Dear Editor,
Lipid disturbances have recently been highlighted as a possible pathway in COVID-19 pathogenicity [1]. Indeed, a decrease in serum levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c) and different apolipoproteins is associated with poor prognosis in patients with COVID-19, and may be an important feature to consider in understanding the pathophysiology of COVID-19.
To explore this pathway, we conducted a retrospective single centre study on prospectively collected data. Every patient admitted in Saint-Louis Hospital’s Surgical Intensive Care Unit (ICU) (Assistance Publique - Hôpitaux de Paris, Paris, France) for respiratory failure related to COVID-19 and who had an exploration of lipid abnormalities at ICU admission was included. Exclusion criteria were age under 18, pregnancy or moribund patient at admission.
Methods to determine cholesterol blood levels (TC, LDL-c, HDL-c, triglycerides) were enzymatic colorimetry and immunoturbidimetry for apolipoproteins (A1 and B).
All patients or their surrogate had information about the data collection and gave their non-opposition to the study (Ethical committee of the French Society of Anaesthesia and Intensive Care [SFAR] IRB 00010254 - 2019 – 203). Continuous variables were described as median with their interquartile ratio (IQR) while categorical variables were expressed as frequencies (%). After a normality test, data were analysed with a Mann-Whitney or a Student t-test according to their distribution with a 5% first species risk. Spearman correlation test was used.
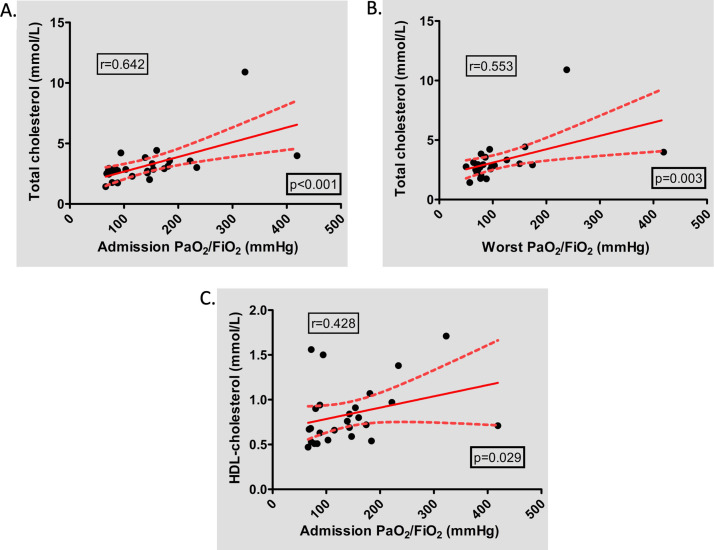
Of 54 COVID-19 patients admitted in our ICU from March 20, 2020 to April 15, 2020, thirty-one patients had an exploration of lipid abnormalities at admission (LDL-c, HDL-c, TC, apolipoproteins A1 and B (ApoA1 and B)). Patients’ characteristics are summarised in Table 1 , and biological results of lipid profile in Table 2 . Among the 31 patients included, dyslipidaemia was not associated with mortality (Table 1). Pre-admission lipid lowering drugs prescription was associated with lower LDL-c, TC and Apo on admission (0.72 vs 1.33 mmol/L; p = 0.043, 2.46 vs 2.95 mmol/L; p = 0.049 and 0.55 vs 0.74 mmol/L; p = 0.037, respectively) and a trend for a higher in-hospital mortality (42.9% vs 12.5%, p = 0.110). Only 27 patients had a lipid exploration and PaO2/FiO2 data available. In these patients, we observed a correlation between PaO2/FiO2 at admission and TC and HDL-c (Fig. 1 A and C; r = 0.642, p < 0.001 and r = 0.428, p = 0.029, respectively) and between worst PaO2/FiO2 and TC (Fig. 1B; r = 0.553, p = 0.003). Furthermore, in bivariate analysis including age and LDL-c level, LDL-c level was associated with 28-day mortality, whereas age was not (OR = 0.0233 [CI 95% 0.0006−0.8835], p = 0.0427 vs OR = 1.0291 [CI 95% 0.8794–1.2044], p = 0.7203, respectively).
Table 1.
Characteristics of patients.
| All patients (n = 31) | Survivors (n = 24) | Non-survivors (n = 7) | p | |
|---|---|---|---|---|
| Age (y) | 63 [60−68] | 62 [60−67] | 74 [64−76] | 0.039 |
| Weight (kg) | 83 [71−88] | 83 [72−92] | 83 [77−86] | 0.232 |
| Size (cm) | 172 [162−176] | 173 [165−176] | 167 [159−174] | 0.858 |
| BMI (kg/m²) | 27 [26−30] | 27 [26−30] | 27 [26−33] | 0.646 |
| Sex male, n (%) | 24 (77.4) | 19 (79.2) | 5 (71.4) | 0.642 |
| Comorbidities | ||||
| Tobacco use, n (%) | 2 (6.5) | 2 (8.3) | 0 (0.0) | 1 |
| Hypertension, n (%) | 17 (54.8) | 12 (50.0) | 5 (71.4) | 0.412 |
| ACEi or ARBS, n (%) | 9 (29.0) | 7 (29.2) | 2 (28.6) | 1 |
| Diabetes mellitus, n (%) | 10 (32.3) | 6 (25.0) | 4 (57.1) | 0.172 |
| Dyslipidaemia, n (%) | 8 (25.8) | 4 (16.7) | 4 (57.1) | 0.053 |
| Coronary disease, n (%) | 1 (3.2) | 1 (4.2) | 0 (0.0) | 1 |
| Chronic pulmonary disease, n (%) | 4 (12.9) | 3 (12.5) | 1 (14.3) | 1 |
| Severity of illness | ||||
| Radiological lesions > 50%, n (%) | 19 (61.3) | 13 (54.2) | 6 (85.7) | 0.201 |
| SAPS II | 37 [29−44] | 37 [31−44] | 37 [29−42] | 0.920 |
| SOFA | 4 [4−7] | 5 [4−7] | 4 [4−7] | 0.820 |
| Organ failure during ICU stay | ||||
| ARDS, n (%) | 26 (83.9) | 20 (83.3) | 6 (85.7) | 1 |
| Admission PaO2/FiO2 (mmHg) | 127 [80−176] | 143 [82−183] | 88 [80−104] | 0.122 |
| Worst PaO2/FiO2 (mmHg) | 81 [72−109] | 86 [75−138] | 72 [71−79] | 0.062 |
| AKI, n (%) | 16 (51.6) | 10 (41.7) | 6 (85.7) | 0.083 |
| RRT, n (%) | 4 (12.9) | 1 (4.2) | 3 (42.9) | 0.028 |
| Norepinephrine in first 48 h, n (%) | 14 (45.2) | 10 (41.7) | 4 (57.1) | 0.671 |
ACEi: angiotensin converting enzyme inhibitor; AKI: acute kidney injury; ARBS: angiotensin receptor blockers; ARDS: acute respiratory distress syndrome; BMI: body mass index; ICU: intensive care unit; SAPS II: simplified acute physiology score II; SOFA: simplified organ failure assessment, RRT: renal replacement therapy.
