Abstract
Background
The majority of the children with SARS-CoV-2 infection present with respiratory symptoms, hence various chest imaging modalities have been used in the management. Knowledge about the radiological findings of coronavirus disease (COVID-19) in children is limited. Hence, we systematically synthesized the available data that will help in better management of COVID-19 in children.
Methods
Four different electronic databases (MEDLINE, EMBASE, Web of Science and CENTRAL) were searched for articles reporting radiological findings in children with COVID-19. Studies reporting thoracic radiological findings of COVID-19 in patients aged <19 years were included. A random-effect meta-analysis (wherever feasible) was performed to provide pooled estimates of various findings.
Results
A total of 1984 records were screened of which forty-six studies (923 patients) fulfilled the eligibility criteria and were included in this systematic review. A chest computed tomography (CT) scan was the most frequently used imaging modality. While one-third of the patients had normal scans, a significant proportion (19%) of clinically asymptomatic children had radiological abnormalities too. Unilateral lung involvement (55%) was frequent when compared with bilateral and ground-glass opacities were the most frequent (40%) definitive radiological findings. Other common radiological findings were non-specific patchy shadows (44%), consolidation (23%), halo sign (26%), pulmonary nodules and prominent bronchovascular marking. Interstitial infiltration being the most frequent lung ultrasound finding.
Conclusion
CT scan is the most frequently used imaging modality for COVID-19 in children and can detect pneumonia before the appearance of clinical symptoms. Undefined patchy shadows, grand-glass opacities and consolidation are commonly observed imaging findings in COVID-19 pneumonia.
Keywords: computed tomography, lung ultrasound, ground-glass opacity, pneumonia, imaging, COVID-19
INTRODUCTION
Coronavirus infection has a predilection towards the respiratory system, therefore, chest imaging is frequently used for initial evaluation and follow-up in suspect/confirmed COVID-19 cases in adults. Although RT-PCR is diagnostic for COVID-19, chest imaging has been used extensively for prediction and prognostication of the disease. Some of the findings can be seen on computerized tomography (CT) even before the onset of symptoms [1]. Due to the mild pattern of disease in children findings are usually subtle when compared with adults, and therefore may not be detected on chest X-ray [2]. Thorough knowledge of the choice of imaging modality and radiological findings in various stages of the disease shall help the paediatricians in early recognition and appropriate clinical management. Therefore, we aimed to compile the radiological findings of COVID-19 pneumonia in children in a systematic manner.
MATERIALS AND METHODS
Search strategy
We conducted a comprehensive literature search using PubMed, EMBASE, Web of Science and Cochrane library for the original articles published between 01 December 2019 and 20 May 2020. This study was conducted following the Meta-analysis Of Observational Studies in Epidemiology guidelines [3]. Keywords and MeSH terms used for literature search were COVID -19, coronavirus, SARS-CoV-2, 2019-nCoV, severe acute respiratory syndrome coronavirus 2, paediatric, children, adolescent and infant. The search strategy is provided in Supplementary Appendix S1. No language restrictions were applied.
Study selection
A pre-defined set of the criterion was used for study selection for this systematic review. Initially, two researchers (J.Y. and J.M.) independently screened the title and abstract for the eligibility. Later all the authors examined full-text articles for inclusion and exclusion criteria. We included all types of studies (case report, case series and observational) describing radiological findings of confirmed SARS-CoV-2 infection in children aged <19 years. Studies reporting other serotypes of coronavirus, narrative or systematic review, editorial, perspective, etc. not meeting the inclusion criteria were excluded.
Data extraction and quality assessment
A well-structured, standardized proforma was used for data extraction. Four investigators (J.Y., J.K., J.M. and A.Y.) independently extracted data from the full text of the eligible studies. Extracted data include first author name, year, country, journal, study design, study population information for risk of bias assessment of the study, method and type of sample used for confirmation of SARS-CoV-2 infection, age and gender distribution of cases, the clinical status of the subjects (symptomatic/asymptomatic) and radiological findings.
