Abstract
Background
The role of tracheostomy in coronavirus disease 2019 (COVID-19) is unclear, with several consensus guidelines advising against this practice. We developed both a dedicated airway team and coordinated education programme to facilitate ward management of tracheostomised COVID-19 patients. Here, we report outcomes in the first 100 COVID-19 patients who underwent tracheostomy at our institution.
Methods
This was a prospective observational cohort study of patients confirmed to have COVID-19 who required mechanical ventilation at Queen Elizabeth Hospital, Birmingham, UK. The primary outcome measure was 30-day survival, accounting for severe organ dysfunction (Acute Physiology and Chronic Health [APACHE]-II score>17). Secondary outcomes included duration of ventilation, ICU stay, and healthcare workers directly involved in tracheostomy care acquiring COVID-19.
Results
A total of 164 patients with COVID-19 were admitted to the ICU between March 9, 2020 and April 21, 2020. A total of 100 patients (mean [standard deviation] age: 55 [12] yr; 29% female) underwent tracheostomy; 64 (age: 57 [14] yr; 25% female) did not undergo tracheostomy. Despite similar APACHE-II scores, 30-day survival was higher in 85/100 (85%) patients after tracheostomy, compared with 27/64 (42%) non-tracheostomised patients {relative risk: 3.9 (95% confidence intervals [CI]: 2.3–6.4); P<0.0001}. In patients with APACHE-II scores ≥17, 68/100 (68%) tracheotomised patients survived, compared with 12/64 (19%) non-tracheotomised patients (P<0.001). Tracheostomy within 14 days of intubation was associated with shorter duration of ventilation (mean difference: 6.0 days [95% CI: 3.1–9.0]; P<0.0001) and ICU stay (mean difference: 6.7 days [95% CI: 3.7–9.6]; P<0.0001). No healthcare workers developed COVID-19.
Conclusion
Independent of the severity of critical illness from COVID-19, 30-day survival was higher and ICU stay shorter in patients receiving tracheostomy. Early tracheostomy appears to be safe in COVID-19.
Keywords: COVID-19, ICU, safety, SARS-CoV-2, tracheostomy
Editor's key points.
-
•
The role of tracheostomy in patients with COVID-19 is unclear, with some non-evidence-based guidelines advising against this practice.
-
•
In a prospective observational cohort study, the authors report outcomes after tracheostomy in COVID-19 patients at a major UK institution where a coordinated education programme supported safe discharge of tracheostomised patients from the ICU.
-
•
A total of 100/164 patients underwent tracheostomy safely, with no transmission of COVID-19 infection in healthcare workers involved in their care.
-
•
Despite similar characteristics and APACHE II scores, 30-day survival was higher after tracheostomy compared with non-tracheotomised patients [relative risk of surviving after tracheostomy: 3.9 (95% confidence intervals [CI]: 2.3–6.4)].
-
•
Early tracheostomy in COVID-19 patients appears to be safe and associated with a shorter ICU stay.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a worldwide pandemic spreading to more than 213 countries, and affecting more than six million people worldwide.1 Although the majority of individuals experience mild symptoms, approximately 10–17% develop acute respiratory distress syndrome requiring invasive mechanical ventilation.2 , 3 In the UK, Intensive Care National Audit and Research Centre (ICNARC) data have reported a mortality rate up to 40.4% in patients admitted to intensive care.4, 5, 6
Before the onset of the SARS-CoV-2 pandemic, early tracheostomy was considered helpful in shortening the duration of ventilation and length of stay in ICUs for any patient requiring prolonged mechanical ventilation.7 , 8 Although the evidence base for tracheostomy improving overall survival remains unclear, a lower incidence of pneumonia with early tracheostomy was suggested by a recent meta-analysis.9 Tracheostomy decreases sedation requirements, avoids pressure-induced trauma (both to the trachea and oral cavity), and may reduce the severe physical deconditioning associated with prolonged mechanical ventilation.10 However, the role and timing of tracheostomy for patients requiring critical care for coronavirus disease 2019 (COVID-19) is unclear.
The emergence of SARS-CoV-2 necessitated the rapid development of guidelines, predominantly based on expert opinion. Early guidelines from both the UK and national bodies proposed a cautious approach to tracheostomy in patients with COVID-19—avoiding before 10 days of intubation and giving serious consideration before performing at all between 10 and 21 days after intubation, allowing for a sufficient decline in viral load.11, 12, 13, 14, 15, 16, 17 Recommendations were also made for patients to test negative before proceeding with a tracheostomy, and that healthcare workers should wear powered respirators; a scarce resource in many settings.14 , 16 Later guidelines from an international working group highlighted the need to balance potential risks against benefits of weaning patients from invasive ventilation.18 , 19 These guidelines were also based on expert opinion and highlighted the need for robust ICU outcome data.