Table 2.
Lipid profile for COVID-19 patients.
| All patients (n = 31) | Survivors (n = 24) | Non-survivors (n = 7) | p | |
|---|---|---|---|---|
| LDL-c (mmol/L) | 1.40 [1.22−2.20] | 1.63 [1.37−2.52] | 0.82 [0.53−1.37] | 0.004 |
| HDL-c (mmol/L) | 0.72 [0.61−0.93] | 0.85 [0.47−1.30] | 0.67 [0.62−1.10] | 0.285 |
| Total cholesterol (mmol/L) | 2.89 [2.56−3.57] | 3.12 [2.78−4.11] | 2.38 [1.95−2.75] | 0.007 |
| Triglycerides (mmol/L) | 1.43 [1.10−1.86] | 1.43 [1.03−1.87] | 1.48 [0.81−1.62] | 0.563 |
| Apolipoprotein A1 (mmol/L) | 0.69 [0.56−0.80] | 0.74 [0.55−0.88] | 0.59 [0.57−0.85] | 0.567 |
| Apolipoprotein B (mmol/L) | 0.71 [0.61−0.89] | 0.79 [0.67−0.95] | 0.60 [0.46−0.69] | 0.008 |
HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol.
Fig. 1.
Correlation between lipid abnormalities at ICU admission and PaO2/FiO2 during ICU stay.
HDL: high-density lipoprotein.
Our results suggest a role of lipid disorders in COVID-19 severity as proposed by Cao and colleagues [1]. The decrease in TC could result from vasculopathy induced by SARS-CoV-2. Indeed, we observed a correlation between TC blood level and COVID-19 severity, assessed by PaO2/FiO2 ratio. Our series suggests that COVID-19 severity is linked with lipid level. It might be interesting to practice lipid dosage in broncho-alveolar lavage to assess if there is an intra alveolar lipid extravasation, alveolar obstruction and inflammation. Whether lipid-lowering treatments (i.e., statins) could be associated with COVID-19 severity remains to be explored in larger studies. Experimental data suggest that statins have potential benefits in acute respiratory distress syndrome (ARDS), including anti-inflammatory properties, immunomodulatory, antioxidant, and antithrombotic effects. Nevertheless, clinical trials failed to show a benefit of statins administration in patients with ARDS [2]. A possible explanation of failure of statins therapy in patients with ARDS could be partly explained by an increase in interleukin-18 level induced by statin therapy [3]. In COVID-19, interleukin-18 is associated with severity of the disease [4] and is also believed to be a potential therapeutic target [5]. More explorations are required to better understand and explain the role of lipid pathways in COVID-19 pathophysiology.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
None.
Authors’ contribution
QR, ED and FD designed the study, collected the data and drafted the manuscript.
NM conducted the assays and drafted the manuscript.
MC drafted the manuscript.
All authors approved the final version of the manuscript.
References
- 1.Cao X., Yin R., Albrecht H., Fan D., Tan W. Cholesterol: a new game player accelerating endothelial injuries caused by SARS-CoV-2? Am J Physiol-Endocrinol Metab. 2020;(June) doi: 10.1152/ajpendo.00255.2020. https://journals.physiology.org/doi/10.1152/ajpendo.00255.2020 [cited 2020 June 12]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The National Heart, Lung, and Blood Institute ARDS Clinical Trials Network Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(June (23)):2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers A.J., Guan J., Trtchounian A., Hunninghake G.M., Kaimal R., Desai M. Association of elevated plasma interleukin-18 level with increased mortality in a clinical trial of statin treatment for acute respiratory distress syndrome*. Crit Care Med. 2019;47(August (8)):1089–1096. doi: 10.1097/CCM.0000000000003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi Y., Ge Y., Wu B., Zhang W., Wu T., Wen T. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 (COVID-19) in China. J Infect Dis. 2020;(June) doi: 10.1093/infdis/jiaa363. https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiaa363/5860445 [cited 2020 June 21]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golonka R.M., Saha P., Yeoh B.S., Chattopadhyay S., Gewirtz A.T., Joe B. Harnessing innate immunity to eliminate SARS-CoV-2 and ameliorate COVID-19 disease. Physiol Genom. 2020;52(5):217–221. doi: 10.1152/physiolgenomics.00033.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]