Abstract
Background
The performance of real-time reverse transcription polymerase chain reaction (rRT-PCR) for SARS-CoV-2 varies with sampling site(s), illness stage, and infection site.
Methods
Unilateral nasopharyngeal, nasal midturbinate, throat swabs, and saliva were simultaneously sampled for SARS-CoV-2 rRT-PCR from suspected or confirmed cases of COVID-19. True positives were defined as patients with at least 1 SARS-CoV-2 detected by rRT-PCR from any site on the evaluation day or at any time point thereafter, until discharge. Diagnostic performance was assessed and extrapolated for site combinations.
Results
We evaluated 105 patients; 73 had active SARS-CoV-2 infection. Overall, nasopharyngeal specimens had the highest clinical sensitivity at 85%, followed by throat, 80%, midturbinate, 62%, and saliva, 38%–52%. Clinical sensitivity for nasopharyngeal, throat, midturbinate, and saliva was 95%, 88%, 72%, and 44%–56%, respectively, if taken ≤7 days from onset of illness, and 70%, 67%, 47%, 28%–44% if >7 days of illness. Comparing patients with upper respiratory tract infection (URTI) vs pneumonia, clinical sensitivity for nasopharyngeal, throat, midturbinate, and saliva was 92% vs 70%, 88% vs 61%, 70% vs 44%, 43%–54% vs 26%–45%, respectively. A combination of nasopharyngeal plus throat or midturbinate plus throat specimen afforded overall clinical sensitivities of 89%–92%; this rose to 96% for persons with URTI and 98% for persons ≤7 days from illness onset.
Conclusions
Nasopharyngeal specimens, followed by throat specimens, offer the highest clinical sensitivity for COVID-19 diagnosis in early illness. Clinical sensitivity improves and is similar when either midturbinate or nasopharyngeal specimens are combined with throat specimens. Upper respiratory specimens perform poorly if taken after the first week of illness or if there is pneumonia.
Keywords: illness duration, pneumonia, rRT-PCR, sample site, SARS-CoV-2
Since its emergence in December 2019, Coronavirus disease 2019 (COVID-19) has infected over 14 million people across 213 countries and territories, as of mid-July 2020. Real-time reverse transcriptase polymerase chain reaction (rRT-PCR) offers definitive diagnosis for COVID-19, but the diagnostic yield of rRT-PCR for SARS-CoV-2 may vary by sampling site(s), stage of illness, and whether disease involves predominantly the upper or lower respiratory tract. Nasopharyngeal specimens, including flocked swabs, washes, or aspirates, are generally considered the optimal specimen type for the diagnosis of respiratory virus infections [1], but compared with oropharyngeal or nasal swabs, these are more technically complex to perform and often unpleasant for patients [2].
While SARS-CoV-2 shedding from various sites may be prolonged, it is maximal in the upper respiratory tract in the first week of illness, as evidenced by studies on viral kinetics [3–6]. Besides more conventional respiratory specimens (nasopharyngeal, oropharyngeal, or throat swabs), recent reports also indicate that SARS-CoV-2 can also be detected in saliva, which has been suggested as an alternate specimen for diagnostics [7–12]. The limitations of available data include a lack of simultaneous sampling, smaller study numbers, and variability in collection techniques.
The National Centre for Infectious Diseases (NCID) is a 330-bed facility (able to ramp up to 586 beds) and has admitted the majority of COVID-19 patients in Singapore. From the start of the COVID-19 outbreak, bilateral nasopharyngeal swabs were the primary specimen type used for SARS-CoV-2 diagnostics at our facility. Given the limited comparative data on simultaneously obtained clinical specimens, we sought to assess the clinical sensitivity of various specimen types for the diagnosis of SARS-CoV-2 in relation to duration of onset of illness and the presence of pneumonia.
METHODS
Audit Population
As part of a clinical audit, specimens from multiple sites (unilateral nasopharyngeal, midturbinate, throat swabs, and saliva) were simultaneously taken on a single audit day for rRT-PCR for SARS-CoV-2 prospectively from a convenience sample of suspected or confirmed cases of COVID-19 admitted to the National Centre for Infectious Diseases, Singapore, from February 27 to March 19, 2020. Suspected COVID-19 cases were defined as patients with acute respiratory symptoms and epidemiological risk factors admitted for evaluation of COVID-19. Confirmed COVID-19 infection was defined as suspected cases who tested positive for SARS-CoV-2 by PCR at any point during their admission. Recovered COVID-19 infection was defined as confirmed cases who tested negative on the day of audit and on all subsequent repeat SARS-CoV-2 PCRs until discharge.
Besides the samples taken on the day of the clinical audit, a standard bilateral nasopharyngeal swab was done upon admission, as per protocol, for all patients admitted for suspected COVID-19, and this was repeated 24 hours later if initial testing was negative [13]. If SARS-CoV-2 was detected (confirmed case of COVID-19), rRT-PCR was performed daily upon clinical recovery to ascertain virologic clearance (clinical recovery was defined as being afebrile for at least 24 hours, with improvement of clinical symptoms, and being deemed medically fit for discharge by managing clinicians). Two negative PCR results 24 hours apart were required as a prerequisite for discharge.