The evolution of a multidisciplinary COVID-19 airway team at the Queen Elizabeth Hospital Birmingham, with surgical, anaesthetic, critical care, and speech and language therapy (SLT) representation occurred at the outbreak of the SARS-CoV-2 pandemic in the UK. We developed agreed parameters for patient selection, procedural strategies, weaning/decannulation policies, organisational re-configuration, and training for managing tracheostomy patients on discharge from critical care. This report describes patient selection, survival, complications, and safety to healthcare workers for the first 100 COVID-19 cases undergoing tracheostomy at our large tertiary hospital.
Methods
Ethical approval
This study was a service evaluation (Supplementary material). All data were routinely recorded contemporaneously on to the hospital electronic systems and then retrospectively analysed.
Setting
All patients admitted to the ICU with severe respiratory failure requiring mechanical ventilation at the Queen Elizabeth Hospital Birmingham, UK, from March 9, 2020 to April 21, 2020 were included. SARS-CoV-2 positivity was confirmed by real time polymerase chain reaction testing of nasopharyngeal swabs or non-directed bronchial lavage/aspirate.
Tracheostomy team
The daily tracheostomy team comprised two head and neck surgeons along with an Operating Department Practitioner (ODP). Critical care and anaesthetic clinicians managed the tracheal tube during tracheostomy insertion and ensured adequate sedation and paralysis. All procedures were performed in personal protective equipment (PPE) for aerosol-generating procedures, as defined by Public Health England (FFP3 masks with fluid repellent gowns, gloves, and eye protection).20 No powered respirators were worn by the tracheostomy team and negative pressure rooms were not used in ICUs or operating theatres.
Patient selection
Primary extubation was the preferred option for all patients, with tracheostomy considered when this was deemed not possible. Through multidisciplinary agreement, parameters to guide selection for tracheostomy were defined before the study period (Table 1 ). Patients with physiology outside of these parameters were still considered for tracheostomy on a case-by-case basis. The decision regarding tracheostomy was made by a multidisciplinary team (MDT) of critical care physicians, anaesthetists, and surgeons. Acute Physiology and Chronic Health (APACHE II) scores were calculated for patients on admission to ICU but did not form part of the decision-making process for tracheostomy. With a dedicated 24 h on call tracheostomy service competent in percutaneous and surgical techniques, and access to dedicated COVID emergency theatres, the choice of performing a surgical or percutaneous tracheostomy depended only on patient body habitus, adequate neck extension, and grade of direct laryngoscopy.
Table 1.
Parameters used by the COVID-19 airway team to guide patient selection for tracheostomy.
| Isolated respiratory failure except for acute renal failure on dialysis or continuous renal replacement therapy |
| Prolonged intubation and mechanical ventilation for 10 or more days |
| Multiple failed sedation holds, failed extubation, or anticipated prolonged respiratory wean |
| Improving oxygen requirements: fraction of inspired oxygen (FiO2) <0.4 and PEEP<10 cm H2O. |
| Appropriate coagulation with no evidence of coagulopathy |
| Unlikely to require further prone position ventilation |
Decannulation protocol
Decannulation was not a prerequisite for discharge from the ICU. Decannulation while on the ICU was performed as a multidisciplinary decision between critical care staff and SLT. Where patients were discharged to the ward with a tracheostomy in situ, a ward-based decannulation protocol was used. This decision was made autonomously by the tracheostomy MDT (SLT, physiotherapy, and Clinical Nurse Specialist).
Training and institutional reconfiguration
To create operational readiness, an MDT led by SLT provided comprehensive education and clinical support to personnel outside of ICU caring for tracheostomised patients. All staff on receiving wards received theoretical and practical training (the latter comprising simulation training observed by the trainers). A standardised intensive training was provided over 68 sessions for 829 members of nursing, therapy, and medical staff, delivered by experienced staff who manage tracheostomy patients regularly. Staff were trained to manage inner tube changes, suction, humidification, tape changes, and cuff pressure. An online MoodleTM training module allowed staff to refresh if anxious, and ward-based practical training was provided when required. Further clinical support was provided by the ear, nose, and throat nurses deployed to the three specialist tracheostomy wards to support unfamiliar staff. A robust and detailed governance framework supported this work stream.
Primary clinical outcome
The primary outcome measure was 30-day survival (from date of ICU admission), compared between tracheotomised patients and those who had no tracheostomy (primarily extubated).
