Graphical abstract
Keywords: Vitamin D, COVID-19, Angiotensin converting enzyme
Highlights
-
•
Insufficient levels of Vitamin D could be seen in COVID-19 patients.
-
•
Increase in the ACE could be seen in COVID-19 patients with higher quantities in the individuals who died from the COVID-19.
-
•
The Neutrophil to Lymphocyte ratio (NLR) is higher in COVID-19 than the control group.
-
•
Serum levels of vitamin D and ACE are associated with the progression and severity of the COVID-19.
Abstract
In late 2019, SARS-CoV-2 started to spread throughout the world causing the COVID-19 that has taken a considerable number of lives. Results obtained from several investigations have explained the virus origin, pathogenicity, and transmission. Similar to SARS coronavirus, the pulmonary angiotensin converting enzyme (ACE) 2 was introduced as the virus receptor for entering the cell. An increased body of epidemiological and clinical evidences has shown modulating effects of vitamin D in lung injuries through several mechanisms. Several clinical symptoms as well as molecular factors have shown to be related to the disease transmission and severity. In this study, vitamin D, ACE concentrations, and neutrophil to lymphocyte ratio (NLR) were measured in patients with confirmed COVID-19 in comparison with control group. Results demonstrated significant alterations in vitamin D and ACE levels as well as NLR in the patients’ group. Contribution of those factors with the prognosis and severity of the disease has been shown.
1. Introduction
In December 2019, several cases of pneumonia occurred in Wuhan, Hubei province, China, caused by a new type of beta-coronavirus. The disease and the causative coronavirus were originally named by the World Health Organization as COVID-19 and nCoV-2019 respectively. On February 11, 2020, Coronavirus Study Group (CSG) of the International Committee on Taxonomy of Viruses tentatively named the novel coronavirus as SARS-CoV-2 (Coronaviridae Study Group of the International Committee on Taxonomy of, V., 2020). Chinese scientists promptly identified the viral sequence isolated from patients and confirmed the human-to-human transmission of the virus. The R0 (the basic reproduction number) of the virus was computed by scientists of various countries and declared to be about 2.2 and even higher (from 1.4 to 6.5) (D’Arienzoa and Coniglio, 2020; Muniz-Rodriguez et al., 2020). Clinically, patients with COVID-19 showed respiratory symptoms that were initially very similar to those of other respiratory viral infections. They were also characterized by ground-glass opacity in lung x-ray, which was even detectable in patients with a milder form of the disease. Recent research on the involvement of laboratory parameters in predicting of COVID-19 cases has suggested that the level of LDH, CRP, ALT and NEU can contribute to the clinical outcomes of the disease (Mardani et al., 2020). The new SARS-CoV-2 structure was recognized as a coated virus possessing an RNA genome with positive polarity, closely related to other SARS coronaviruses and more distant from common respiratory viruses circulating in humans (Coronaviridae Study Group of the International Committee on Taxonomy of, V., 2020). The new virus has 79.5 % genetic similarity with SARS-CoV (SARS agent in 2002) and 96.2 % with the RaTG13 bat coronavirus. Structural studies of the virus receptor protein sequences have revealed that SARS-CoV-2 can recognize angiotensin converting enzyme 2 (ACE2) from humans and other animal species such as ferrets, cats, and others as intermediate hosts (Wan et al., 2020). Inside the lungs, the ACE2 protein has more expression in the apical surface of the deep alveolar epithelial cells. This receptor is expressed in multiple human organs. It assists the human-to-human and cross-species transmission of the virus (Andersen et al., 2020; Hussain et al., 2020a). ACE2 is a zinc-metallopeptidase which is an antagonist of the angiotensin converting enzyme (ACE). ACE converts the angiotensin (Ang) I to Ang II, a vasoconstrictor, by removing a dipeptide from its C-terminal. Besides, ACE is a destroyer of bradykinin which is a vasodilator. The ACE2 acts, unlike ACE, to remove a single amino acid from the end of a protein, hence unable to convert Ang I to Ang II or inactivate bradykinin. The main role of ACE2 is to convert Ang II to Ang-(1–7) and to promote the relevant pathway. From the physiological point of view, it counteracts the activities of Ang II (pressor, proliferative effect, and pro-fibrotic effect). In fact, both enzymes appear to balance the rennin-angiotensin-aldosterone system (RAAS). Certain researches have shown that RAAS inhibitors, namely angiotensin receptor blockers (ARBs) and ACE inhibitors can increase the ACE2 expression (Zheng et al., 2020). RAAS inhibitors have heterogeneous effects by affecting different enzymes and peptides involved in the system. Various laboratory animal models have shown that ARBs and mineralocorticoid receptor blockers increase both ACE2 expression and activity. Increased ACE has a positive regulatory effect on the production of angiotensin II, which in turn results in positive feedback on the activity of the ACE2 (Tikellis and Thomas, 2012). Studies show that an increase in the level of ACE2 could activate the angiotensin II-Mas receptor axis, which acts as a cardiopulmonary protector through its anti-inflammatory and antioxidant effects (Shenoy et al., 2010). Renin is the initial hormone in the cascading process of the RAS system, on which vitamin D plays a moderating role.