As CT scan was the predominant modality used for imaging, we extracted data using a structured proforma mentioning the side of the lesion (left/right/both), lobes (upper, middle and lower), location (central/peripheral) and characteristic of the lesion (consolidation, ground glass, etc.). Any disagreement between two investigators was resolved through discussion with the third investigator (A.Y.). One researcher (J.K.) independently rechecked the extracted data for its accuracy as well as completeness and ensured that there is no duplication of included studies. The quality of the included studies in this systematic review was assessed using the Newcastle Ottawa scale. Three investigators (J.Y., J.K. and J.M.) independently assigned an overall risk of bias to each eligible study, and if they disagreed, another researcher (A.Y.) was called to resolve the discrepancy. Publication bias was considered high since all the included studies were either case series or case reports.
Data synthesis and statistical analysis
We presented the data with descriptive statistics and provided pooled estimates of various parameters, wherever it was feasible to meta-analyses the data. Percentages and mean values were calculated to describe categorical and continuous variables, respectively. Meta-analysed parameters were presented as pooled estimates with a 95% confidence interval (CI). Meta-analysis was performed using Stata version 14.2 (College Station, TX, USA). We pooled data from individual studies using a random effect model with the assumption that the frequency of radiological findings will be variable across the studies. Heterogeneity in studies was explored by inspection of forest plot as well as using the χ2 test on Cochran’s Q statistics. Study heterogeneity was assessed by using the Higgins and Thompson I2 method [4]. The I2 heterogeneity was categorized as follows: 0–50% low, 50–75% moderate and >75% considerable heterogeneity. To explore the heterogeneity, we performed sensitivity and subgroup analysis.
RESULTS
Characteristics of included studies
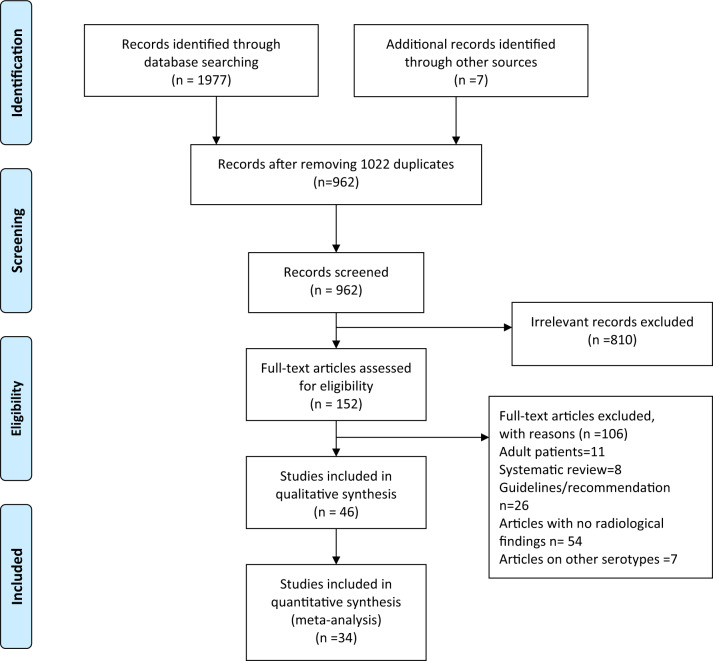
After removing 1022 duplicates, 962 records were screened through titles and abstracts for eligibility for this systematic review (Fig. 1). After the removal of 810 irrelevant articles, 152 full-text articles were assessed for inclusion in this study. Finally, we included 46 articles; of these 34 were case series (three or more cases) [5–38] and 12 case reports [39–50], with 923 patients with COVID-19. Characteristics of all eligible studies are described in Table 1. Among included studies, 17 are of fair quality and rest 19 are of poor quality. Forty-two of the included studies are from China, two from Italy and one each from Korea and Lebanon.
Fig. 1.
PRISMA flowchart.
Table 1.