Secondary outcomes
Secondary outcome measures were time to waking after ceasing sedation, duration of sedation and mechanical ventilation, discharge from ICU, tracheostomy decannulation rate, and complications. The endpoint for ventilatory support was defined as when the patient wore a tracheostomy mask for at least 24 h.
Sensitivity analyses
Thirty-day survival was compared for patients with APACHE scores of <17 and >17, based on this threshold being relevant in other studies.21 , 22 Guidelines had raised concerns about performing tracheostomy before day 10, and suggested performing after day 14 may be preferable. Subgroup analyses were therefore performed based on these cut-offs to explore whether these timings affected survival, time on ventilator, and length of ICU stay.
Statistics
Data were analysed using XLSTAT (Addinsoft, New York, USA) and IBM SPSS Statistics (version 26; IBM Corp, Armonk, NY). The distribution of continuous variables was tested using the one-sample Kolmogorov–Smirnov test, and if normal, variables were presented as mean (standard deviation [sd]). Means of two continuous, normally distributed variables were compared by an independent Student's t-test. Frequencies of categorical variables were compared using the χ2 test. A P-value <0.05 was considered significant. The Kaplan–Meier method was used to assess survival with significance calculated using the log rank test.
Results
Patient characteristics
The 30-day outcomes were available for 164 patients admitted to the ICU at the Queen Elizabeth Hospital. One hundred patients (61%) underwent a tracheostomy during this period. Twenty-seven (16%) patients were extubated without the need for a tracheostomy. Baseline characteristics are shown in Table 2 . All patients were intubated on admission to the ICU. The 30-day survival was worse in 19/42 (45%) patients with an APACHE-II score of ≥17 compared with 93/122 (76%) with a score <17 (relative risk [RR]: 2.3, 95% confidence interval [CI]: 1.5–3.5, P=0.0001).
Table 2.
Characteristics of patients admitted to the Intensive Care Unit (ICU). Means expressed for continuous variables with standard deviation shown in parentheses. P<0.05 used for significance. APACHE, Acute Physiology and Chronic Health.
| No tracheostomy | Tracheostomy | P | By type of tracheostomy |
|||
|---|---|---|---|---|---|---|
| Percutaneous | Open | P | ||||
| Number | 64 | 100 | NA | 75 | 25 | |
| Age (yr) | 56.9 (24–80) | 55.2 (21–78) | 0.40 | 56.2 (27–78) | 52.1 (21–71) | 0.12 |
| Sex (M:F) | 48:16 | 71:29 | 0.39 | 52:23 | 18:7 | 0.80 |
| Ethnicity | White: 38 | White: 53 | 0.63 | White: 39 | White: 14 | 0.79 |
| Asian: 22 | Asian: 41 | Asian: 32 | Asian: 9 | |||
| Black: 5 | Black: 6 | Black: 4 | Black: 2 | |||
| BMI (kg m−2) | 30.0 (5.4) | 32.0 (7.0) | 0.05 | 30.9 (6.6) | 35.4 (7.2) | 0.005 |
| APACHE II score | 15 (5) | 14 (4) | 0.25 | 14 (4) | 14 (5) | 0.91 |
| Mean FiO2 | NA | 0.39 (0.07) | NA | 0.39 (0.07) | 0.39 (0.07) | 0.89 |
| Mean low PEEP (cm H2O) | NA | 7.8 (1.7) | NA | 7.7 (1.7) | 8.0 (1.7) | 0.58 |
| Days intubated at time of tracheostomy | NA | 13.9 (4.5) | NA | 13.8 (4.5) | 14.1 (4.5) | 0.74 |
Characteristics of patients with tracheostomy
The time from intubation to tracheostomy ranged from 5 to 29 days (Table 2). Seventy-five patients underwent percutaneous tracheostomy in the ICU, while 25 underwent surgical tracheostomy in the operating theatre. Patients undergoing open tracheostomy had significantly higher BMI. The indications for tracheostomy were failed extubation (13%), failed sedation hold (52%), anticipated prolonged respiratory wean (32%), and severe facial oedema from prone-positioning (3%). APACHE II scores were similar between patients who underwent, or avoided, tracheostomy (Table 2).
Primary outcome: 30-day survival
The 30-day survival for the whole cohort was 68.3% (112/164). The 30-day survival was higher in patients who received a tracheostomy compared with those that did not 85/100 (85%) vs 27/64 (42%), (RR: 3.9, 95% CI: 2.3–6.4 P<0.0001).
