | 13 (7) |
| IL-1, IL-6 inhibitors | 13 (7) |
| IVIG or gamma globulin | 9 (5) |
| Convalescent plasma | 4 (2) |
| Maternal outcomes (n = 10 597) | |
| Alive to delivery | 10 437 (98) |
| Died | 33 (0.3) |
| Still hospitalized | 127 (1.0) |
| Pregnancy outcomes (n = 2297) | |
| Live births | 1375 (60) |
| In utero, pregnancy ongoing | 869 (38) |
| Spontaneous miscarriage (<20 wk) | 20 (0.9) |
| Elective abortion | 17 (0.7) |
| Stillbirth (>20 wk) | 14 (0.6) |
| Ectopic pregnancy | 2 (0.08) |
| Gestational age at birth (n = 868) | |
| Term | 695 (80) |
| Preterm (<37 wk) | 173 (20) |
| Delivery type (n = 871) | |
| Vaginal delivery | 259 (30) |
| Cesarean section | 612 (70) |
| Neonatal outcomes (n = 1163) | |
| Alive | 1155 (99) |
| Neonatal death | 8 (0.7) |
Abbreviations: ICU, intensive care unit; IL, interleukin; IVIG, intravenous immunoglobulin.
aOne large cohort study (n = 8207) did not have estimated gestational ages available.
bOther symptoms included diarrhea (n = 596), nausea/vomiting (n = 700), sore throat (n = 1005), loss of taste or smell (n = 803), and chills (n = 1016).
cIncluded asymptomatic cases.
dTreatment received at any time during pregnancy or postpartum after the time of COVID-19 diagnosis. Some women may have received multiple treatments.
Figure 1.
Search strategy of reported cases of COVID-19 in pregnancy in the literature.
The most common symptoms reported were cough (24%, 2704/11 308) and fever (18%, 2051/11 308), followed by fatigue, malaise, or myalgias (13%, 1525/11 308), headache (13%, 1448/11 308), and shortness of breath or dyspnea (12%, 1391/11 308); overall, <5% were reported as entirely asymptomatic (366/11 308 or 3%). Not all studies reported maternal symptoms. The majority of these COVID-19 cases were mild to moderate in severity (79% [1583/1999]), with 21% (416/1999) being defined as severe or critical by the authors, requiring intensive care unit admission or intubation; 82% (9309/11308) did not comment on disease severity. Several women were reported to have developed disease or worsening of symptoms postpartum, though these instances and associated time intervals were not always reported; thus, no conclusions can be drawn related to the possibility of immune reconstitution given the heterogeneity of data. Overall, 98% of women (10 437/10 597) survived to delivery or hospital discharge. There were 33 maternal deaths, with 127 patients still hospitalized at the time of publication; 711 maternal outcomes were not reported.
The majority of cases did not report on pregnancy outcomes (80% [9011/11 308]), and 8% of pregnancies were ongoing at the time of publication (869/11 308). Among those that delivered and reported pregnancy outcomes, 96% (1375/1428) of women delivered a live birth (70% [612/871] via C-section). There were 20 spontaneous miscarriages, 17 elective abortions, 14 stillbirths, and 2 ectopic pregnancies. Overall 99% (1155/1163) of fetuses/neonates survived; there were 8 reported neonatal deaths. Neonatal testing for SARS-CoV-2 varied widely, making it challenging to draw any definitive conclusions about maternal fetal transmission. In many case series and case reports, SARS-CoV-2 was tested but not detected in amniotic fluid, breast milk, umbilical cord blood, placental tissue, or maternal vaginal secretions, nor was SARS-CoV-2 detected in neonatal serum, plasma, whole blood, feces, gastric secretions, or throat swabs. Overall, we only identified 41 neonates with possible SARS-CoV-2 infection based on positive polymerase chain reaction (PCR) or antibody tests performed on neonatal specimens (eg, nasopharyngeal/oropharyngeal swabs, blood, stool, and/or unspecified sites) (Supplementary Table 1) [8–25]. The following specimens were also found to be SARS-CoV-2 PCR or IgG/IgM positive: 9 placental specimens [10, 13, 19, 26–28], 3 umbilical cord blood [18, 19, 27], 3 breast milk [13, 19, 29], 3 maternal stool or rectal/anal swabs [18, 29], and 1 sample of maternal vaginal secretions [13]. Several reports have discussed the placental pathology associated with maternal SARS-CoV-2 infection, but have not specifically assessed the placenta for the microbiologic presence of SARS-CoV-2 [13, 16, 27, 28, 30–32].
