Abstract
The purpose of the present study was to discover the effects of iron on the intestinal development and epithelial maturation of suckling piglets. Twenty-seven newborn male piglets from 9 sows (3 piglets per sow), with similar body weight, were selected. The 3 piglets from the same sow were randomly divided into 1 of the 3 groups. The piglets were orally administrated with 2 mL of normal saline (CON group) or with 25 mg of iron by ferrous sulfate (OAFe group; dissolved in normal saline) on the 2nd, 7th, 12th, and 17th day, respectively, or intramuscularly injected with 100 mg of iron by iron dextran (IMFe group) on the 2nd day. The slaughter was performed on the 21st day and intestinal samples were collected. Compared with the CON group, iron supplementation significantly increased the length (P < 0.001), weight (P < 0.001), relative weight (P < 0.001), and the length:weight ratio (P < 0.001) of the small intestine in both OAFe and IMFe groups. The villus height (P < 0.001), crypt depth (CD) (P < 0.001), villus width (P = 0.002), and surface area (P < 0.001) in the jejunum of IMFe and OAFe piglets were also greater than those in CON piglets. The mRNA expression of trehalase (Treh; P = 0.002) and sucrase isomaltase (Sis; P = 0.043), markers of epithelial maturation, increased in OAFe and IMFe piglets, respectively. Moreover, enterocyte vacuolization, observed in fetal-type enterocyte, was reduced in OAFe and IMFe piglets, compared with CON piglets. However, no significant difference in the expression of the target genes of wnt/β-catenin signaling pathway was observed. The results indicated that both oral administration and intramuscular injection with iron promoted intestinal development and epithelial maturation in suckling piglets and that the effects of iron may be independent of wnt/β-catenin signaling.
Keywords: enterocyte vacuolization, intestinal morphology, jejunum, organ index, wnt/β-catenin signaling
Introduction
Intestinal tract is an important digestive organ and the largest immune organ of animals, and intestinal integrity is the basis of animal growth and development (Yang et al., 2013). The gut development and maturation of piglets change widely during the suckling period, with rapid growth of the gastrointestinal tract, reducing villus height (VH), increasing crypt depth (CD) and mitotic index, reducing maltase and sucrase activities, and reducing vacuolating epithelial cells and vacuole size (Zabielski et al., 2008; Yang et al., 2013). The rate of vacuole disappearance is closely related to the maturation of the intestine, i.e., transformation into adult digestion and absorption (Zabielski et al., 2008). However, due to early weaning in piglet production today, the gut cannot develop naturally (Radberg et al., 2001; Thomsson et al., 2007). Due to the immaturity of the gastrointestinal tract, the piglets are unable to adapt to external environmental stimuli, when weaned at 21 d, resulting in intestinal morphology damage, decreasing feed intake, and diarrhea (Hu et al., 2013).
Iron is one of the most important trace elements in the growth of livestock and poultry. Iron plays key role in transporting oxygen and carbon dioxide, material transport and storage, and nutrient synthesis by combining with oxygen to form hemoglobin or as an activator of enzyme (Blake et al., 1983; Gozzelino et al., 2016; Szudzik et al., 2018). Due to low body storage, iron content in milk and high growth intensity, the suckling piglets commonly suffered from iron deficiency without iron supplementation (Venn et al., 1947; Egeli et al., 1998; Svoboda et al., 2005). Recently, it was reported that iron plays important roles in modulating intestinal stem cell activity, which in turn play a key role in modulating intestinal development and epithelial maturation, and cell proliferation via Wnt/β-catenin signaling (Song et al., 2011; Radulescu et al., 2016). Therefore, iron deficiency may affect intestinal development of suckling piglets by modulating stem cell activity and cell proliferation. In vivo experiments have shown that iron supplementation could improve growth performance, blood iron nutrition levels, reduce duodenal mucosal damage, and intestinal immune development of piglets (Novais et al., 2017; Pu et al., 2018). However, this also shows the effects of iron supplementation on intestinal development and epithelial maturation of suckling piglets. In the present study, we hypothesized that iron supplementation may improve intestinal development and epithelial maturation of piglets during the suckling period. Therefore, the objective of the present study was to determine intestinal organ indices, intestinal morphology, and epithelial maturation markers in control and iron-supplemented suckling piglets.