Details of studies included in systematic review (n=46)
| Author | Publication date (DD-MM-YYYY) | Country | Total patients | Age (range) | Quality of study | |
|---|---|---|---|---|---|---|
| Bai, et al. [5] | 22-04-2020 | China | 25 | 0.6–17 years | Fair | |
| Cai, et al. [6] | 28-02-2020 | China | 10 | 3 months–10.91 years | Poor | |
| Chen, et al. [7] | 22-03-2020 | China | 12 | 6 months–17 years | Poor | |
| Denina, et al. [52] | 21-04-2020 | Italy | 8 | 0–17 years | Poor | |
| Duan W, et al. [9] | 03-02-2020 | China | 30 | 0.5–17 years | Fair | |
| Feng, et al. [11] | 02-05-2020 | China | 5 | 1–18 years | Poor | |
| Feng Kai, et al. [10] | 02-04-2020 | China | 15 | 4–14 years | Fair | |
| Li B, et al. [13] | 07-04-2020 | China | 22 | 2–8 years | Fair | |
| Li W, et al. [13] | 11-03-2020 | China | 5 | 10 months–6 years | Poor | |
| Li Y, et al. [12] | 07-04-2020 | China | 8 | 1–5 years | Poor | |
| Liu M, et al. [17] | 06-05-2020 | China | 5 | 7 months–13 years | Poor | |
| Liu H, et al. [15] | 02-03-2020 | China | 4 | 2 months–11 years | Poor | |
| Liu W, et al. [16] | 12-03-2020 | China | 6 | 1–7 years | Poor | |
| Lu, et al. [18] | 18-03-2020 | China | 111 | 1 day–15 years | Fair | |
| Lu, et al. [19] | 28-04-2020 | China | 9 | 2 months–12 years | Fair | |
| Ma, et al. [20] | 06-05-2020 | China | 50 | 0.9–9.8 years | Fair | |
| Parri, et al. [21] | 01-05-2020 | Italy | 18 | 0–17.5 years | Fair | |
| Qiu, et al. [22] | 25-03-2020 | China | 19 | 1–16 years | Fair | |
| Shen, et al. [23] | 23-03-2020 | China | 9 | 1–12 years | Poor | |
| Song, et al. [24] | 16-04-2020 | China | 16 | 11 months–14 years | Poor | |
| Su, et al. [25] | 12-03-2020 | China | 9 | 2 years 9 months–9 years | Poor | |
| Sun, et al. [26] | 02-03-2020 | China | 8 | 2 months–15 years | Poor | |
| Tan, et al. [27] | 03-04-2020 | China | 10 | 13 months–11 years | Fair | |
| Wu, et al. [28] | 06-05-2020 | China | 74 | 0.10–15.08 years | Fair | |
| Xia, et al. [29] | 05-05-2020 | China | 20 | 1 day–14 years | Fair | |
| Xin, et al. [30] | 30-03-2020 | China | 13 | 2–17 years | Fair | |
| Xing, et al. [31] | 25-03-2020 | China | 3 | 1.5–6 years | Poor | |
| Xu, et al. [32] | 13-03-2020 | China | 10 | 2 months–16 years | Poor | |
| Yao-Ling, et al. [33] | 16-03-2020 | China | 115 | 1 month–15 years | Fair | |
| Zhang, et al. [34] | 30-04-2020 | China | 46 | 7 months–18 years | Fair | |
| Zheng, et al. [35] | 10-03-2020 | China | 24 | 1 month–14 years | Fair | |
| Zhong, et al. [36] | 28-03-2020 | China | 9 | 3 months–12 years | Poor | |
| Zhou, et al. [37] | 01-03-2020 | China | 9 | 0–3 years | Poor | |
| Zhu, et al. [38] | 01-06-2020 | China | 10 | 1–18 years | Fair | |
| Case reports (n-12) | ||||||
| Cui, et al. [40] | 17-03-2020 | China | 1 | 55 days | NA | |
| Ji, et al. [50] | 16-03-2020 | China | 2 | 15 years, 9 years | NA | |
| Jiang, et al. [39] | 05-04-2020 | China | 2 | 6 years 8 months | NA | |
| Mansour, et al. [41] | 04-03-2020 | Lebanon | 1 | 16 months | NA | |
| Li, et al. [46] | 20-04-2020 | China | 1 | 8 months | NA | |
| Lin, et al. [47] | 04-03-020 | China | 1 | 7 years | NA | |
| Mao, et al. [45] | 07-05-2020 | China | 1 | 14 months | NA | |
| Pan, et al. [43] | 19-02-2020 | China | 1 | 3 years | NA | |
| Park, et al. [42] | 16-03-2020 | Korea | 1 | 10 years | NA | |
| Qiu, et al. [48] | 13-04-2020 | China | 1 | 8 months | NA | |
| Xu, et al. [44] | 01-03-2020 | China | 1 | 10 years | NA | |
| Zhang, et al. [49] | 01-03-2020 | China | 2 | 1 year 3 months | NA | |
NA, quality of study not assessed.