Clinical Specimens
The nasopharyngeal swab was collected using a flexible minitip flocked swab (220252, Copan Diagnostics, Brescia, Italy), inserted half the distance from the nares to the base of the ear, or to a depth of ~5 cm, and only unilateral swabs were sampled. Both midturbinate and throat swabs were done using a regular flocked swab (502C201, Copan Diagnostics, Brescia, Italy). For midturbinate sampling, the swab was inserted ~1–2.5 cm, rubbed along the septum of the contralateral nostril for 3–5 seconds around the area of the middle turbinate, and withdrawn [14]. For throat (oropharyngeal) swabs, the posterior oropharynx was swabbed under direct vision. Each specimen was collected in an individual universal transport medium (UTM-RT, Copan Diagnostics, Brescia, Italy).To collect saliva samples, patients were asked to rinse their mouth with plain water at least 30 minutes postmeal and 10 minutes precollection to remove residual food debris. Two milliliters of fresh salivary sample was then spit out (drooling method) by the patient into a sterile container containing an equal amount of nucleic acid stabilization formula (SF; Institute of Bioengineering and Nanotechnology, Singapore), and this was mixed after capping by gently inverting the container 5 times. All specimens were obtained by trained nurses and processed within 24 hours.
Diagnostic Testing
The A*STAR Fortitude Kit (Accelerate Technologies, Singapore) was used for all samples after extraction (NucliSens EasyMAG, biomerieux, Marcy L'Etoile, France), as previously described [15]. Additionally, saliva samples were tested with a second rRT-PCR assay targeting the N and ORF1ab genes, after extraction (EZ1 virus mini kit, version 2.0, Qiagen, Hilden, Germany) [15].
Statistical Analysis
Reference standard true positives were defined as patients with at least 1 positive SARS-CoV-2 result detected from any site on the day of the audit or at any time point thereafter (including extrapulmonary sites), until discharge. These include positive rRT-PCR by the Fortitude kit for nasopharyngeal, nasal, or throat samples and/or positive SARS-CoV-2 from either the N or ORF1ab target genes for saliva specimens. Performers of the rRT-PCR assays were blinded to reference standard results. Index test results on the day of audit and clinical information were assessed by the audit team to determine reference standard positivity.
Diagnostic performance was assessed for each site and compared with true-positive values to determine test sensitivity and specificity. Subanalyses were performed for patients’ day of illness on audit day (early, defined as patients with ≤7 days, and late, defined as >7 days from onset of illness on audit day) and the presence of pneumonia. Pneumonia was defined as reported pulmonary opacities suggesting infection on chest x-ray and/or computed tomography taken during admission.
Diagnostic accuracy for combinations of multiple sites was extrapolated from the primary performance at each individual site. Data analysis was performed using SPSS, version 26.0 (SPSS), and Vassarstats (http://vassarstats.net/). The McNemar test was used for test pairs, and P values <.05 were considered statistically significant.
Patient Consent Statement
This audit was performed as part of clinical operations at the National Centre for Infectious Diseases, and results were used as part of routine clinical care. The reporting of this audit with waiver of written informed consent was approved by the institutional review board (National Healthcare Group Domain Specific Review Board reference number 2020/00338). This audit followed the Standards for Reporting Diagnostic Accuracy study (STARD) guidelines (https://www.equator-network.org/reporting-guidelines/stard/) (Supplementary Data).
RESULTS
Baseline Characteristics
A total of 581 patients were potentially eligible (171 with COVID-19). We included 105 patients in this evaluation, 32 of whom tested negative for SARS-CoV-2 (11 patients recovered from COVID-19 and 21 had alternate diagnoses) and 73 of whom had active SARS-CoV-2 infection (Table 1; STARD flow diagram in Supplementary Figure 1). Twenty-eight (27%) patients had pneumonia as evidenced by radiological changes, while 77 (73%) had upper respiratory tract infection (URTI) with normal radiological results. Overall, the median duration from onset of illness to the day of audit (interquartile range) was 7 (3–10) days. One saliva sample was unavailable for analysis with the Fortitude kit, and 7 were unavailable after a second assay targeting the N and ORF1ab genes, leaving 104 and 98 samples, respectively, for analysis for this particular sample type.
Table 1.