Materials and Methods
The experimental design and procedures were approved by the Animal Protection and Utilization Committee of Hunan Normal University, Changsha City, Hunan Province.
Animals and experimental design
A total of 27 newborn male piglets (Duroc × Landrace × Yorkshire) with similar body weight were selected from 9 sows (3 piglets per sow). The sow diet met the National Research Council (2012) nutrient specifications for lactating sows. The piglets from the same sow were randomly divided into 3 groups (1 piglet per group). All piglets were fed with breast milk. The piglets were orally administrated with 2 mL of normal saline (CON group) or 25 mg of iron by ferrous sulfate (OAFe group; dissolved in normal saline) on the 2nd, 7th, 12th, and 17th day, respectively, or intramuscularly injected with 100 mg of iron by iron dextran (IMFe group) on the 2nd day. The piglets were weighed every week after birth, and, on the 21st day, the average body weight for piglets in CON, OAFe, and IMFe groups were 5.11 ± 0.49, 5.33 ± 0.78, and 5.39 ± 0.55 kg, respectively. There were 9 piglets in each group. During the whole experiment period, all piglets were free to eat and drink.
Sample collection
On the 21st day of the experiment, the piglets were euthanized with a sodium pentobarbital overdose of 40 mg/kg body weight, followed by exsanguination (Yin et al., 2001). Then, their abdomens were opened and the small intestine was taken out. The small intestine was then washed with precooling normal saline (4 °C) immediately to remove its contents, and weighed with an electronic scale; thereafter, the intestines were placed on a table marked with two rows 1 m apart. One end of the small intestine was fixed, while the other was not stretched and measured (Thomsson et al., 2007). About 2 cm segments of jejunum (from the middle part of the small intestine) were fixed in 10% formalin solution and preserved at room temperature until the morphological measurement was done. The samples of jejunal mucosa were collected with tin foil, frozen in liquid nitrogen, and kept in a refrigerator at –80°C for quantitative polymerase chain reaction (PCR) analysis (Wang et al., 2019).
Intestinal morphological analysis
According to the standard paraffin-embedding procedure, jejunal samples immersed in 10% formaldehyde fixed solution were dehydrated by a series of gradients of alcohol and required xylene to make the samples transparent and then embedded in paraffin. Paraffin was then cut into 5 μm thick slices by a slicer (RM2235; Leica, Germany) and placed on a glass slide (CITOGLAS, Jiangsu, China). After hematoxylin and eosin staining (HE), jejunum samples were placed in an optical microscope. The Image-Pro Plus 6.0 software (Media Cybernetics, San Diego, CA) was used to measure the VH, CD, and villi width of the jejunum, and calculate the VH to CD and fluff surface area. At least 30 complete villi and their associated crypts were selected from each intestine segment of each piglet and the corresponding average value for each piglet was calculated and used for further analysis (Wang et al., 2020).
Real-time quantitative PCR
The total RNA was isolated from intestinal mucosal samples, and then the genomic DNA was removed by a primescript Real-Time Kit (Takara, Dalian, China) with gDNA eraser (42 °C, 2 min). The reverse transcription was carried out at 37 °C for 15 min, and the cDNA was obtained at 85 °C for 5 s. Five-fold dilution of RNase free water was used for real-time quantitative PCR (RT-qPCR) analysis. RT-qPCR was carried out under the StepOnePlus™ system (QuantStudio, Thermo Fisher Scientific). Each reaction consisted of 5 μL of SYBR Green mixture, 0.2 μL of positive and negative primers, 0.2 μL of rox reference dye (50×), 3.4 μL of water and 1 μL of 5-fold diluted cDNA. The final volume was 10 μL. The RT-qPCR amplification was done as follows: predenaturation at 95 °C for 30 s, denaturation at 95 °C for 5 s, annealing at 60 °C for 30 s, a total of 40 cycles, followed by melting curve procedure of 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s. The specific operation steps are performed according to the product manual. The primers were designed using Primer Premier 6.0 primer design software and are listed in Supplementary Table S1. Each gene was repeated 3 times, and each sample corresponded to β-actin as a reference. The target gene mRNA relative expression (RE) was calculated as RE = 2–ΔΔCt, where –ΔΔCt = Ct (target gene) – Ct (β-actin) (Kenneth et al., 2001).