Radiology findings
Thirty-four case series reported data of 908 participants of which imaging details are provided for 747 (82.3%) only. Among these 747 patients, the clinical details were available for 722 (97%) patients (521 symptomatic, 201 asymptomatic). Chest CT was the commonest imaging modality (96.1%), followed by chest X-ray (8.2%) and chest ultrasound (3%).
Among the clinically symptomatic ones (n = 521 patients), 92 (17.6%) did not have any identifiable radiological abnormality, whereas 19% of clinically asymptomatic (38 of 201) patients had significant radiological findings. Overall, almost one-third (38%, 95% CI 28–42, I2 = 70%) of the patients who underwent imaging had a normal scan.
All the abnormal scans were further classified according to the type and size of the lesion. The exact description of the side of lung involvement was available for 263 patients only of which 143 (55%) have unilateral lung involvement and rest (45%) have bilateral lung involvement. The location of the lesion was mentioned for 55 only of which 44 (80%) lesions were located in the peripheral zone. The lower lobe of the right lung (40%) was most commonly involved followed by the upper lobe of the right lung (28%), lower lobe of the left lung (17%), middle lobe of the right lung (9%) and upper lobe of the left lung (6%).
Ground-glass opacity (GGO) was the most frequently reported radiological abnormality (32 studies and 706 patients) with pooled estimate in 39% (95% CI 31–48) of the patients. Patchy shadow (of undefined attenuation) was reported in 13 studies (246 patients) with a pooled estimate of 44% (95% CI 32–55). Consolidation was reported in 12 studies (192 patients) with a pooled estimate of 23% (95% CI 12–34). Other commonly reported abnormalities were halo sign, prominent bronchovascular marking, pleural effusion, bronchial wall thickening and pulmonary nodules (Table 2). To explore the high heterogeneity in pooled estimate, we performed sensitivity analysis did not find any specific study affecting the overall pooled estimates. On subgroup analysis according to quality of study, no significant difference was observed in pooled estimated of poor and fair quality study groups.
Table 2.
Common radiological findings in children with SARS-CoV-2 infection
| Radiological finding | No. of studies (patients) | Pooled estimates % (95% CI) | Heterogeneity % (I2) | P-value for heterogeneity |
|---|---|---|---|---|
| Ground glass opacity | 32 (706) | 39 (31–48) | 82 | 0.00 |
| Consolidation | 12 (192) | 23 (12–34) | 82 | 0.00 |
| Halo sign | 6 (78) | 26 (11–41) | 51 | 0.09 |
| Patchy shadow | 13 (246) | 44 (32–55) | 62 | 0.00 |
| Prominent bronchiovascular markings | 5 (97) | 17 (09–24) | 0.0 | 0.83 |
| Bronchial wall thickening | 4 (36) | 11 (1–21) | 0.0 | 1.00 |
| Pleural effusion | 5 (187) | 2 (0.1–4) | 0.0 | 0.60 |
| Interstitial pattern | 4 (187) | 12 (1–23) | 82 | 0.00 |
| Nodules | 5 (63) | 25 (9–41) | 62 | 0.03 |
Three studies directly compared a chest X-ray with a chest CT scan [11, 31, 32]. Of the 18 patients, only 3 patients had positive chest X-ray findings, whereas chest CT identified radiological abnormalities in 10 patients. Point of care ultrasound is an emerging modality in COVID-19. Three studies evaluated its role in a total of 23 patients [11, 51, 52]. The commonest finding was pulmonary interstitial syndrome (seen in 19 of 23 patients) followed by small consolidation. One study directly compared chest CT, X-ray and lung ultrasound (LUS) in five patients and found that ultrasound fares better than X-ray and successfully identified two of the three abnormal cases [11].