Baseline Characteristics of Patients
| Total (n = 105) | COVID-19 | Non-COVID-19 (n = 21) | P Value | P Value | |||
|---|---|---|---|---|---|---|---|
| Total COVID-19 (n = 84) | Active COVID-19 (n = 73) | Recovered COVID-19 (n = 11) | Active vs Recovered COVID-19 | Active vs Non-COVID-19 | |||
| Gender, No. (%) | |||||||
| Male | 57 (54) | 45 (53.6) | 40 (54.8) | 5 (45.5) | 12 (57.1) | .56 | .85 |
| Female | 48 (46) | 39 (46.4) | 33 (45.2) | 6 (54.5) | 9 (42.9) | ||
| Age | |||||||
| Median (IQR), y | 44 (20–79) | 44 (20–79) | 44 (31–56) | 44 (26–79) | 45 (22–73) | .81 | .94 |
| Clinical syndrome, No. (%) | |||||||
| Pneumonia | 28 (26.7) | 26 (31.0) | 23 (31.5) | 3 (27.3) | 2 (9.5) | 1.00a | .045 |
| URTI | 77 (73.3) | 58 (69.0) | 50 (68.5) | 8 (72.7) | 19 (90.5) | ||
| Days of illness | |||||||
| ≤7 d, No. (%) | 57 (54.3) | 46 (54.8) | 43 (58.9) | 3 (27.3) | 11 (52.4) | .21a | .68 |
| >7, No. (%) | 48 (45.7) | 38 (45.2) | 30 (41.1) | 8 (72.7) | 10 (47.6) | ||
| Median (IQR), d | 7 (3–10) | 7 (3–10) | 6 (3–9) | 12 (3–20) | 7 (3–10) | .054 | .71 |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range; URTI, upper respiratory tract infection.
aFisher test.
Overall Results
Data analysis of the clinical sensitivity and specificity of individual sample sites and combination testing is summarized in Table 2. Overall, nasopharyngeal specimens were found to have the highest clinical sensitivity, at 85%, followed by throat, 80%, mid-turbinate, 62%, and saliva, 38%–52%. There was no statistically significant difference between the nasopharyngeal and throat site for rRT-PCR overall, but the nasopharyngeal site was found to be more sensitive compared with midturbinate or saliva (either assay) (P < .01) (Supplementary Table 1).
Table 2.
Overall Positive Clinical Sensitivities and Clinical Specificities of Sample Sites for SARS-CoV-2
| Sample Sitea | Clinical Sensitivity, % (95% CI) | Clinical Specificity, % (95% CI) |
|---|---|---|
| Single site | ||
| Unilateral nasopharyngeal | 84.9 (74.2–91.9) | 100 (86.7–100) |
| Unilateral nasal midturbinate | 61.6 (49.4–72.6) | 100 (86.7–100) |
| Throat | 79.5 (68.1–87.7) | 100 (86.7–100) |
| Saliva (n = 104) | 37.5 (26.6–49.7) | 100 (86.7–100) |
| Saliva (N and ORF1ab assay; n = 98)b | 51.5 (39.0–63.9) | 100 (86.7–100) |
| Combination of sample sitesc | ||
| Unilateral nasopharyngeal + throat | 91.7 (82.4–96.6) | 100 (86.7–100) |
| Unilateral nasal midturbinate + throat | 89.0 (79.0–94.8) | 100 (86.7–100) |
| Unilateral nasal midturbinate + saliva | 72.6 (60.7–82.1) | 100 (86.7–100) |
| Throat + saliva | 83.6 (72.7–90.9) | 100 (86.7–100) |
aAll samples (unless otherwise stated) were tested with the A*STAR Fortitude Kit (Accelerate Technologies, Singapore).
bA second polymerase chain reaction assay targeting the N and ORF1ab genes was used for saliva samples.
cFor data for sample site combinations, data from the A*STAR Fortitude Kit were used for saliva.
The clinical sensitivity of the combined sites was extrapolated from the results of individual sites. While the midturbinate site alone was less sensitive compared with nasopharyngeal, when either was combined with throat swabs, clinical sensitivity was similar, at 89% vs 92%, respectively (P = .5); either combination offered the highest yield compared with others.
Subgroup Analyses
Upper Respiratory Tract Infection vs Pneumonia
We performed a subgroup analysis of data based on the presence or absence of radiologic evidence of pneumonia and day of illness (Tables 2 and 3). For patients with upper respiratory tract infection (URTI) who by definition had normal radiological findings (n = 77), a similar pattern was found, with nasopharyngeal swabs having the highest clinical sensitivity (92%), followed by throat (88%) and midturbinate (70%). Saliva had poor clinical sensitivity (43–54%). Combined nasopharyngeal and throat swabs or midturbinate and throat swabs showed the best performance, at 96% and 94%, respectively (P = 1.00). Nasopharyngeal specimens were found to be more sensitive than both midturbinate or saliva in patients with URTIs (P = .003 and P < .001, respectively) (Supplementary Table 1).
Table 3.