Statistical analysis
The SPSS 20.0 (IBM Corp., Chicago, IL) was used for statistical analysis. A one-way analysis of variance was used to calculate the statistical differences in basic characteristics between the groups. Values are expressed as standard error of the mean. P < 0.05 was considered statistically significant.
Results and Discussion
Intestinal indexes and morphology
Lactation period is a period of rapid growth and development of piglets and a key stage of piglet intestinal development. Studies have shown that weight, length, diameter, and mucosal weight of the small intestine increased by 72%, 24%, 15%, and 115%, respectively, on the first day of life (Xu et al., 1992). In the present study, although no significant difference in growth performance was observed among the 3 treatments, the length, weight, and relative weight of small intestine in OAFe and IMFe piglets were significantly greater (P < 0.001) than those in CON piglets; the relative length also tended to be greater (P = 0.072) in OAFe and IMFe piglets than CON piglets (Table 1). These results indicate that iron supplementation could promote the growth and development of small intestine in suckling piglets. Moreover, the piglets from both OAFe and IMFe treatments had a greater ratio of intestinal weight to intestinal length (P < 0.001; Table 1), indicating that iron-supplemented piglets have greater weight per unit length of small intestine than CON piglets. To test whether the increase in weight per unit length of small intestine was due to the growth of intestinal morphology, we measured the ratio of intestinal weight to intestinal length. As expected, iron supplementation increased VH, CD, and villus width and villus surface area in both OAFe and IMFe treatments (P < 0.05), compared with the CON group (Table 1). The results were in agreement with those of previous experiments, which showed that iron supplementation could increase the height of duodenal villi, the depth of duodenal crypt, and thicken the duodenal wall of piglets (Pu et al., 2018; Chen et al., 2019). The increase in CD means increase in cell proliferation and more cells migrating to villi; thus, iron supplementation may improve intestinal structure by promoting cell proliferation in suckling piglets. These results indicate that iron supplementation promotes intestinal development in suckling piglets by enhancing organ indices and intestinal morphology. Intestinal length and VH are positively related to digestive and absorptive capacity of pigs (Wang et al., 2020). The effects of iron on intestinal development may not only improve intestinal functions of piglets but also have lifetime effects on nutrient digestibility.
Table 1.
Effects of iron supplementation on organ indexes, morphology, and gene expression in the small intestine of suckling piglets1
| Treatments2 | ||||
|---|---|---|---|---|
| Items | CON | IMFe | OAFe | P-value |
| Organ indexes | ||||
| Intestine length, m | 7.59 ± 0.62b | 8.99 ± 0.46a | 9.12 ± 0.75a | 0.000 |
| Intestine weight, g | 150.44 ± 16.52c | 254.67 ± 21.37a | 227.78 ± 29.21b | 0.000 |
| Weight:length, g/m | 19.61 ± 1.51c | 28.28 ± 2.34a | 24.84 ± 2.76b | 0.000 |
| Relative length, m/kg | 1.50 ± 0.17 | 1.65 ± 0.16 | 1.75 ± 0.29 | 0.072 |
| Relative weight, g/kg | 29.43 ± 3.11bb | 46.64 ± 5.92a | 42.87 ± 8.84a | 0.000 |
| Intestinal morphology | ||||
| VH, μm | 289.15 ± 37.45b | 389.73 ± 27.48a | 382.96 ± 41.60a | 0.000 |
| CD, μm | 179.23 ± 19.74b | 232.88 ± 18.64a | 234.08 ± 26.10a | 0.000 |
| Villus width, μm | 119.09 ± 8.74b | 135.85 ± 6.96a | 132.93 ± 9.99a | 0.002 |
| VH:CD | 1.67 ± 0.26 | 1.66 ± 0.16 | 1.61 ± 0.16 | 0.839 |
| Surface area, μm2 | 108268.1 ± 17503.8b | 166533.0 ± 17332.9a | 159994.9 ± 22072.4a | 0.000 |
| Expresssion of enterocytes marker genes | ||||
| Treh | 1.24 ± 0.30c | 9.03 ± 0.96a | 4.77 ± 0.71b | 0.002 |
| Arg2 | 1.23 ± 1.07 | 0.70 ± 0.21 | 1.41 ± 1.28 | 0.378 |
| Sis | 1.09 ± 0.43b | 1.65 ± 0.53ab | 2.33 ± 1.49a | 0.043 |
| Ada | 1.06 ± 0.37 | 0.86 ± 0.21 | 0.87 ± 0.37 | 0.391 |
| Ass1 | 1.20 ± 0.84 | 1.39 ± 0.82 | 1.19 ± 0.49 | 0.838 |
| Gleb-β | 1.02 ± 0.21 | 0.94 ± 0.44 | 1.08 ± 0.51 | 0.790 |
| Expression of Wnt/β-catenin target genes | ||||
| Need8 | 1.07 ± 0.37 | 0.76 ± 0.35 | 1.01 ± 0.41 | 0.267 |
| Bmp4 | 1.11 ± 0.52 | 0.78 ± 0.36 | 0.90 ± 0.2 | 0.250 |
| Sgk1 | 1.05 ± 0.37 | 1.01 ± 0.51 | 1.11 ± 0.67 | 0.933 |
| JAG1 | 1.04 ± 0.28 | 0.84 ± 0.32 | 0.87 ± 0.53 | 0.564 |
| JAG2 | 1.08 ± 0.42 | 0.95 ± 0.49 | 1.04 ± 0.69 | 0.889 |
1 n = 9.