Reports in the literature on treatment received by peripartum women with COVID-19 have varied greatly, or peripartum treatment was not reported. Overall, we found reports of 106 women who received hydroxychloroquine, 39 lopinavir/ritonavir, 13 remdesivir, 13 interleukin-1 (IL-1) or IL-6 inhibitors, and 9 intravenous immunoglobulin (IVIG) or gamma globulin either during pregnancy or during the postpartum period.
Inclusion of Pregnant Women in Ongoing Clinical Trials
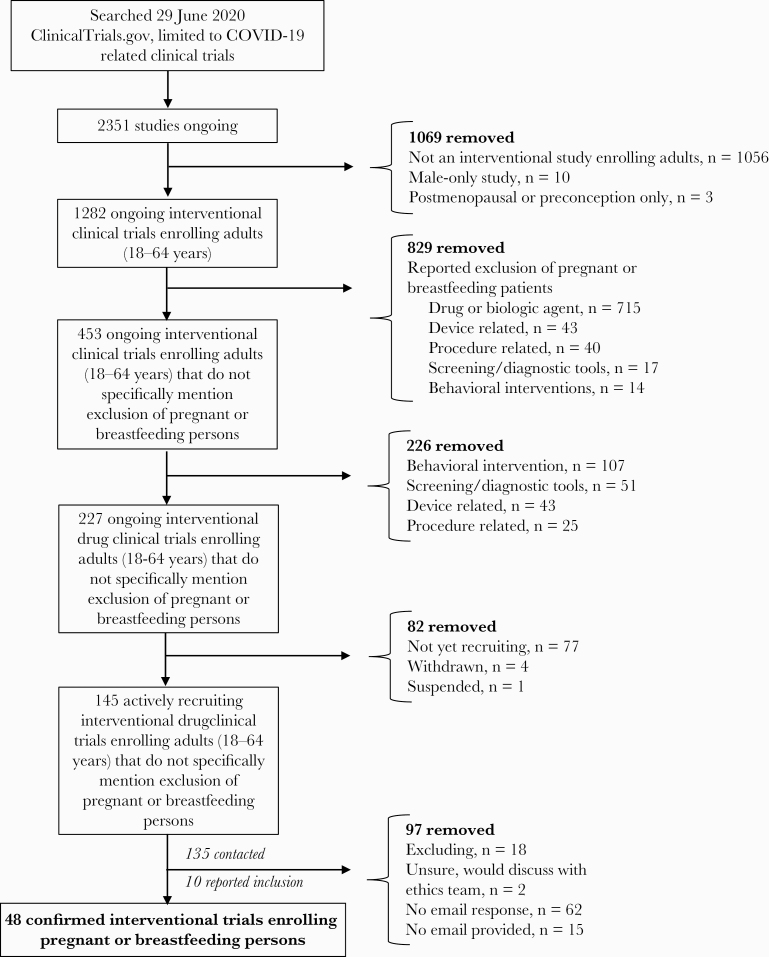
As of June 29, 2020, there were 2351 ongoing COVID-19-related clinical trials registered on ClinicalTrials.gov [33]. Approximately 55% (1282/2351) were interventional clinical trials investigating the therapeutic use of a drug, device, procedure, screening/diagnostic tool, or behavioral intervention in reproductive-age adults (18–64 years); the remainder were observational studies or patient registries. Of the interventional studies, 65% (829/1282) specifically reported the exclusion of pregnant or breastfeeding women (76% [715/942] drug, dietary supplement, or biologic agent, 50% [43/86] device, 62% [40/65] procedure, 25% [17/68] screening/diagnostic tool, 12% [14/121] behavioral intervention). Of the chloroquine/hydroxychloroquine and lopinavir/ritonavir studies, at least 68% (75/109) and 80% (28/35), respectively, excluded pregnant or breastfeeding women despite widespread use in pregnancy for treatment of systemic lupus erythematosus and HIV [34–37]. For convalescent plasma-related studies, at least 48% (44/91) reported the exclusion of pregnant or breastfeeding persons (Supplementary Table 2).