Subanalysis Based on Clinical Syndromes
| Test Site(s) | Clinical Sensitivity, % (95% CI) | Clinical Specificity, % (95% CI) |
|---|---|---|
| Upper Respiratory Tract Infection (n = 77) | ||
| Single sitea | ||
| Unilateral nasopharyngeal | 92.0 (79.9–97.4) | 100 (84.5–100) |
| Unilateral nasal midturbinate | 70.0 (55.2–81.7) | 100 (84.5–100) |
| Throat swab | 88.0 (74.9–95.0) | 100 (84.5–100) |
| Saliva (n = 76) | 42.9 (29.1–57.7) | 100 (84.5–100) |
| Saliva (N and ORF1ab assay; n = 73) b | 54.3 (39.2–68.8) | 100 (84.5–100) |
| Combination of sitesc | ||
| Unilateral nasopharyngeal + throat | 96.0 (85.1–99.3) | 100 (84.5–100) |
| Unilateral nasal midturbinate + throat | 94.0 (82.5–98.4) | 100 (84.5–100) |
| Unilateral nasal midturbinate + saliva | 82.0 (68.1–91.00 | 100 (84.5–100) |
| Throat + saliva | 94.0 (82.5–98.4) | 100 (84.5–100) |
| Pneumonia (n = 28) | ||
| Single sitea | ||
| Unilateral nasopharyngeal | 69.5 (47.0–85.9) | 100 (46.3–100) |
| Unilateral nasal midturbinate | 43.5 (23.9–65.1) | 100 (46.3–100) |
| Throat | 60.9 (38.8–79.5) | 100 (46.3–100) |
| Saliva (n = 28) | 26.1 (11.1–48.7) | 100 (46.3–100) |
| Saliva (N and ORF1ab assay; n = 25) b | 45.0 (23.8–68.0) | 100 (46.3–100) |
| Combination of sitesc | ||
| Unilateral nasopharyngeal + throat | 82.6 (60.5–94.3) | 100 (46.3–100) |
| Unilateral nasal midturbinate + throat | 78.2 (55.8–91.7) | 100 (46.3–100) |
| Unilateral nasal midturbinate + saliva | 52.2 (31.1–72.6) | 100 (46.3–100) |
| Throat + salivaa | 60.9 (38.8–79.5) | 100 (46.3–100) |
Abbreviations: URTI, upper respiratory tract infection.
aAll samples (unless otherwise stated) were tested with the A*STAR Fortitude Kit (Accelerate Technologies, Singapore).
bA second polymerase chain reaction assay targeting the N and ORF1ab genes was used for saliva samples.
cFor data for sample site combinations, data from the A*STAR Fortitude Kit were used for saliva.
For patients with radiographic evidence of pneumonia (n = 28), performance of upper respiratory tract samples was overall much less sensitive, with nasopharyngeal swabs having the highest clinical sensitivity, but only at 70%, and the other upper respiratory samples having 26%–61%. Testing with a nasopharyngeal or midturbinate swab combined with a throat swab improved clinical sensitivity to 82% and 78%, respectively. Nasopharyngeal specimens were found to be more sensitive than saliva, but not midturbinate specimens, in patients with pneumonia (P = .002 and P = .11, respectively) (Supplementary Table 1).
Day of Illness
In patients with onset of symptoms of ≤7 days (n = 57), unilateral nasopharyngeal swab showed the highest clinical sensitivity for COVID-19 diagnosis (95%), followed by throat swab (88%) and midturbinate swab (72%; nasopharyngeal vs throat P = .25; nasopharyngeal vs midturbinate P = .006) (Table 4; Supplementary Table 1). Combination testing from patients in their first week of COVID-19 showed the best performance, with a clinical sensitivity of 98% for either midturbinate or nasopharyngeal swabs combined with throat swabs.
Table 4.
Subanalysis Based on Duration of Illness
| Test Site(s) | Clinical Sensitivity, % (95% CI) | Clinical Specificity, % (95% CI) |
|---|---|---|
| Days of Illness | ||
| ≤7 d (n = 57) | ||
| Single sitea | ||
| Unilateral nasopharyngeal | 95.3 (83.0–99.1) | 100 (73.2–100) |
| Unilateral nasal midturbinate | 72.1 (56.1–84.2) | 100 (73.2–100) |
| Throat | 88.4 (74.1–95.6) | 100 (73.2–100) |
| Saliva (n = 57) | 44.2 (29.4–60.0) | 100 (73.2–100) |
| Saliva (N and ORF1ab assay; n = 53)5 | 56.4 (39.8–71.8) | 100 (73.2–100) |
| Combination of sitesc | ||
| Unilateral nasopharyngeal + Throat | 97.7 (86.2–99.9) | 100 (73.2–100) |
| Unilateral nasal midturbinate + Throat | 97.7 (86.2–99.9) | 100 (73.2–100) |
| Unilateral nasal midturbinate swab + salivaa | 79.1 (63.5–89.4) | 100 (73.2–100) |
| Throat swab + salivaa | 90.7 (76.9–97.0) | 100 (73.2–100) |
| >7 d (n = 48) | ||
| Single sitea | ||
| Unilateral nasopharyngeal | 70.0 (50.4–84.6) | 100 (78.1–100) |
| Unilateral nasal midturbinate | 46.7 (28.8–65.4) | 100 (78.1–100) |
| Throat | 66.7 (47.1–82.1) | 100 (78.1–100) |
| Saliva (n = 47) | 27.6 (13.4–47.5) | 100 (78.1–100) |
| Saliva (N and ORF1ab assay; n = 45)b | 44.4 (26.0–64.4) | 100 (78.1–100) |
| Combination of sitesc | ||
| Unilateral nasopharyngeal + throat | 83.3 (64.5–93.7) | 100 (78.1–100) |
| Unilateral nasal midturbinate + throat | 76.7 (57.3–89.4) | 100 (78.1–100) |
| Unilateral nasal midturbinate + saliva | 63.3 (43.9–79.5) | 100 (78.1–100) |
| Throat + saliva | 73.3 (53.8–87.0) | 100 (78.1–100) |
aAll samples (unless otherwise stated) were tested with the A*STAR Fortitude Kit (Accelerate Technologies, Singapore).