2CON, control; OAFe, orally administrated ferrous sulfate; IMFe, intramuscularly injected with iron dextran.
a,bValues in the same row with different superscript letters are significantly different (P < 0.05).
Intestinal epithelial maturation
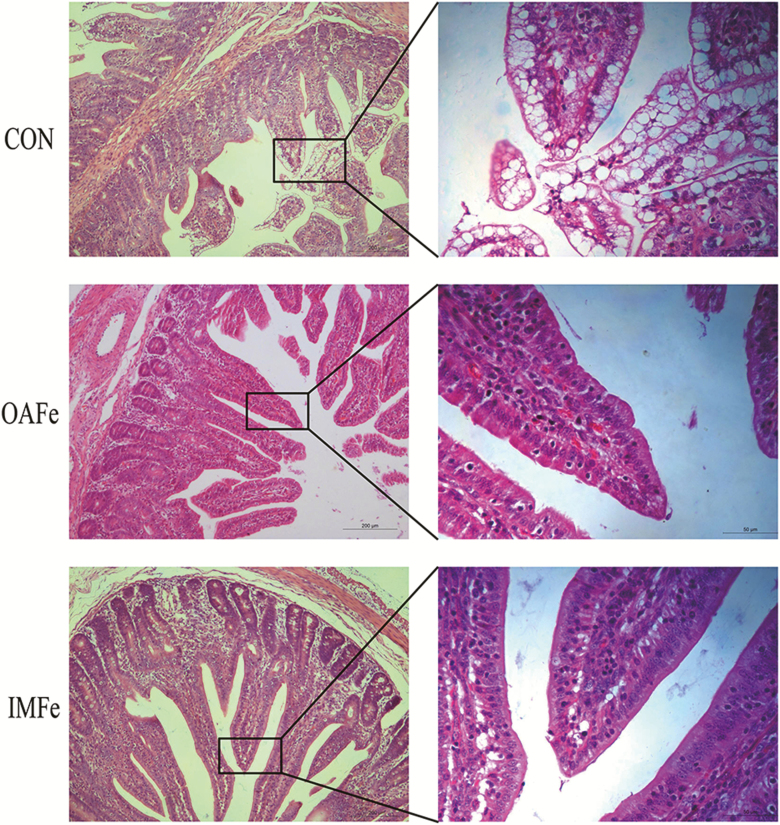
The development of intestine in suckling piglets not only includes increase in its length and weight and changes in intestinal morphology but also intensive processes in intestinal epithelium (Yang et al., 2013). In order to absorb immunoglobulins and other large biologically active molecules from mother’s milk, especially colostrum, the piglets are born with vacuolating fetal-type enterocytes, gradually replaced by nonvacuolating adult-type enterocytes during lactation (Zabielski et al., 2008; Yang et al., 2013). In piglets, the vacuolating fetal-type enterocytes gradually disappeared from the proximal jejunum to the ileum; the entire process takes about 3 to 4 wk (Skrzypek et al., 2007). The rate of vacuole disappearance is closely related to the maturation of the intestine, i.e., transformation into adult digestion and absorption (Radberg et al., 2001; Skrzypek et al., 2007). The HE slices showed that the number of vacuolating enterocytes in CON piglets were greater than those in OAFe and IMFe piglets (Figure 1), suggesting that iron supplementation promoted intestinal epithelial maturation of suckling piglets. The change in intestinal enterocytes is associated with changing the expression of marker genes of different intestinal enterocytes (Harper et al., 2011; Muncan et al., 2011). Argininosuccinate synthetase 1 (Ass1) and β-galactosidase (Gleb) are reported to be marker genes of immature fetal-type enterocytes, while Sis, Treh, adenosine deaminase (Ada), and arginase 2 (Arg2) are expressed in mature adult-type enterocytes and considered as the marker genes of mature adult-type enterocytes (Harper et al., 2011; Muncan et al., 2011). To confirm the results of HE slices, we measured the mRNA expression of marker genes of fetal-type and adult-type enterocytes. Consistent with HE slices results, the expression of Treh was greater (P < 0.05) in OAFe and IMFe treatments than that in the CON group and the expression of Sis in OAFe piglets was greater (P < 0.05) than that in CON piglets (Table 1). These results indicated that iron supplementation could promote intestinal epithelial maturation of piglets during lactation. However, as no significant differences in the expression of Ass1, Gleb, Ada, and Arg2 were observed among the 3 treatments, further research is needed to study why iron supplementation has different