Of the 453 interventional studies registered on ClinicalTrials.gov that did not specifically mention the exclusion of pregnant or breastfeeding women, only 50% (227/453) were studies investigating the therapeutic use of a drug. Of these, 77 were not yet recruiting, 4 were withdrawn, and 1 was suspended—these studies were removed from further analyses. Of the remaining 145 trials, only 7% (10/145) reported specific inclusion of pregnant or breastfeeding patients. The remainder were contacted by email to verify whether pregnant and breastfeeding persons could be enrolled.
A further 38 studies whose investigators were contacted by email verified the inclusion of pregnant and breastfeeding patients; 18 were investigating use of convalescent plasma, 16 chloroquine/hydroxychloroquine, 10 various other drugs, 2 IL-6 inhibitors, and 2 remdesivir. The 2 remdesivir studies clarified that pregnant patients could be enrolled on a request for compassionate use basis only. One study assessing thromboprophylaxis clarified that only breastfeeding patients could be enrolled, and 1 hydroxychloroquine postexposure prophylaxis study reported inclusion of pregnant persons into the control arm only. Overall, 62 trials did not respond to email inquiries, 18 reported the exclusion of pregnant or breastfeeding women, 15 had no email address provided and could not be contacted, and 2 trials were either unsure or reported that they would discuss with an ethics committee (Figure 2). A total of 48 completed or actively recruiting clinical trials reported the inclusion of pregnant or breastfeeding persons (Table 2).
Figure 2.
Search strategy of ongoing COVID-19-related clinical trials permitting the inclusion of pregnant or breastfeeding persons.
Table 2.
COVID-19-Related Clinical Trials Reporting Inclusion of Pregnant or Breastfeeding Women
| NCT Number | Location | Study Name | Recruitment Number | Medication/Therapy Under Investigation |
|---|---|---|---|---|
| Convalescent plasma | ||||
| NCT04411602 | United States | Feasibility Study of Anti-SARS-CoV-2 Plasma Transfusions in COVID-19 Patients With SRD | 90 | |
| NCT04342182 | Netherlands | Convalescent Plasma as Therapy for Covid-19 Severe SARS-CoV-2 Disease (CONCOVID Study) | 426 | |
| NCT04345523 | Spain | Convalescent Plasma Therapy vs. SOC for the Treatment of COVID19 in Hospitalized Patients | 278 | |
| NCT04347681 | Saudi Arabia | Potential Efficacy of Convalescent Plasma to Treat Severe COVID-19 and Patients at High Risk of Developing Severe COVID-19 | 40 | |
| NCT04348656 | United States, Canada | CONvalescent Plasma for Hospitalized Adults With COVID-19 Respiratory Illness (CONCOR-1) | 1200 | |
| NCT04361253 | United States | Evaluation of SARS-CoV-2 (COVID-19) Antibody-containing Plasma thErapy | 220 | |
| NCT04373460 | United States | Convalescent Plasma to Limit SARS-CoV-2 Associated Complications | 1344 | |
| NCT04385199 | United States | Convalescent Plasma for Patients With COVID-19 | 30 | |
| NCT04405310 | Mexico | Convalescent Plasma of Covid-19 to Treat SARS-COV-2 a Randomized Double Blind 2 Center Trial | 80 | |
| NCT04412486 | United States | COVID-19 Convalescent Plasma (CCP) Transfusion | 100 | |
| NCT04356482 | Mexico | Convalescent Plasma for Ill Patients by COVID-19 | 90 | |
| NCT04376034 | United States | Convalescent Plasma Collection and Treatment in Pediatrics and Adults | 240 | |
| NCT04388527 | United States | COVID-19 Convalescent Plasma for Mechanically Ventilated Population | 50 | |