bA second polymerase chain reaction assay targeting the N and ORF1ab genes was used for saliva samples.
cFor data for sample site combinations, data from the A*STAR Fortitude Kit were used for saliva.
As the illness progresses to day 8 and beyond, the clinical sensitivity of individual sample sites decreased significantly and ranged from 28% to 70%, with nasopharyngeal swabs still showing the best clinical sensitivity among the upper respiratory specimens. Notably, among the true positives in this audit, 3 were detected on postaudit of lower respiratory specimens (2 rRT-PCR positive by sputum, and another via endotracheal aspirate).
Cycle Threshold
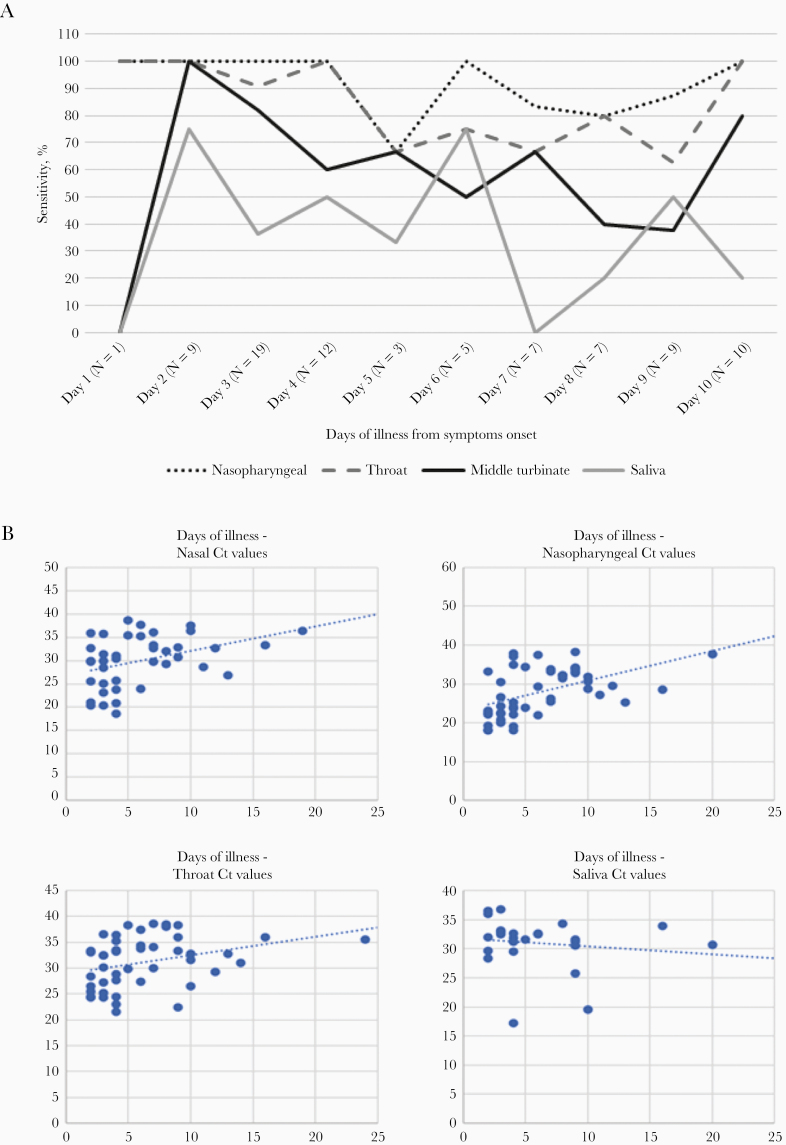
The performance of each sampled site and their associated rRT-PCR cycle threshold (Ct) values (Fortitude kit) with days of illness are summarized in Figure 1. Nasopharyngeal and throat swabs showed generally good performance results across the first week of illness up to day 10 after onset of symptoms, but saliva samples did not yield consistent results even in the first week of illness in our study. These findings were corroborated by lower Ct values (a surrogate marker for higher viral loads) in the corresponding sampling sites, demonstrating higher sensitivity in earlier illness (≤7 days) as opposed to later illness (>7 days). The median Ct values for nasopharyngeal, midturbinate, throat, and saliva samples by the Fortitude assay were 24.05, 29.89, 30.10, and 32.49 (≤7 days) and 32.20, 32.69, 33.03, and 30.98 (>7 days), respectively (Supplementary Table 2).