In this systematic review, we also included 12 case reports [39–50] with COVID-19. Of these 12 patients had chest CT alone, one had X-ray only and one had both. Of the 15 patients, 8 did not have any radiological abnormality while among the remaining 2 had GGO alone, 2 had GGO and consolidation, 2 each have consolidation and shadows and 1 had GGO along with consolidation and pleural effusion.
DISCUSSION
We found that the chest CT scan was the commonest imaging modality performed in children with confirmed COVID-19. Overall, one-third of patients did not have any radiological abnormality. Significant proportions (19%) of clinically asymptomatic but RT-PCR proven COVID-19 patients have radiological findings, suggesting that the chest CT can detect the infection before the appearance of clinical signs. GGOs are the commonest radiological lesion seen in COVID-19. Most of the lesions are located peripherally with the lower lobe of the right lung being the most common location. A chest X-ray is insensitive in identifying COVID-19 pneumonia. LUS fares better than X-ray but more evidence is needed.
Similar to adults, CT scan is the most frequently used radiological imaging modality. Alike adults, children too have GGOs as the most frequently reported radiological finding [1, 53]. Also, similar to adults the pulmonary lesions are peripherally distributed and have multilobar involvement in most of the cases. However, the incidence of radiological abnormalities is higher in adults. As high as one-third of the chest CT may not show any radiological evidence in children, whereas only 10–15% cases in adults may show a normal CT [1, 53]. Also, unlike adults where most of the lesions (∼80%) are bilateral, the distribution seems in children almost same in both lungs individually [1, 53]. Chest CT has been recommended as a screening tool in adults due to its higher sensitivity than RT PCR (98% vs. 71%, respectively) in initial stages of disease, however, similar data for children are lacking [54]. But a significant proportion of clinically asymptomatic children can be detected on chest CT.
The concern with the use of CT scan in children lies in the radiation exposure, which leads to exploring LUS as an imaging modality in COVID-19 [51, 52]. Since it is a point of care tool and most of the intensivists are more experienced with bedside LUS than CT, its role in the emergency department is likely to increase further [51]. Also, COVID-19 causes diffuse interstitial pneumonia with lesions involving mainly the peripheral lung, which makes it particularly suitable for use of LUS. The ‘light beam sign’ is considered an early as well as a specific marker of COVID-19 pneumonia on LUS [55]. However, this modality is still evolving and further research on it is likely to enlighten us further.
As many of the included studies were limited by sample size, methodological quality and variation in reporting of imaging findings leading to significant heterogeneity, the results (particularly pooled estimates) should be interpreted cautiously. Also, due to very limited data availability, we could not correlate the imaging findings with the disease severity, disease progression and outcomes.
CONCLUSIONS
In the paediatric age group, a chest CT scan is the most common imaging modality used in COVID pneumonia. GGO followed by patchy shadows and consolidation are the most frequently reported radiological findings. LUS is an emerging modality and needs further exploration.