There is a body of epidemiological and clinical evidence showing that vitamin D can reduce lung injuries through several mechanisms, including inducing the antimicrobial peptides, reducing the concentrations of pro-inflammatory cytokines and increasing the anti-inflammatory cytokines (Foley et al., 1998). In several observational studies, vitamin D deficiency has been shown to have an independent association with increased risk of acute viral respiratory infections. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are among the leading causes of mortality in intensive care units. They have common characteristics, including increased permeability of the epithelium of the alveoli and endothelium of the pulmonary capillaries, edema, and pulmonary fibrosis. It is also likely that vitamin D can exert protective effects against COVID-19 through suppression of cytokine response and reduce the risk of ARDS (Martineau et al., 2017). Research on mice lacking vitamin D receptors (VDR-null mice) has shown increased renin production and hyper reninemia in those animals, suggesting the negative regulation of renin by 125(OH)2 D3. Renin production in the kidney during the RAAS process breaks down the angiotensinogen and converts it to Ang I. The latter compound is converted to Ang II by ACE as mentioned. Calcitriol has been shown to reduce the risk of lung damage through the RAS system by negative regulation of the renin gene. Decrease in ACE and ACE2 in lung has been shown in animal models with increased mRNA levels of proinflammatory cytokines, and AT1R levels, associated with activation of the ACE-AngII-AT1R (angiotensin II receptor type 1) axis, pulmonary injury, and progression of cytokine storms. In this study, serum levels of vitamin D and ACE in patients with confirmed COVID-19 have been measured and results were discussed in relation to the certain possible pathways that could be involved in the progression of the disease.
2. Materials and methods
2.1. Patient participation and data collection
The study protocol was accepted by the Review Board and the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1399.131). All individuals participated in the study voluntarily and provided signed informed consent. Individuals were consisted of 65 male and 58 female outpatients, aged between 18–78 years, referred to Behpooyan clinic (Tehran, Iran) during March 2020 with respiratory difficulties including cough and shortness of breath and/or with CT-scan showing ground glass opacity in lungs. Oropharyngeal (OP) specimens were collected and samples were transferred to the laboratory using viral transport medium (VTM). All samples were tested for SARS-CoV-2 using probe based real-time RT-PCR (PishtazTeb, IRAN). Briefly, the viral RNA extractions followed by cDNA synthesis were performed on the OP specimens. The qRT-PCR was carried out using specific probes and primers conforming to the kit instructions. Based on the RT-PCR results, samples were divided into two groups of positive or negative for COVID-19.
Patients with comorbidities such as chronic lung diseases, hematological diseases, liver disease, having undergone radiotherapy, and chemotherapy were excluded from the study.
We do not have the sample at the peak of the disease and there was no data on patients’ clinical condition subsequent to this study since their samples were collected on admittance to the hospital, except knowing that one female and three male patients have deceased during the study.
2.2. Laboratory testings
Blood tests were performed using routine methods for the detection of Lymphocyte (LYM), and Neutrophil (NEU) counts. The angiotensin converting enzyme (ACE) was measured in the sera using ELISA method (Biorexfars, Iran) conforming to the instructions of the manufacturer. The serum level of vitamin D was measured using ELISA method (Monobind, USA), based on the kit instructions. In this regard, four levels for vitamin D concentrations were initially considered in the present study as deficient (<10 ng/mL), insufficient (10−30 ng/mL), sufficient (30−100 ng/mL), and potential toxic (>300 ng/mL). According to the previously reported test protocols used for the vitamin D quantification, two ranges of sufficient and insufficient vitamin D levels (>30 ng/mL and <30 ng/mL respectively) have been considered in this study (Holick, 2009).
2.3. Statistical analysis
The acquired data obtained were expressed as mean values with ± standard deviations (SD). Statistical differences between or among groups were calculated using Mann-Whitney and Kruskal-Wallis tests the GraphPad Prism Statistical Software V6.
3. Results
3.1. Laboratory findings in the COVID-19 patients and the control groups
In the present study, 123 (65 males and 58 females) individuals have participated comprised of 63 confirmed COVID-19 patients and 60 COVID-19 negative controls with an average of 42 and a median age of 39 years old. Suspected patients showed shadows or ground-glass opacities in their CT scans. However, they were undergone confirmatory diagnosis through the collection of oropharyngeal swab specimens and nucleic acid analysis tests (Xie et al., 2020). The results of two groups of positive and negative COVID-19 in terms of age, the absolute value of neutrophils, lymphocytes, nucleic acid analysis, as well as analyses of their relationships with COVID-19 are shown in Table 1 and Fig. 1, Fig. 2 , and he Supplementary data.
Table 1.