| NCT04392232 | United States | A Study of COVID 19 Convalescent Plasma in High Risk Patients With COVID 19 Infection | 100 | |
| NCT04397757 | United States | COVID-19 Convalescent Plasma for the Treatment of Hospitalized Patients With Pneumonia Caused by SARS-CoV-2 | 80 | |
| NCT04421404 | United States | Effects of COVID-19 Convalescent Plasma (CCP) on Coronavirus-associated Complications in Hospitalized Patients | 30 | |
| NCT04364737 | United States | Convalescent Plasma to Limit COVID-19 Complications in Hospitalized Patients | 300 | |
| NCT04381858 | Mexico | Convalescent Plasma vs Human Immunoglobulin to Treat COVID-19 Pneumonia | 500 | Convalescent plasma vs human immunoglobulin |
| Hydroxychloroquine/chloroquinea | ||||
| NCT04308668 | United States | Post-exposure Prophylaxis/Preemptive Therapy for SARS-Coronavirus-2 | 3000 | Hydroxychloroquine |
| NCT04328467 | United States | Pre-exposure Prophylaxis for SARS-Coronavirus-2 | 3500 | Hydroxychloroquine |
| NCT04323527 | Brazil | Chloroquine Diphosphate for the Treatment of Severe Acute Respiratory Syndrome Secondary to SARS-CoV2 | 440 | Chloroquine diphosphate |
| NCT04328493 | Vietnam | The Vietnam Chloroquine Treatment on COVID-19 | 250 | Chloroquine |
| NCT04328961 | United States | Hydroxychloroquine for COVID-19 Post-exposure Prophylaxis (PEP) | 2000 | Hydroxychloroquine |
| NCT04334148 | United States | Healthcare Worker Exposure Response and Outcomes of Hydroxychloroquine | 15000 | Hydroxychloroquine |
| NCT04335552 | United States | Pragmatic Factorial Trial of Hydroxychloroquine, Azithromycin, or Both for Treatment of Severe SARS-CoV-2 Infection | 500 | Hydroxychloroquine, azithromycin |
| NCT04350281 | Hong Kong | Double Therapy With IFN-beta 1b and Hydroxychloroquine | 80 | Hydroxychloroquine, IFN-beta 1b |
| NCT04342169 | United States | University of Utah COVID-19 Hydroxychloroquine Trial | 400 | Hydroxychloroquine |
| NCT04352933 | United Kingdom | PROLIFIC Chemoprophylaxis Trial (COVID-19) | 1000 | Hydroxychloroquine |
| NCT04354428 | United States | Treatment for COVID-19 in High-Risk Adult Outpatients | 630 | Hydroxychloroquine, azithromycin |
| NCT04363450 | United States | Hydroxychloroquine as Prophylaxis for COVID-19 in Healthcare Workers (HCQPreP) | 1700 | Hydroxychloroquine |
| NCT04364022 | Switzerland | Efficacy of Pragmatic Same-day COVID-19 Ring Prophylaxis for Adult Individuals Exposed to SARS-CoV-2 in Switzerland | 420 | Hydroxychloroquine, lopinavir/ritonavir |
| NCT04435808 | United States | Efficacy of Hydroxychloroquine Prophylaxis for Health Care Workers at High Risk for COVID-19 | 350 | Hydroxychloroquine |
| NCT04410562 | Spain | Hydroxychloroquine Efficacy and Safety in Preventing SARS-CoV-2 Infection and COVID-19 Disease Severity During Pregnancy | 714 | Hydroxychloroquine |
| Biologics | ||||
| NCT04327388 | Canada | Sarilumab COVID-19 | 300 | Sarilumab SAR153191 |
| NCT04317092 | Italy | Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) | 400 | Tocilizumab injection |
| Antivirals | ||||
| NCT04292899b | United States | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19) | 2400 | Remdesivir |
| NCT04292730b | United States | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment | 1600 | Remdesivir |
| Other/multidrug combination | ||||
| NCT02735707c | Australia | Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia | 6800 | Various drug treatments |
| NCT04381936 | United Kingdom | Randomised Evaluation of COVID-19 Therapy | 12000 | Various drug treatments |