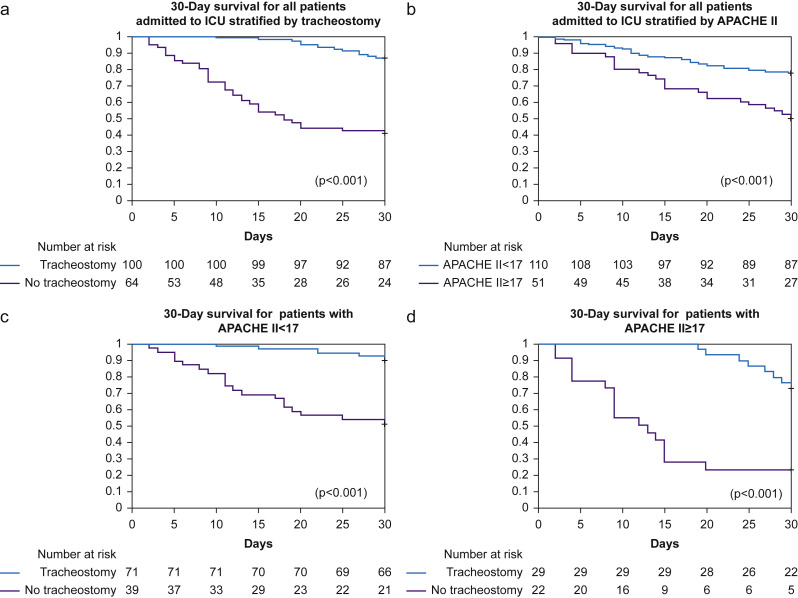
The likelihood of receiving a tracheostomy with APACHE-II≥17 was 20/42 (48%) which was similar to 80/122 (66%) patients with APACHE-II<17 (RR: 0.7, 95% CI: 0.5–1.0, P=0.07). For 14/20 (68%) patients with APACHE II score≥17, tracheostomy was associated with higher survival at 30 days, compared with 4/22 (19%) patients who did not undergo tracheostomy (RR: 2.7, 95% CI: 1.4–5.5, P=0.005) (Fig. 1 ).
Fig 1.
Kaplan–Meier plot for 30-day survival from date of intubation. Number at risk detailed below chart. (a) All patients stratified by tracheostomy. (b) All patients stratified by APACHE II score. (c) All patients with APACHE II score<17, stratified by tracheostomy. (d) All patients with APACHE II score ≥17, stratified by tracheostomy. P<0.05 used for significance as calculated by the log rank test. APACHE, Acute Physiology and Chronic Health; ICU, Intensive Care Unit.
Secondary outcomes
Timing of tracheostomy and survival
Nine patients underwent tracheostomy before 10 days, 55 between 10 and 14 days, and 36 after 14 days of intubation. There was no difference in survival between those undergoing tracheostomy before or after day 10 (11% vs 15%, respectively, P=0.73), or before or after day 14 (19% vs 12%, respectively, P=0.18).
Duration of ventilatory support
The mean time to tracheostomy was 13.9 (4.5) days. Total duration of ventilatory support and mean ventilator duration are presented in Table 3 . For surviving patients, tracheostomy at ≤14 days was associated with reduced duration of ventilatory support compared with tracheostomy at >14 days (21 [6.0] days, vs 27 [6.3] days, P=0.0001). There was no difference between the groups in time from tracheostomy to 24 h tracheostomy mask. Three patients required prone-position ventilation after tracheostomy because of deterioration of oxygenation, with none of this group surviving past 30 days. These patients had not required prone-positioning for at least 48 h before tracheostomy, and although they appeared to be progressing well after tracheostomy, they had a deterioration in oxygenation at 72, 96, and 96 h, respectively.
Table 3.
Ventilatory/recovery data for patients admitted to the Intensive Care Unit (ICU). Means expressed for continuous variables with standard deviation shown in parentheses. P<0.05 used for significance.