effects on the expression of marker genes. Iron deficiency was reported to be associated with diarrhea and intestinal dysfunction in suckling piglets (Svoboda et al., 2005; Streyl et al., 2015), which may be because iron deficiency impaired intestinal epithelial maturation. Therefore, iron supplementation may be used to improve intestinal functions of piglets and we may be able to regulate intestinal epithelial maturation during lactation to alleviate weaning stress, but more research on the effects of iron doses and sources is needed.
Figure 1.
Effects of iron supplementation on jejunal vacuolating enterocytes of suckling piglets. CON, control; OAFe, orally administrated ferrous sulfate; IMFe, intramuscularly injected with iron dextran.
The Wnt/β-catenin signaling plays crucial roles in modulating intestinal development and epithelial renewal by affecting the activity of intestinal stem cell (de Santa Barbara et al., 2003; Theodosiou et al., 2003), and, recently, iron was shown to trigger Wnt/β-catenin signaling via an independent cadherin pathway (Song et al., 2011; Radulescu et al., 2016). Therefore, we have hypothesized that iron may, via Wnt/β-catenin signaling, affect intestinal development and epithelial maturation. However, there were no significant differences in the expression of Wnt/β-catenin target genes, neural precursor cell expressed, developmentally downregulated 8 (Nedd8), bone morphogenetic protein 4 (Bmp4), serum/glucocorticoid regulated kinase 1 (Sgk1), jagged 1 (Jag1), and jagged 2 (Jag2) among the 3 treatments. These results indicate that the effects of iron on intestinal development and epithelial maturation are independent of Wnt/β-catenin signaling in suckling piglets, and more studies are needed to reveal the mechanism of iron on intestinal development and epithelial maturation.
In conclusion, the present study indicates that iron supplementation is capable of modulating intestinal development by increasing intestinal length and weight, improving intestinal morphology, and promoting epithelial maturation in suckling piglets. The effects of iron on intestinal development and epithelial maturation are independent of Wnt/β-catenin signaling. More studies are needed to reveal the mechanism of iron on intestinal development and epithelial maturation.
Supplementary Material
Acknowledgments
This work was supported by National Key R & D Program (2016YFD0501201), Changsha Zhuzhou Xiangtan High-level Talent Concentration Project (2017XK2022), Natural Science Foundation of Hunan Province (2017JJ1020), Scientifc Research Foundation of Hunan Provincial Education Department (17B164).