| NCT04342689 | United States | The Role of Resistant Starch in COVID-19 Infection | 1500 | Dietary supplement |
| NCT04374461 | United States | A Study of N-acetylcysteine in Patients With COVID-19 Infection | 86 | N-acetylcysteine |
| NCT04366960 | Italy | Comparison of Two Doses of Enoxaparin for Thromboprophylaxis in Hospitalized COVID-19 Patients | 2712 | Enoxaparin |
| NCT04367831 | United States | Intermediate or Prophylactic-Dose Anticoagulation for Venous or Arterial Thromboembolism in Severe COVID-19 | 100 | Enoxaparin, heparin |
| NCT0430824d | United States | Covid-19 Associated Coagulopathy | 170 | Intermediate-dose thromboprophylaxis |
| NCT04381962 | United Kingdom | A Multicentre Open-label Two-arm Randomised Superiority Clinical Trial of Azithromycin Versus Usual Care In Ambulatory COVID19 (ATOMIC2) | 800 | Azithromycin |
| NCT04319731 | United States | A Pilot Study of Human Amniotic Fluid for COVID19 Associated Respiratory Failure | 10 | Human amniotic fluid |
| NCT04444271 | Pakistan | Mesenchymal Stem Cell Infusion for COVID-19 Infection | 20 | Mesenchymal stem cells |
aOne study (NCT04408456) reported that pregnant or breastfeeding persons would be included in the control arm only (not displayed here).
bCompassionate use basis only.
cPregnant or breastfeeding women are excluded from particular study arms.
dPregnancy is an exclusion criterion; breastfeeding is not.
DISCUSSION
Data regarding the clinical presentation, disease evolution, treatment, and outcomes for COVID-19 in pregnancy and lactation are scarce. Despite nearly 14 million cases of COVID-19 worldwide [38], we identified only a few thousand reported cases of pregnant or breastfeeding persons with COVID-19. In general, published data mainly consist of case series and reports, and the information is heterogeneous, with outcome reporting subject to major bias and sometimes missing entirely. From the limited available literature, we found that when outcomes were reported, maternal and fetal/neonatal survival was overall excellent (98% and 99%) and that fetal/neonatal infection was rare but described.
We found that nearly two-thirds of completed or ongoing interventional COVID-19-related clinical trials have excluded pregnant or breastfeeding persons. This finding reaffirms prior reports [39]. Despite available safety data in pregnancy for hydroxychloroquine and lopinavir/ritonavir, we were surprised to find that 68% and 80% of the respective clinical trials had excluded pregnant or breastfeeding persons. While some clinical trials may have had multiple study arms that might have prohibited the enrollment of this population due to known teratogenicity of other medications, many trials were singularly investigating hydroxychloroquine or lopinavir/ritonavir. Both the American College of Obstetrics and Gynecology and the American College of Rheumatology endorse the use of hydroxychloroquine in pregnancy for the treatment and management of systemic lupus erythematosus [34, 35] and lopinavir/ritonavir is one of the few drugs with an actual indication for pregnancy per the US Food and Drug Administration (FDA) [40]. While recent evidence suggests that these drugs may not be effective in treating or preventing COVID-19 [41–44], the large percentage of clinical trials that excluded pregnant persons should serve as a wake-up call to us all, including institutional review boards, regulatory authorities, and clinical trial investigators.