| No tracheostomy | Tracheostomy | P | By timing of insertion |
|||
|---|---|---|---|---|---|---|
| ≤14 days | >14 days | P | ||||
| Number of patients | 27 | 85 | NA | 56 | 29 | NA |
| Days on ventilator | 8.0 (4.7) | 22.9 (6.7) | <0.0001 | 21.0 (6.0) | 27.0 (6.3) | 0.0001 |
| Total ICU stay (days) | 11.4 (5.4) | 25.3 (6.6) | <0.0001 | 23.1 (5.6) | 29.5 (6.7) | <0.0001 |
| Days from tracheostomy to 24 h tracheostomy mask | NA | 9.5 (5.6) | NA | 9.6 (5.9) | 9.4 (4.8) | 0.26 |
| Days from tracheostomy/extubation to ICU discharge | 3.9 (3.5) | 12.1 (5.5) | NA | 11.9 (5.5) | 12.6 (5.4) | 0.60 |
| Days from tracheostomy to decannulation | NA | 12.7 (6.1) | NA | 12.3 (6.7) | 13.5 (4.6) | 0.49 |
Length of critical care stay
Those patients who underwent successful extubation had a mean length of ICU stay of 12 (5.4) days. Tracheostomy before 14 days was associated with shorter length of ICU stay compared with tracheostomy after 14 days (23 [5.6] days vs 30 [6.7] days, respectively, P<0.0001). There was no difference between the groups in time from tracheostomy to discharge from critical care. All surviving tracheostomised patients were successfully discharged from the ICU, and 97% were discharged from hospital at 60 days. The mean overall length of hospital stay for surviving tracheostomised patients was 34 (8.9) days and for surviving primarily extubated patients was 16 (9.5) days.
Tracheostomy complications
Complications in the 77 percutaneous tracheostomies performed included self-limiting bleeding (n=3), false passage (n=2), and conversion to surgical tracheostomy (n=2). Complications in the 25 surgically inserted tracheostomies were self-limiting bleeding (n=1), tube dislodgement (n=1), and air leak postoperatively (n=2). Late complications encountered included two cases of vocal cord palsy, the aetiology of which was uncertain.
Sedation weaning outcomes
All 85 surviving tracheostomy patients underwent weaning from sedation. Sedation was stopped within 48 h of tracheostomy insertion in 65/85 patients (76.5%) and within 96 h in 10/85 (12%). Sedation hold was delayed >96 h in 10/85 (12%) because of worsening oxygenation (n=5) or delirium (n=5). Once sedation was ceased, 63/85 (74%) patients regained consciousness (Richmond-Agitation-Sedation-Score [RASS] score of –1, 0, or 1) within 24 h, or within 72 h in 79/85 (93%). The remaining six patients regained consciousness between 4 and 21 days after ceasing sedation. Four of those patients were diagnosed with COVID-related encephalopathy, and the remaining two had multiple cerebral infarcts.
Decannulation
Decannulation was successfully completed in 84/85 surviving patients (99%). The mean time to decannulation for all tracheostomy patients was 14 (7.9) days with no difference seen between the early vs late tracheostomy groups (13 vs 15 days, P=0.72). Out of the 84 decannulated patients, 41 (49%) were decannulated without downsizing the tube or tube fenestration. Downsizing was required in 25 (30%) patients, whereas downsizing and fenestration were needed in 18 (21%) to support their weaning.
Healthcare worker safety
During the study period, none of the tracheostomy, SLT, or ODP teams developed COVID-19 symptoms. One surgeon developed COVID-19 infection before having performed any tracheostomies. The remaining eight members of the tracheostomy team were negative for SARS-CoV-2 antibodies 14 days after the study period.
Discussion
This is the largest single institution observational cohort study of patients undergoing tracheostomy with COVID-19. Using the selection criteria utilised by our institution, patients who underwent a tracheostomy were more likely to survive, compared with those that did not. This appeared to be independent of baseline prognosis, with no difference in APACHE II scores between the two groups. Survival was higher for patients with an APACHE score of <17 compared with a score of ≥17. However, the survival advantage of those receiving a tracheostomy was seen in both groups, including in the sickest patients.
During the study period, primary extubation was always the preferred option, usually occurring within 10 days of intubation. For those intubated for >10 days, or those expected to have a slow respiratory wean by expert MDT consensus, a tracheostomy was felt to offer the advantage of safely weaning sedation, improving patient comfort, and allowing effective pulmonary toilet and proactive rehabilitation. Tracheostomy reduces airway trauma from prolonged tracheal intubation23 and perioral pressure sores exacerbated by prone-positioning that were observed in some of our patients. COVID-19-related laryngeal oedema,24 and upper airway oedema from prone-positioning also increased the risk of failure of extubation. These factors all guided a more interventionalist approach adopted by our COVID-19 airway team.