Glossary
Abbreviations
- PCR
polymerase chain reaction
- RT-qPCR
real-time quantitative PCR
- RE
relative expression
- HE
hematoxylin-eosin staining
- Ass1
argininosuccinate synthetase 1
- Gleb
β-galactosidase
- Sis
sucrase isomaltase
- Treh
trehalase
- Ada
adenosine deaminase
- Arg2
arginase 2
- Bmp4
bone morphogenetic protein 4
- Nedd8
neural precursor cell expressed, developmentally down-regulated 8
- Sgk1
serum/glucocorticoid regulated kinase 1
- Jag1
jagged 1
- Jag2
jagged 2
- VH
villus height
- CD
crypt depth
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Blake D R, Hall N D, Bacon P A, Dieppe P A, Halliwell B, and Gutteridge J M. . 1983. Effect of a specific iron chelating agent on animal models of inflammation. Ann. Rheum. Dis. 42:89–93. doi: 10.1136/ard.42.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X Y, Zhang X F, Zhao J, Tang X Y Wang F Q, and Du. H H. 2019. Split iron supplementation is beneficial for newborn piglets. Biomed. Pharmacother. 120:109479. doi: 10.1016/j.biopha.2019.109479. [DOI] [PubMed] [Google Scholar]
- Egeli A K, and Framstad T. . 1998. Evaluation of the efficacy of perorally administered glutamic acid-chelated iron and iron-dextran injected subcutaneously in Duroc and Norwegian Landrace piglets. Zentralbl Veterinarmed A 45:53–61. doi: 10.1111/j.1439-0442.1998.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Gozzelino R, and Arosio P. . 2016. Iron homeostasis in health and disease. Int. J. Mol. Sci. 17:130. doi: 10.3390/ijms17010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J, Mould A, Andrews R M, Bikoff E K, and Robertson E J. . 2011. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc. Natl. Acad. Sci. USA. 108:10585–10590. doi: 10.1073/pnas.1105852108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C H, Xiao K, Luan Z S, and Song J. . 2013. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 91:1094–1101. doi: 10.2527/jas.2012-5796. [DOI] [PubMed] [Google Scholar]
- Kenneth J L, and Schmittgen T D. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ CT method. Methods. 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Muncan V, Heijmans J, Krasinski S D, Büller N V, Wildenberg M E, Meisner S, Radonjic M, Stapleton K A, Lamers W H, Biemond I, . et al. 2011. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat. Commun. 2:452. doi: 10.1038/ncomms1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council 2012. Nutrient requirements of swine, 11th revised edition. Washington, DC: National Academy Press. [Google Scholar]
- Novais A. K., da Silva C. K., dos Santos R. D. K. S., Dias C. P., Callegari M. A., and de Oliveira E. R.. 2017. The effect of supplementing sow and piglet diets with different forms of iron. Rev. Bras. Zootec. 45:615–621. doi: 10.1590/S1806-92902016001000006. [DOI] [Google Scholar]
- Pu Y T, Li S H, Xiong H T, Zhang X F, Wang Y Z, and Du H H. . 2018. Iron promotes intestinal development in neonatal piglets. Nutrients. 10:726. doi: 10.3390/nu10060726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rådberg K, Biernat M, Linderoth A, Zabielski R, Pierzynowski S G, and Weström B R. . 2001. Enteral exposure to crude red kidney bean lectin induces maturation of the gut in suckling pigs. J. Anim. Sci. 79:2669–2678. doi: 10.2527/2001.79102669x. [DOI] [PubMed] [Google Scholar]
- Radulescu S, Brookes M J, Salgueiro P, Ridgway R A, McGhee E, Anderson K, Ford S J, Stones D H, Iqbal T H, Tselepis C, . et al. 2016. Luminal iron levels govern intestinal tumorigenesis after Apc loss in vivo. Cell Rep. 17:2805–2807. doi: 10.1016/j.celrep.2016.10.028. [DOI] [PubMed] [Google Scholar]
- de Santa B P, Van Den Brink G R, and Roberts D J. . 2003. Development and differentiation of the intestinal epithelium. Cell. Mol. Life Sci. 60:1322–1332. doi: 10.1007/s00018-003-2289-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypek T, Valverde J L P, Skrzypek H, Kazimierczak W, Biernat M, and Zabielski R. . 2007. Gradual disappearance of vacuolated enterocytes in the small intestine of neonatal piglets. J. Physiol. Pharmacol. 58Suppl. 3: 87–95. doi: 10.2170/physiolsci.SC001607. [DOI] [PubMed] [Google Scholar]