While we recognize that the inclusion of this population in clinical trials of investigational drugs is not without risk of harm to the fetus/neonate, the blanket exclusion of pregnant or breastfeeding persons from COVID-19 clinical trials has serious implications [39]. Although we found that severe complications and death were rare (1%–2%), uncommon complications are important on a population level during a pandemic because of the sheer volume of cases (a 1% mortality rate still equates to 10 000 deaths if one were to examine 1 million cases). Furthermore, while published outcomes were reassuring, outcomes may be worse in developing countries with less access to critical care resources, making a safe and effective drug therapy all the more important.
Only when drugs are intended to be used in pregnancy does the FDA require pharmaceutical companies to demonstrate safety and efficacy in pregnancy [45]. Between 1960 and 2013, only 1% of pharmacokinetic clinical trials were conducted with the inclusion of pregnant women [46]. Through observation alone, it can take an average of 27 years to develop enough data to estimate the teratogenicity risk of a drug [45], which is a prohibitively long timeline during a pandemic. Enrolling pregnant persons into clinical trials with their informed consent can help circumvent this delay. In our search, we came across 3 observational cohort studies specifically capturing information regarding the treatment of pregnant women (NCT04323839, NCT04319016, NCT04315870). While these studies will greatly increase our knowledge of the treatment and outcomes of this population, mores studies are needed, including examining the safety and efficacy of any potential vaccine.
To improve care for pregnant persons with COVID-19, pregnancy-specific adaptive clinical trials, similar to those for HIV [47], should be considered. These can allow for pregnancy-specific expertise in the recruitment, consent, and assessment of pregnancy-related outcomes. Such studies could assess pharmacokinetics at varying stages in pregnancy to ensure dose optimization and can accommodate the rapid inclusion of promising new drugs that may emerge. These studies would complement ongoing clinical trials enrolling pregnant persons. A similar approach is currently underway for children (NCT04278404). For future and ongoing trials that include or enroll pregnant persons, there should be a mechanism in place to systematically capture longitudinal pregnancy outcomes. This may include consenting women for long-term follow-up should they become pregnant during the course of the trial. The availability of safety and efficacy data for investigational drugs for use in COVID-19 is paramount in order to properly counsel people who become pregnant during infection and/or following an unintentional exposure.
Our review of the literature was timely and is the first study (to our knowledge) to systematically examine and compile the available data related to treatment and outcomes of COVID-19 in pregnancy and related clinical trials. While we conducted a comprehensive search, our study has limitations. The major limitation relates to the data included in the published studies. There are several inherent biases (eg, recollection and publication bias) present in case series and observational studies. These may be especially prevalent in our review, given the extent of missing data and the lack of uniform reporting on important pregnancy and neonatal outcomes. Asymptomatic or mild cases of COVID-19 may have been underreported, and for cases that did not report outcomes, we cannot assume that none of these were without severe complications. Furthermore, not all studies reported detailed treatment information, and the majority of published cases described only the peripartum/postpartum period. Lastly, we only analyzed clinical trials reported on ClinicalTrials.gov and may have missed other important ongoing clinical trials registered elsewhere.
CONCLUSION
While we found reassuring maternal and fetal/neonatal outcomes with COVID-19, more rigorous, harmonized data collection and publication regarding all aspects of the disease, its treatment, and outcomes in pregnant and breastfeeding individuals are needed. Consideration should be given to establishing an international registry and pregnancy-specific adaptive clinical trials with coordinated data collection for important outcomes. We urge investigators and regulatory health agencies to work together to find a sound ethical approach to include this population of consenting adults in interventional trials. This will ensure that trial results are generalizable and that we have the necessary knowledge to guide safe, effective treatment in order to counsel the many pregnant and breastfeeding individuals who may go on to develop COVID-19 during this evolving pandemic.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of Minnesota. Katelyn Pastick is a Doris Duke International Clinical Research Fellow. Dr. McDonald receives salary support from the Fonds de Recherche du Québec - Santé. Drs. Nicol and Rajasingham are supported by the National Institute of Allergy and Infectious Disease (K08AI134262, K23AI138851). Dr. Zash is supported by the National Institute of Health/National Institute of Child Health and Human Development (K23 HD088230-01A1).