Supplementary Material
REFERENCES
- 1. Salehi S Abedi A Balakrishnan S, et al. . Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am J Roentgenol 2020;215:87–93. [DOI] [PubMed] [Google Scholar]
- 2. Liguoro I, Pilotto C, Bonanni M, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 4. Higgins JPT, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Statist Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 5. Bai K, Liu W, Liu C, et al. Clinical analysis of 25 novel coronavirus infections in children. Pediatr Infect Dis J 2020. [DOI] [PubMed] [Google Scholar]
- 6. Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis 2020. https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa198/5766430 (11 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J, Zhang Z-Z, Chen Y-K, et al. The clinical and immunological features of pediatric COVID-19 patients in China. Genes Dis 2020. https://linkinghub.elsevier.com/retrieve/pii/S2352304220300507 (25 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Denina M, Scolfaro C, Silvestro E, et al. Lung ultrasound in children with COVID-19. Pediatrics 2020; e20201157. [DOI] [PubMed] [Google Scholar]
- 9. Duan Y, Zhu Y, Tang L, et al. CT features of novel coronavirus pneumonia (COVID-19) in children. Eur Radiol 2020. http://link.springer.com/10.1007/s00330-020-06860-3 (25 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng K, Yun YX, Wang XF, et al. Analysis of CT features of 15 Children with 2019 novel coronavirus infection. Chin J Pediatr 2020;58:E007. [DOI] [PubMed] [Google Scholar]
- 11. Feng XY, Tao XW, Zeng LK, et al. Application of pulmonary ultrasound in the diagnosis of COVID-19 pneumonia in neonates. Chin J Pediatr 2020;58:347–50. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Cao J, Zhang X, et al. Chest CT imaging characteristics of COVID-19 pneumonia in preschool children: a retrospective study. BMC Pediatr 2020;20:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li B, Shen J, Li L, et al. Radiographic and clinical features of children with 2019 novel coronavirus (COVID-19) pneumonia. Indian Pediatr 2020;57:423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li W, Cui H, Li K, et al. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol 2020;50:796–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu H, Liu F, Li J, et al. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect 2020;80:e7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu W, Zhang Q, Chen J, et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med 2020;382:1370–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu M, Song Z, Xiao K.. High-resolution computed tomography manifestations of 5 pediatric patients with 2019 novel coronavirus. J Comput Assist Tomogr 2020;44:311–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med 2020;382:1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu Y, Wen H, Rong D, et al. Clinical characteristics and radiological features of children infected with the 2019 novel coronavirus. Clin Radiol 2020;75:520–5. https://linkinghub.elsevier.com/retrieve/pii/S0009926020301665 (25 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma H, Hu J, Tian J, et al. A single-center, retrospective study of COVID-19 features in children: a descriptive investigation. BMC Med 2020;18 https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-020-01596-9 (11 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parri N, Lenge M, Buonsenso D.. Children with Covid-19 in Pediatric Emergency Departments in Italy. N Engl J Med 2020. http://www.nejm.org/doi/10.1056/NEJMc2007617 (11 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qiu H, Wu J, Hong L, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 2020;20:689–96. https://linkinghub.elsevier.com/retrieve/pii/S1473309920301985 (11 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shen K-L, Yang Y-H.. Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. World J Pediatr 2020. 10.1007/s12519-020-00344-6 (12 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song W, Li J, Zou N, et al. Clinical features of pediatric patients with coronavirus disease (COVID-19). J Clin Virol 2020;127:104377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Su L, Ma X, Yu H, et al. The different clinical characteristics of corona virus disease cases between children and their families in China – the character of children with COVID-19. Emerg Microbes Infect 2020;9:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun D, Li H, Lu X-X, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan Y, Tan B, Pan J, et al. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol 2020;127:104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Q, Xing Y, Shi L, et al. Co-infection and other clinical characteristics of COVID-19 in children. Pediatrics 2020; e20200961. [DOI] [PubMed] [Google Scholar]