- Song S, Christova T, Perusini S, Alizadeh S, Bao R Y, Miller B W, Hurren R, Jitkova Y, Gronda M, Isaac M, . et al. 2011. Wnt inhibitor screen reveals iron dependence of β-catenin signaling in cancers. Cancer Res. 71:7628–7639. doi: 10.1158/0008-5472.CAN-11-2745. [DOI] [PubMed] [Google Scholar]
- Streyl K, Carlstron J, Dantos E, Mendoza R, Islas J A, and Bhushan C. . 2015. Field Evaluation of the effectiveness of an oral toltrazuril and iron combination (Baycox® Iron) in maintaining weaning weight by preventing coccidiosis and anaemia in neonatal piglets. Parasitol. Res. 114(Suppl. 1:S193–S200. doi: 10.1007/s00436-015-4525-9. [DOI] [PubMed] [Google Scholar]
- Svoboda M, and Drabek J. . 2005. Iron deficiency in suckling piglets: etiology, clinical aspects and diagnosis. Folia Vet. 49:104–111. [Google Scholar]
- Szudzik M, Starzyński R R, Jończy A, Mazgaj R, Lenartowicz M, and Lipiński P. . 2018. Iron supplementation in suckling piglets: An ostensibly easy therapy of neonatal iron deficiency anemia. Pharmaceuticals. 11:128. doi: 10.3390/ph11040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosiou N A, and Tabin C J. . 2003. Wnt signaling during development of the gastrointestinal tract. Dev. Biol. 259:258–271. doi: 10.1016/s0012-1606(03)00185-4. [DOI] [PubMed] [Google Scholar]
- Thomsson A, Rantzer D,Weström B R, Pierzynowski S G, and Svendsen J.. 2007. Effects of crude red kidney bean lectin (phytohemagglutinin) exposure on performance, health, feeding behavior, and gut maturation of pigs at weaning. J. Anim. Sci. 85:477–485. doi: 10.2527/jas.2006-250 [DOI] [PubMed] [Google Scholar]
- Venn J A J, McCance R A, and Widdowson E A. . 1947. Iron metabolism in piglet anemia. Comp. Pat. 57:314–325. doi: 10.1016/s0368-1742(47)80037-2. [DOI] [PubMed] [Google Scholar]
- Wang ZB, Li J, Wang Y, Wang L, Yin Y B, Yin L M, Yang H S, Yin Y L. . 2020. Dietary vitamin A affects growth performance, intestinal development, and functions in weaned piglets by affecting intestinal stem cells. J. Anim. Sci. 98:1–11. doi: 10.1093/jas/skaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang C, Wang Q, Li J, Huang P, Li Y, Ding X, Yang H, and Yin Y. . 2020. The relationship between villous height and growth performance, small intestinal mucosal enzymes activities and nutrient transporters expression in weaned piglets. J. Anim. Physiol. Anim. Nutr. (Berl.). 104:606–615. doi: 10.1111/jpn.13299. [DOI] [PubMed] [Google Scholar]
- Wang M, Yang C, Wang Q Y, Li J Z, Li Y L Ding X Q, Yin J, Yang H S, and Yin Y L. . 2019. The growth performance, intestinal digestive and absorptive capabilities in piglets with different lengths of small intestines. Animal 14:1196–1203. doi: 10.1017/S175173111900288X. [DOI] [PubMed] [Google Scholar]
- Xu R J, Mellor D J, Tungthanathanich P, Birtles M J, Reynolds G W, and Simpson H V. . 1992. Growth and morphological changes in the small and the large intestine in piglets during the first three days after birth. J. Dev. Physiol. 18:161–172. [PubMed] [Google Scholar]
- Yang H, Xiong X, and Yin Y. . 2013. Development and renewal of intestinal villi in pigs. In Nutritional and physiological functions of amino acids in pigs. Vienna: Springer; 29–47. doi: 10.1007/978-3-7091-1328-8_3 [DOI] [Google Scholar]
- Yin Y L, Baidoo S K, Schulze H, and Simmins P H. . 2001. Effects of supplementing diets containing hulless barley varieties having different levels of non-starch polysaccharides with β-glucanase and xylanase on the physiological status of the gastrointestinal tract and nutrient digestibility of weaned pigs. Livest. Prod. Sci. 71:97–107. doi: 10.1016/S0301-6226(01)00214-7 [DOI] [Google Scholar]
- Zabielski R, Godlewski M M, and Guilloteau P. . 2008. Control of development of gastrointestinal system in neonates. J. Physiol. Pharmacol. 59(Suppl. 1:35–54. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.