Figure 1.
Performance of testing sites and Ct values over days of illness.
Bilateral vs Unilateral Nasopharyngeal Swabs
Although the audit was not designed to address this primarily, we also examined the performance of bilateral nasopharyngeal swabs done on the pre-audit day (24 hours prior, with likely higher viral loads) as compared with the unilateral nasopharyngeal swabs done on the audit day. Given that viral shedding from the upper respiratory tract is highest in the first week of illness, we limited this subanalysis to patients with ≤7 days from onset of symptoms (n = 57, 54%). We found the clinical sensitivity of bilateral nasopharyngeal swabs to be 98% (95% CI, 87%–100%), vs unilateral nasopharyngeal swabs at 89% (95% CI, 76%–96%); however, this difference was not statistically significant (P = .13).
DISCUSSION
Our study highlights several important findings and provides clinicians with a practical guidance as to optimal sampling sites for SARS-CoV-2 detection. First, consistent with reports on higher viral shedding in early COVID-19 [4, 9, 16–18], the clinical sensitivity of PCR diagnostics is highest across upper respiratory specimen types in early illness (≤7 days from symptom onset), compared with later illness. Given that pneumonia occurs in later illness, the sensitivity of PCR was also found to be correspondingly diminished when upper respiratory tract specimens were sampled. This phenomenon is not peculiar to SARS-CoV-2 [19], as viral loads in various anatomic sites are related to pathology at the site and degree of viral replication. With regard to SARS-CoV-2, this is likely related to distribution of ACE2 (the receptor for viral entry) in the respiratory tract and the stage of disease [20, 21].
Second, among upper respiratory specimens, nasopharyngeal swabs were found to offer the best clinical sensitivity, followed by oropharyngeal specimens, although statistically significant differences between both sites were not found overall, or in subanalyses for early illness or the presence of pneumonia. Midturbinate swabs were less sensitive than either naso- or oropharyngeal swabs. From clinical sensitivities extrapolated from the performance of individual sites, we determined that a nasopharyngeal or midturbinate swab combined with a throat swab offered the highest sensitivity (and similar clinical sensitivities). Midturbinate swabs may be less uncomfortable or invasive compared with nasopharyngeal swabs [2] and, if used, should be combined with oropharyngeal sampling.
Besides clinical sensitivity, the choice of sampling site may also depend on other factors including familiarity and training of staff who are obtaining samples, patient preference, comfort, and adequate supply of required materials (such as the appropriate swabs). In the face of increased global demand on available trained staff and in areas with limited supply of personal protective equipment, patient-collected midturbinate samples may be considered as they offer up to 96% clinical sensitivity for the detection of SARS-CoV-2 when compared with health care worker–collected NP samples, as suggested in a study by Tu et al. [22], although based on our findings we suggest that this be combined with a oropharyngeal specimen to increase sensitivity.
Third, although saliva was previously reported as a promising sample and a more convenient diagnostic sample for COVID-19, our data suggest otherwise. Several factors may account for this. Studies reporting higher sensitivities had small numbers, ranging from 12–39 SARS-CoV-2-positive patients studied [7–12]. The method of collection and what constituted “saliva” were also different in various studies. To et al. collected “early morning saliva” from the posterior oropharynx (“coughed up by clearing throat”), which is technically a combination of saliva, nasopharyngeal, and bronchopulmonary secretions [9, 11]. Kojima et al. studied both self-collected and clinician-supervised oral fluid collection, which was comprised of expectorated sputum and secretions and an oral swab of the buccal mucosa, tongue, gums, and the hard palate [7]. Also, the severity of illness differed in some studies. For example, Azzi et al. studied 25 patients who had severe COVID-19, collecting salivary specimens by the “drool technique” or pipetting pooled oral secretions, and found SARS-CoV-2 by PCR in all patients studied. This group also found that higher lactate dehydrogenase (LDH) levels (indicative of tissue damage) were correlated with lower Ct values with salivary samples. Another reason for the differences in our study for saliva was that sensitivity could also have been influenced by prerinsing, as instructed by the developer of the transport medium. Nonetheless, given the findings of the various studies on saliva as a diagnostic for COVID-19 and older supportive data for saliva as a diagnostic specimen in humans and a rhesus macaque infection model [23, 24] for SARS-CoV-1 (which also uses ACE2 as its receptor), further standardization of methods for salivary diagnostics and larger confirmatory studies are needed.