- 29. Xia W, Shao J, Guo Y, et al. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr Pulmonol 2020;55:1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xin T, Juan H, Fen Z, et al. Analysis of clinical characteristics of 13 cases of novel coronavirus infection in children in Changsha. Chin J Contemp Pediatr 2020;22:294–8. [Google Scholar]
- 31. Xing Y-H, Ni W, Wu Q, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect 2020;53:473–80. https://linkinghub.elsevier.com/retrieve/pii/S1684118220300815 (25 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 2020;26:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yaoling M, Shengying X, Min W, et al. Analysis of clinical characteristics of 115 children infected with novel coronavirus. Chin J Contemp Pediatr 2020;290–3. [Google Scholar]
- 34. Zhang B, Liu S, Zhang J, et al. Children hospitalized for coronavirus disease 2019 (COVID-19): a multicenter retrospective descriptive study. J Infect 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng F, Liao C, Fan Q, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci 2020;40:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhong Z, Xie X, Huang W, et al. Chest CT findings and clinical features of coronavirus disease 2019 in children. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020;45:236–42. [DOI] [PubMed] [Google Scholar]
- 37. Zhou Y, Yang G-D, Feng K, et al. Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children. Zhongguo Dang Dai Er Ke Za Zhi 2020;22:215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhu L, Wang J, Huang R, et al. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol 2020;55:1430–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiang S Liu P Xiong G, et al. . Coinfection of SARS-CoV-2 and multiple respiratory pathogens in children. Clinical Chemistry and Laboratory Medicine (CCLM) 58:1160–1. [DOI] [PubMed] [Google Scholar]
- 40. Cui Y, Tian M, Huang D, et al. A 55-day-old female infant infected with 2019 novel coronavirus disease: presenting with pneumonia, liver injury, and heart damage. J Infect Dis 2020;221:1775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mansour A, Atoui R, Kanso K, et al. First case of an infant with COVID-19 in the Middle East. Cureus 2020;12:e7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park JY, Han MS, Park KU, et al. First pediatric case of coronavirus disease 2019 in Korea. J Korean Med Sci 2020;35:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis 2020;20:410–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu R, Du M, Li L, et al. CT imaging of one extended family cluster of corona virus disease 2019 (COVID-19) including adolescent patients and “silent infection”. Quant Imaging Med Surg 2020;10:800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mao L, Xu J, Xu Z, et al. A child with household transmitted COVID-19. BMC Infect Dis 2020;20:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li J, Feng J, Liu T, et al. An infant with a mild SARS-CoV-2 infection detected only by anal swabs: a case report. Braz J Infect Dis 2020. http://www.sciencedirect.com/science/article/pii/S141386702030043X (25 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin J, Duan J, Tan T, et al. The isolation period should be longer: lesson from a child infected with SARS-CoV-2 in Chongqing. Pediatr Pulmonol 2020;55:E6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qiu L, Jiao R, Zhang A, et al. A case of critically ill infant of coronavirus disease 2019 with persistent reduction of T lymphocytes. Pediatr Infect Dis J 2020;39: e87–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang G-X, Zhang A-M, Huang L, et al. Twin girls infected with SARS-CoV-2. Zhongguo Dang Dai Er Ke Za Zhi 2020;22:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ji L-N, Chao S, Wang Y-J, et al. Clinical features of pediatric patients with COVID-19: a report of two family cluster cases. World J Pediatr 2020. http://link.springer.com/10.1007/s12519-020-00356-2 (25 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parri N, Lenge M, Buonsenso D, Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group. Children with Covid-19 in Pediatric Emergency Departments in Italy. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Denina M, Scolfaro C, Silvestro E, et al. Lung ultrasound in children with COVID-19. Pediatrics 2020. http://pediatrics.aappublications.org/lookup/doi/10.1542/peds.2020-1157 (22 May 2020, date last accessed). [DOI] [PubMed] [Google Scholar]
- 53. Sun Z, Zhang N, Li Y, et al. A systematic review of chest imaging findings in COVID-19. Quant Imaging Med Surg 2020;10:1058–79. http://qims.amegroups.com/article/view/41327/html (27 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology 2020. http://pubs.rsna.org/doi/10.1148/radiol.2020200432 (22 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Volpicelli G, Gargani L.. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J 2020;12:22 https://theultrasoundjournal.springeropen.com/articles/10.1186/s13089-020-00171-w (27 May 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.