Potential conflicts of interest. Drs. Radha Rajasingham and David Boulware are principal investigators on 3 randomized clinical trials investigating use of hydroxychloroquine for the prevention or treatment of COVID-19 in Canada and the United States. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Women of reproductive age (15–49 years) population (thousands). Available at: https://www.who.int/data/maternal-newborn-child-adolescent/indicator-explorer-new/mca/women-of-reproductive-age-(15-49-years)-population-(thousands). Accessed 20 April 2020.
- 2. World Health Organization. Women in the health workforce. Available at: https://www.who.int/hrh/events/2018/women-in-health-workforce/en/. Accessed 20 April 2020.
- 3. CDC COVID-19 Response Team. Preliminary estimates of the prevalence of selected underlying health conditions among patients with Coronavirus Disease 2019—United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis 2008; 14:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong SF, Chow KM, de Swiet M. Severe acute respiratory syndrome and pregnancy. BJOG 2003; 110:641–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz DA, Graham AL. Potential Maternal and Infant Outcomes from (Wuhan) Coronavirus 2019-nCoV Infecting Pregnant Women: Lessons from SARS, MERS, and Other Human Coronavirus Infections. Viruses 2020; 12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu H, Wang LL, Zhao SJ, et al. . Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol 2020; 139:103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu W, Wang J, Li W, et al. . Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med 2020; 14:193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Savasi VM, Parisi F, Patanè L, et al. . Clinical Findings and Disease Severity in Hospitalized Pregnant Women With Coronavirus Disease 2019 (COVID-19). Obstet Gynecol 2020; 136:252–8. [DOI] [PubMed] [Google Scholar]
- 10. Patanè L, Morotti D, Giunta MR, et al. . Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am J Obstet Gynecol MFM 2020; 2:100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehta H, Ivanovic S, Cronin A, et al. . Novel coronavirus-related acute respiratory distress syndrome in a patient with twin pregnancy: a case report. Case Rep Womens Health 2020; 27:e00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knight M, Bunch K, Vousden N, et al. ; UK Obstetric Surveillance System SARS-CoV-2 Infection in Pregnancy Collaborative Group Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ 2020; 369:m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirtsman M, Diambomba Y, Poutanen SM, et al. . Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ 2020; 192:E647–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kayem G, Lecarpentier E, Deruelle P, et al. . A snapshot of the Covid-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod 2020; 49:101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Govind A, Essien S, Karthikeyan A, et al. . Re: novel coronavirus COVID-19 in late pregnancy: outcomes of first nine cases in an inner city London hospital. Eur J Obstet Gynecol Reprod Biol 2020; 251:272–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Futterman I, Toaff M, Navi L, Clare CA. COVID-19 and HELLP: overlapping clinical pictures in two gravid patients. AJP Rep 2020; 10:e179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferrazzi E, Frigerio L, Savasi, et al. Vaginal delivery in SARS‐CoV‐2‐infected pregnant women in Northern Italy: a retrospective analysis. BJOG 2020; 127:1116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carosso A, Cosma S, Borella F, et al. . Pre-labor anorectal swab for SARS-CoV-2 in COVID-19 pregnant patients: is it time to think about it? Eur J Obstet Gynecol Reprod Biol 2020; 249:98–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buonsenso D, Costa S, Sanguinetti M, et al. . Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am J Perinatol 2020; 37:869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alzamora MC, Paredes T, Caceres D, et al. . Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol 2020; 37:861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng H, Xu C, Fan J, et al. . Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA 2020; 323:1848–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu N, Li W, Kang Q, et al. . Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis 2020; 20:559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang S, Guo L, Chen L, et al. . A Case Report of Neonatal 2019 Coronavirus Disease in China. Clin Infect Dis 2020; 71:853–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khan S, Jun L, Nawsherwan, et al. Association of COVID-19 with pregnancy outcomes in health-care workers and general women. Clin Microbiol Infect 2020; 26:788–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong L, Tian J, He S, et al. . Possible vertical transmission of SARS-CoV-2 from an infected mother to her Newborn. JAMA 2020; 323:1846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Penfield CA, Brubaker SG, Limaye MA, et al. . Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM 2020; 2:100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hosier H, Farhadian SF, Morotti RA, et al. . SARS-CoV-2 infection of the placenta. J Clin Invest. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferraiolo A, Barra F, Kratochwila C, et al. . Report of Positive Placental Swabs for SARS-CoV-2 in an Asymptomatic Pregnant Woman with COVID-19. Medicina (Kaunas) 2020; 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu Y, Liu C, Dong L, et al. . Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. Bjog 2020; 127:1109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shanes ED, Mithal LB, Otero S, et al. . Placental pathology in COVID-19. Am J Clin Pathol 2020; 154:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuhrt K, McMicking J, Nanda S, Nelson-Piercy C, Shennan A. Placental abruption in a twin pregnancy at 32 weeks’ gestation complicated by coronavirus disease 2019 without vertical transmission to the babies. Am J Obstet Gynecol MFM 2020; 2:100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson J, Schauer J, Bryant S, Graves CR. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Rep Womens Health 2020; 27:e00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. NIH US National Library of Medicine. Available at: https://clinicaltrials.gov/. Accessed 22 May 2020.
- 34. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. ACOG Committee Opinion No. 776: immune modulating therapies in pregnancy and lactation. Obstet Gynecol 2019; 133:e287–95. [DOI] [PubMed] [Google Scholar]
- 35. Sammaritano LR, Bermas BL, Chakravarty EE, et al. . 2020 American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2020; 72:529–56. [DOI] [PubMed] [Google Scholar]
- 36. Pasley MV, Martinez M, Hermes A, et al. . Safety and efficacy of lopinavir/ritonavir during pregnancy: a systematic review. AIDS Rev 2013; 15:38–48. [PubMed] [Google Scholar]
- 37. Antiretroviral Pregnancy Registry. Antiretroviral pregnancy registry interim report. Available at: http://www.apregistry.com/forms/exec-summary.pdf. Accessed 20 April 2020.
- 38. World Health Organization. Coronavirus disease (COVID-19) situation report–180. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200718-covid-19-sitrep-180.pdf?sfvrsn=39b31718_2. Accessed 19 July 2020.
- 39. LaCourse S, John-Stewart G, Adams Waldorf KM. Importance of Inclusion of Pregnant and Breastfeeding Women in COVID-19 Therapeutic Trials. Clin Infect Dis 2020; 71:879–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. US Food and Drug Administration. Kaletra (lopinavir and ritonavir). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021251s052_021906s046lbl.pdf. Accessed 26 April 2020.
- 41. Boulware DR, Pullen MF, Bangdiwala AS, et al. . A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020; 383:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Skipper CP, Pastick KA, Engen NW, et al. . Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Horby P, Mafham M, Linsell L, et al. . Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv 20151852 [Preprint]. 15 July 2020. Available at: 10.1101/2020.07.15.20151852. Accessed 20 July 2020. [DOI] [Google Scholar]
- 44. Cao B, Wang Y, Wen D, et al. . A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382:1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Briggs GG, Polifka JE, Wisner KL, et al. . Should pregnant women be included in phase IV clinical drug trials? Am J Obstet Gynecol 2015; 213:810–5. [DOI] [PubMed] [Google Scholar]
- 46. McCormack SA, Best BM. Obstetric pharmacokinetic dosing studies are urgently needed. Front Pediatr 2014; 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. IMPAACT Network. International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT). Available at: https://impaactnetwork.org/. Accessed 21 July 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.