The strengths of our study include its large sample size and simultaneous sampling from multiple sites of each patient. The limitations of our study were that results from a combination of sites were extrapolated from the results of each site. We were also not able to systematically study lower respiratory tract or nonrespiratory samples and are thus not able to assess the comparative performance of these. It is noted, however, that not all patients with COVID-19 have a productive cough (and thus are unable to produce sputum) or they may be ill enough to warrant more invasive sampling (eg, endotracheal aspirates or bronchoalveolar lavage). Stool shedding, on the other hand, has been reported to persist for as long as 5 weeks in some patients. SARS-CoV-2 may be detected in stool beyond the duration of respiratory shedding and could be an alternate diagnostic specimen if upper respiratory samples are negative, although shedding may be not be detectable in all patients or may be intermittent [25–27]. Anecdotally, in our institution, we have diagnosed several cases of COVID-19 in the pneumonic phase, with stool being the only positive sample by PCR. Stool PCR, along with other adjuncts to diagnosis such as serology or computed tomography (which may have characteristic findings, albeit not specific), may assist clinicians in the diagnosis of COVID-19. Another limitation is that although 1 nasopharyngeal swab is sufficient for the detection of SARS-CoV-2, 2 swabs from each nostril are recommended for midturbinate swabs [28]. This was not performed in our audit so as to minimize discomfort and trauma from repetitive sampling of the same nostrils, as sampling of all 4 sites (nasopharyngeal, midturbinate, throat, and saliva) was done in the same setting during audit day. Lastly, the design of our audit did not allow us to fully compare the relative performance of unilateral vs bilateral nasopharyngeal swabs. Although the clinical sensitivity of bilateral nasopharyngeal swabs was found to be slightly higher than unilateral in early illness, our comparison was biased toward bilateral nasopharyngeal swabs (as these were obtained ~24 hours before the unilateral swab). Reassuringly, these differences were not statistically different.
CONCLUSIONS
In summary, nasopharyngeal and throat specimens offer the best clinical sensitivity for the molecular diagnosis of COVID-19 in early illness. More data and standardization of collection techniques are required to assess the utility of saliva as a diagnostic specimen. Although the sensitivity of midturbinate specimens was lower than nasopharyngeal and throat specimens, test sensitivities improve and are similar when midturbinate or nasopharyngeal specimens are combined with throat specimens. Midturbinate combined with throat swabs may thus be an alternative to nasopharyngeal swabs, which are more invasive and may cause more patient discomfort. In the pneumonic stage or later disease (>7 days), upper respiratory specimens perform poorly, and clinicians should be aware and seek alternate specimen types and other adjuncts to support a diagnosis of COVID-19.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank all clinical and nursing staff who provided care for the patients at the National Centre for Infectious Diseases, Singapore, and staff at the Department of Laboratory Medicine, Tan Tock Seng Hospital, and the National Public Health Laboratory for their excellent work during this pandemic. We also thank Dr. Tan Min Han (Institute of Bioengineering and Nanotechnology, A*STAR) for kindly supplying the saliva nucleic acid stabilization formula used for this study.
National Centre for Infectious Diseases COVID-19 Outbreak Research Team
Sean Wei Xiang Ong, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Brenda Sze Peng Ang, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
David Chien Lye, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Poh Lian Lim, MD, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Cheng Chuan Lee, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Li Min Ling, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Lawrence Lee, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Barnaby Edward Young, MB Bchir, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Tau Hong Lee, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Chen Seong Wong, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Sapna Sadarangani, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Ray Lin, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Deborah Hee Ling Ng, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Mucheli Sadasiv, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Po Ying Chia, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore
Chiaw Yee Choy, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Glorijoy Shi En Tan, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Frederico Dimatatac, MD, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Isais Florante Santos, MD, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Chi Jong Go, MD, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Yeo Tsin Wen, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Yu Kit Chan, MBBS, National Centre for Infectious Diseases, Singapore, Tan Tock Seng Hospital, Singapore
Pooja Rao, MBBS, Department of Laboratory Medicine, Tan Tock Seng Hospital, Singapore
Jonathan WZ Chia, MBBS, Department of Laboratory Medicine, Tan Tock Seng Hospital, Singapore
Constance Yuan Yi Chen, MD, Department of Laboratory Medicine, Tan Tock Seng Hospital, Singapore
Boon Kiat Toh, MBBS, Department of Laboratory Medicine, Tan Tock Seng Hospital, Singapore
Financial support. None.