In the absence of an evidence base, many guidelines based on expert opinion were published referencing the role of tracheostomy in COVID-19. Early guidelines proposed avoiding a tracheostomy completely before 10 days of intubation and to only consider it carefully before performing at all between 10 and 21 days after intubation.11, 12, 13, 14, 15, 16 , 25 Indeed, a recent report of outcomes of patients from the USA shows only 17/203 (8%) received a tracheostomy.26 Later guidelines from an expert working group adopted a more moderate stance and the results of this study provide support for this approach.18 , 19 Tracheostomy timing in COVID-19 patients should take into consideration the safety and ideal timing for patient outcomes, and the safety of healthcare personnel performing the tracheostomy.18 , 19
The timing of tracheostomy in patients requiring prolonged mechanical ventilation has been the subject of debate even before the outbreak of the SARS-CoV-2 pandemic. The landmark TracMan trial found no advantage of early (<4 days) tracheostomy in relation to 30-day mortality, duration of mechanical ventilation, or length of time in critical care.8 However, tracheostomy occurred in both trial arms much earlier after intubation than is recommended in the COVID-19 setting. Other studies have demonstrated a reduction in the duration of mechanical ventilation, and a reduction in critical care stay with early tracheostomy.7 , 23 , 27 This also has implications for resource planning in a global pandemic, where ventilator capacity is a defining factor in hospitals not becoming overwhelmed. Our findings also suggest that tracheostomy at ≤14 days compared with tracheostomy at >14 days, was associated with shorter periods of ventilation and ICU stay. In our cohort of 64 patients undergoing earlier tracheostomy, this equates to approximately 448 bed days gained over delaying until >14 days as per the guidelines.
There was no difference in time from tracheostomy to either not requiring ventilatory support or being discharged from the ICU. This suggests the reductions were specifically as a result of shortening the period from intubation to tracheostomy. Hence, it appears safe and reasonable to perform a tracheostomy when clinically indicated and physiologically ready, and not wait for a defined time to pass. This may help to reduce the overall length of time required on mechanical ventilation and in the setting of a surge in SARS-CoV-2 patients, the ability to more efficiently move patients through the ICU allows hospitals to maximise their ventilator capacity.
Healthcare personnel safety has influenced the development of tracheostomy guidelines. Some advocate delaying tracheostomy to allow time for the viral load to reduce, or after a negative SARS-CoV-2 swab result.14 , 17 , 25 , 28 To lessen the potential viral exposure, guidelines have also stipulated a limit of two tracheostomies performed per day by the procedural team and on the number of days worked by any team member.13 , 28 In our hospital, the core tracheostomy team were required to wear PPE, although this did not include the use of powered respirators or negative pressure rooms as advised in some current guidelines. At the peak of cases, more than 10 tracheostomies were being performed per day. All members of the team were head and neck cancer surgeons regularly performing open tracheostomies and were also trained to perform percutaneous tracheostomy without bronchoscopy to minimise aerosol generation. Among the tracheostomy team, none of the clinicians developed COVID-19 symptoms during the study period and subsequent antibody testing at 2 weeks after the study period was negative in all but one surgeon, who developed COVID-19 before commencing on the team. In addition, none of the SLT or ODP team members involved developed COVID-19 symptoms. In a review of 23 open tracheostomies performed during the 2003 SARS epidemic, no healthcare professionals contracted SARS with the use of appropriate PPE and care applied to minimise aerosol production.29 Our experience in a much larger cohort supports the assertion that tracheostomy can be performed with low risk to COVID-19 patients and healthcare workers. Indeed, delaying tracheostomy over concerns for healthcare personnel safety may prolong patients' time on a ventilator and ICU stay, without any benefit of improved safety for either the clinicians involved, or the patient.
Prolonged continuous sedation requirements are a recognised feature of ventilated COVID-19 patients.30 Tracheostomy provides an opportunity to reduce sedation requirements,8 , 10 which allows patient participation in physiotherapy, earlier return to oral alimentation, reduced delirium, earlier identification of neurological dysfunction, and improved communication with staff and relatives. Lighter sedation is known to be a factor in reducing delirium in ICU patients and has been shown to reduce overall length of stay.31 , 32 Early tracheostomy in our cohort was associated with rapid cessation of sedation in 77% of patients. This is likely to have impacted positively on the degree of delirium and may also have contributed to the reduced length of ICU stay seen in this group.
Our approach to decannulation was developed by a multidisciplinary tracheostomy team led by SLT. Although guidelines suggest management of patients with cuffed non-fenestrated tubes,15 and awaiting negative SARS-CoV-2 swabs before deflating the cuff, we found this was not appropriate for the rehabilitative needs of this cohort. The need for downsizing and fenestration was required for decannulation in almost half of patients. The patients who required downsizing and fenestration of their tracheostomy tubes presented with symptoms of upper airway obstruction and oedema such as poor cough, ineffective voice, difficulty breathing around their tracheostomy tube, or the inability to tolerate a speaking valve.
Interpretation of our results needs to consider several limitations. The inherent bias of any non-randomised study will affect the results, often in favour of the intervention being investigated. However, this is the first large cohort study with survival outcomes evaluating the role of tracheostomy in COVID-19 patients, indicating that current guidelines are too conservative. Despite the limitations, the findings suggest that the patient selection criteria implemented in our institution were able to successfully predict the patients who were likely to benefit from a tracheostomy. Furthermore, with the baseline APACHE II scores being the same for the two groups, it would imply not just a selection advantage, but the possibility that tracheostomy may confer benefit in patients with COVID-19.
Conclusions
In patients requiring mechanical ventilation for COVID-19-related pneumonitis, tracheostomy was associated with an improved 30-day survival. This benefit appeared to be independent of the severity of baseline illness, including the sickest patients. With appropriate patient selection, training of healthcare personnel, and allocation of resources, tracheostomies can be performed safely in patients with COVID-19. This may assist healthcare planning for future COVID-19 pandemic surges.
Authors' contributions
Writing group, development of tracheostomy protocol, data collection and analysis, statistical analysis, delivery of tracheostomy service (Surgery): OB, PN, NS
Development of tracheostomy protocol, data collection, writing, review and editing of final manuscript, delivery of tracheostomy service (SLT): CD
Development of tracheostomy protocol, delivery of tracheostomy service (Surgery/ICU/Anaesthetics), review and editing of final manuscript: MI, CJ, TM, RM, DP, SP, PP, PrP, CR, SS, SS
Delivery of tracheostomy service (ICU/Anaesthetics), review and editing of final manuscript: MB, PI, MM, NM, JP, RS, LT
Development and validation of antibody testing, review and editing of final manuscript: AR, AS
All authors have verified the accuracy and authenticity of the data. All authors have agreed with submission of the manuscript.
Acknowledgements
This work uses data provided by patients and collected by the NHS as part of their care and support at University Hospitals Birmingham NHS Foundation Trust. It has been approved as a service evaluation by University Hospitals Birmingham NHS Foundation Trust COVID-19 research facilitation group under application reference COV191.
Handling editor: Gareth Ackland
Footnotes
This article is accompanied by an editorial: Tracheostomy for COVID-19: business as usual? by McGrath et al., Br J Anaesth 2020:125:867–871, doi: 10.1016/j.bja.2020.08.048
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.08.023.
Contributor Information
Queen Elizabeth Hospital Birmingham COVID-19 airway team:
Omar Breik, Paul Nankivell, Neil Sharma, Mansoor N. Bangash, Camilla Dawson, Matthew Idle, Peter Isherwood, Christopher Jennings, Damian Keene, Mav Manji, Tim Martin, Rob Moss, Nick Murphy, Dhruv Parekh, Sat Parmar, Jaimin Patel, Paul Pracy, Prav Praveen, Carla Richardson, Alex Richter, Rajneesh Sachdeva, Adrian Shields, Somiah Siddiq, Simon Smart, and Laura Tasker
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
No funding was received for this study.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Johns Hopkins University Coronavirus Resource Center Coronavirus COVID-19 global cases by the center for systems science and engineering (CSSE) at Johns Hopkins University. https://coronavirus.jhu.edu/map.html Available from:
- 2.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Intensive care national audit and research centre. COVID-19 report. Available from: https://www.icnarc.org/DataServices/Attachments/Download/96b455be-059e-ea11-9126-00505601089b. [Accessed 14 July 2020].