Potential conflicts of interest. Dr. Vasoo has received research support from bioMerieux and Rosco Diagnostica. Dr. Barkham is a co-inventor of the Fortitude Kit, with a patent, “DETECTING A VIRUS,” and royalties paid to Tan Tock Seng Hospital. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
National Centre for Infectious Diseases COVID-19 Outbreak Research Team:
Sean Wei Xiang Ong, Brenda Sze Peng Ang, David Chien Lye, Poh Lian Lim, Cheng Chuan Lee, Li Min Ling, Lawrence Lee, Barnaby Edward Young, Tau Hong Lee, Chen Seong Wong, Sapna Sadarangani, Ray Lin, Deborah Hee Ling Ng, Mucheli Sadasiv, Po Ying Chia, Chiaw Yee Choy, Glorijoy Shi En Tan, Frederico Dimatatac, Isais Florante Santos, Chi Jong Go, Yeo Tsin Wen, Yu Kit Chan, Pooja Rao, Jonathan W Z Chia, Constance Yuan Yi Chen, and Boon Kiat Toh
References
- 1. Ginocchio CC, McAdam AJ. Current best practices for respiratory virus testing. J Clin Microbiol 2011; 49:44–8. [Google Scholar]
- 2. Frazee BW, Rodríguez-Hoces de la Guardia A, Alter H, et al. . Accuracy and discomfort of different types of intranasal specimen collection methods for molecular influenza testing in emergency department patients. Ann Emerg Med 2018; 71:509–17.e1. [DOI] [PubMed] [Google Scholar]
- 3. Pan Y, Zhang D, Yang P, et al. . Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 2020; 20:411–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He X, Lau EHY, Wu P, et al. . Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–5. [DOI] [PubMed] [Google Scholar]
- 5. Wölfel R, Corman VM, Guggemos W, et al. . Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. [DOI] [PubMed] [Google Scholar]
- 6. Zheng S, Fan J, Yu F, et al. . Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ 2020; 369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kojima N, Turner F, Slepnev V, et al. . Self-collected oral fluid and nasal swabs demonstrate comparable sensitivity to clinician collected nasopharyngeal swabs for Covid-19 detection. medRxiv 20062372 [Preprint]. 15 April 2020. Available at: 10.1101/2020.04.11.20062372. Accessed 26 August 2020. [DOI] [Google Scholar]
- 8. Williams E, Bond K, Zhang B, et al. . Saliva as a non-invasive specimen for detection of SARS-CoV-2. J Clin Microbiol 2020; 58:e00776–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. To KK-W, Tsang OT-Y, Leung W-S, et al. . Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; 20:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wylie LA, Fournier J, Casanovas-Massana A, et al. . Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. medRxiv 20067835 [Preprint]. 22 April 2020. Available at: 10.1101/2020.04.16.20067835. Accessed 26 August 2020. [DOI] [Google Scholar]
- 11. To KK-W, Tsang OT-Y, Yip CC-Y, et al. . Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis 2020; 71:841–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Azzi L, Carcano G, Gianfagna F, et al. . Saliva is a reliable tool to detect SARS-CoV-2. J Infect 2020; 81:e45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tay J-Y, Lim PL, Marimuthu K, et al. . De-isolating coronavirus disease 2019 suspected cases: a continuing challenge. Clin Infect Dis 2020; 71:883–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Irving SA, Vandermause MF, Shay DK, Belongia EA. Comparison of nasal and nasopharyngeal swabs for influenza detection in adults. Clin Med Res 2012; 10:215–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun Y, Koh V, Marimuthu K, et al. . Epidemiological and clinical predictors of COVID-19. Clin Infect Dis 2020; 71:786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Y, Chen S, Yang Z, et al. . SARS-CoV-2 viral load in clinical samples of critically ill patients. Am J Respir Crit Care Med 2020; 201:1435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young BE, Ong SWX, Kalimuddin S, et al. . Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020; 323:1488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lirong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients to. N Engl J Med 2020; 382:1175–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh K, Vasoo S, Stevens J, et al. . Fast-track communication pitfalls in diagnosis of pandemic (novel) A/H1N1 2009 influenza. J Clin Microbiol 2010; 48:1501–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winichakoon P, Chaiwarith R, Liwsrisakun C, et al. . Negative nasopharyngeal and oropharyngeal swabs do not rule out COVID-19. J Clin Microbiol 2020; 58:e00297–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamming I, Timens W, Bulthuis ML, et al. . Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tu Y-P, Jennings R, Hart B, et al. . Patient-collected tongue, nasal, and mid-turbinate swabs for SARS-CoV-2 yield equivalent sensitivity to health care worker collected nasopharyngeal swabs. medRxiv 20050005 [Preprint]. 6 April 2020. Available at: 10.1101/2020.04.01.20050005. Accessed 26 August 2020. [DOI] [Google Scholar]
- 23. Wang WK, Chen SY, Liu IJ, et al. . Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis. Emerg Infect Dis 2004; 10:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu L, Wei Q, Alvarez X, et al. . Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of Rhesus macaques. J Virol 2011; 85:4025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheung KS, Hung IF, Chan PP, et al. . Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology 2020; 159:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu Y, Guo C, Tang L, et al. . Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020; 5:434–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu Y, Li X, Zhu B, et al. . Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 2020; 26:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons under investigation (PUIs) for coronavirus disease 2019 (COVID-19). 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Accessed 18 July 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.