- 6.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffiths J., Barber V.S., Morgan L., Young J.D. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. Br Med J. 2005;330:1243–1246. doi: 10.1136/bmj.38467.485671.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young D., Harrison D.A., Cuthbertson B.H., Rowan K. Effect of early vs late tracheostomy. JAMA. 2013;309:2121–2129. doi: 10.1001/jama.2013.5154. [DOI] [PubMed] [Google Scholar]
- 9.Siempos, Ntaidou T.K., Filippidis F.T., Choi A.M.K. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:150–158. doi: 10.1016/S2213-2600(15)00007-7. [DOI] [PubMed] [Google Scholar]
- 10.Nieszkowska A., Combes A., Luyt C.-E. Impact of tracheotomy on sedative administration, sedation level, and comfort of mechanically ventilated intensive care unit patients. Crit Care Med. 2005;33:2527–2533. doi: 10.1097/01.ccm.0000186898.58709.aa. [DOI] [PubMed] [Google Scholar]
- 11.Chao T.N., Braslow B.M., Martin N.D. Tracheotomy in ventilated patients with COVID-19. Ann Surg. 2020;272:e30–e32. doi: 10.1097/SLA.0000000000003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miles B.A., Schiff B., Ganly I. Tracheostomy during the SARS-CoV-2 pandemic: recommendations from the New York head and neck society. Head Neck. 2020;42:1282–1290. doi: 10.1002/hed.26166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takhar A., Walker A., Tricklebank S. Recommendation of a practical guideline for safe tracheostomy during the COVID-19 pandemic. Eur Arch Oto-Rhino-Laryngology. 2020;277:2173–2184. doi: 10.1007/s00405-020-05993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engels P.T., Weitzel E., Witterick I.J. 2020. Canadian society of otolaryngology – head and neck surgery - recommendations from the CSO-HNS taskforce on performance of tracheotomy during the COVID-19 pandemic.https://www.entcanada.org/wp-content/uploads/COVID-19-Guidelines-CSOHNS-Task-Force-Mar-23-2020.pdf Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ENT-UK ENT-UK COVID-19 Tracheostomy guidance. https://www.entuk.org/sites/default/files/files/COVID tracheostomy guidance_compressed.pdf Available from:
- 16.American Academy of Otolaryngology and Head and Neck Surgery . 2020. AAO position statement: tracheostomy recommendations during the COVID-19 pandemic.https://www.entnet.org/content/aao-position-statement-tracheotomy-recommendations-during-covid-19-pandemic Available from: [Google Scholar]
- 17.Michetti C.P., Burlew C.C., Bulger E.M., Davis K.A., Spain D.A. Performing tracheostomy during the covid-19 pandemic: guidance and recommendations from the critical care and acute care surgery committees of the American association for the surgery of trauma. Trauma Surg Acute Care Open. 2020;5 doi: 10.1136/tsaco-2020-000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGrath B.A., Brenner M.J., Warrillow S.J. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8:717–725. doi: 10.1016/S2213-2600(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGrath B.A., Ashby N., Birchall M. Multidisciplinary guidance for safe tracheostomy care during the COVID-19 pandemic: the NHS national patient safety improvement programme (NatPatSIP) Anaesth. May 12 2020 doi: 10.1111/anae.15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Public Health England COVID-19: infection, prevention control guidance. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/886668/COVID-19_Infection_prevention_and_control_guidance_complete.pdf Available from:
- 21.Zou X., Li S., Fang M. Acute Physiology and Chronic Health Evaluation II Score as a predictor of hospital mortality in patients of coronavirus disease 2019. Crit Care Med. 2020;48:e657–e665. doi: 10.1097/CCM.0000000000004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang G., Hu C., Luo L. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rumbak M.J., Newton M., Truncale T., Schwartz S.W., Adams J.W., Hazard P.B. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med. 2004;32:1689–1694. doi: 10.1097/01.ccm.0000134835.05161.b6. [DOI] [PubMed] [Google Scholar]
- 24.McGrath B.A., Wallace S., Goswamy J. Laryngeal oedema associated with COVID-19 complicating airway management. Anaesthesia. 2020;75:972. doi: 10.1111/anae.15092. [DOI] [PubMed] [Google Scholar]
- 25.Heyd C.P., Desiato V.M., Nguyen S.A. Tracheostomy protocols during COVID-19 pandemic. Head Neck. 2020;42:1297–1302. doi: 10.1002/hed.26192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch T., Hecker B., Hecker A. Early tracheostomy decreases ventilation time but has no impact on mortality of intensive care patients: a randomized study. Langenbecks Arch Surg. 2012;397:1001–1008. doi: 10.1007/s00423-011-0873-9. [DOI] [PubMed] [Google Scholar]
- 28.British Laryngological Association British laryngological association tracheostomy guideline COVID 19. https://www.britishlaryngological.org/sites/default/files/BLA Tracheostomy guideline -BLA April 2020 FINAL.pdf Available from:
- 29.Tay J.K., Khoo M.L., Loh W.S. Surgical considerations for tracheostomy during the COVID-19 pandemic: lessons learned from the severe acute respiratory syndrome outbreak. JAMA Otolaryngol Head Neck Surg. 2020;146:517–518. doi: 10.1001/jamaoto.2020.0764. [DOI] [PubMed] [Google Scholar]
- 30.Hanidziar D., Bittner E.A. Sedation of mechanically ventilated COVID-19 patients: challenges and special considerations. Anesth Analg. July 2020;131:e40–e41. doi: 10.1213/ANE.0000000000004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kress J.P., Pohlman A.S., O’Connor M.F., Hall J.B. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 32.Pun B.T., Balas M.C., Barnes-Daly M.A. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 Adults. Crit Care Med. 2019;47:3–14. doi: 10.1097/CCM.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.