The comparison between the two groups in terms of the average age, Neutrophil, Lymphocyte, ACE and vitamin D is presented here. These data showed that the differences between the two groups were statistically significant (p < 0.0001).
| Covid-19 | Average |
||||
|---|---|---|---|---|---|
| Age | Neutrophil | Lymphocyte | ACE | Vitamin D | |
| Positive | 43.3 | 59.5 | 38.1 | 39.8 | 18.5 |
| Negative | 40.1 | 47.4 | 52.1 | 31.2 | 30.2 |
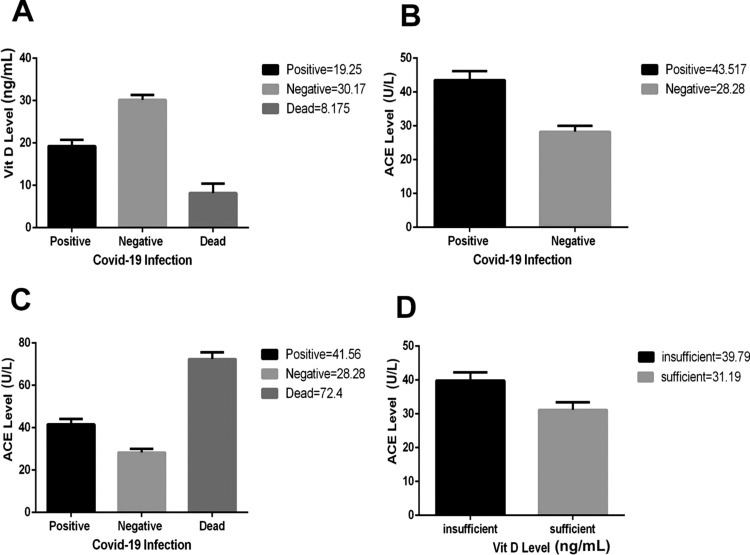
Fig. 1.
Analysis of the COVID-19, in terms of correlation with vitamin D and ACE modifications, in comparison with the control group. Higher levels of Vitamin D were seen in non−COVID-19 individuals (A). Increase in the ACE was seen in COVID-19 (B) with higher quantities in dead individuals (C). Such increase in ACE showed relationship with insufficient amounts of vitamin D in patient group (D).
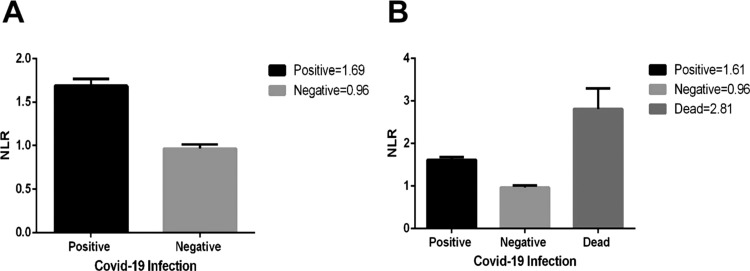
Fig. 2.
Association of the NLR with the vitamin D concentrations in COVID-19 patients. (A) The Neutrophil to Lymphocyte ratio (NLR) is higher in COVID-19 than the non−COVID-19 group (p < 0.0001). (B) The mean value of the NLR shows a significant increase in the individuals who died from the COVID-19 (p < 0.0001).
3.2. Association of vitamin D and ACE concentrations with the COVID-19 patients
The differences between COVID-19 positive and negative groups, in terms of the vitamin D and angiotensin converting enzyme (ACE) concentrations, are depicted in Fig. 1. The results show that vitamin D had an important change in the group of COVID-19 positive individuals. Four patients have unfortunately deceased during this study for whom, the mean vitamin D concentration was significantly decreased compared to the control group and other COVID-19 patients (p < 0.0001). Serum ACE concentration showed a significant increase in patients group, compared to the control group (p < 0.0001). The ACE concentration was significantly higher in deceased individuals even compared to the other COVID-19 patients (p < 0.0001). Looking at individuals with different vitamin D levels, the ACE concentration showed a higher quantity among individuals with insufficient vitamin D concentration (p < 0.0039). Those results have been depicted in Fig. 1A to D.
3.3. Association between neutrophil‐to‐lymphocyte ratio and the COVID-19 patients
Significant decrease in lymphocyte count and lymphopenia has been observed in this study (p < 0.0001). There was also a significant increase in the patients’ group in terms of the neutrophil count (p < 0.0001). In the case of the deceased patients, the change in lymphocyte and neutrophil count had an important difference not only with the control group but also with other COVID-19 patients (p < 0.0001). Consequently, the ratio between neutrophils and lymphocytes (NLR) was also considerably higher in the COVID-19 group, and beyond in the case of the deceased patients (p < 0.0001). The results are demonstrated in the Fig. 2.
4. Discussions
By the end of 2019, COVID-19 started in Wuhan, China, and emerged rapidly as a pandemic all over the world. Quickly after its appearance, COVID-19 was detected in Iran during the winter 2020. In the present work, patients under study were individuals who had been contaminated by the SARS-CoV-2 and needed hospitalization after confirmation of the clinical COVID-19. Among them, 4 individuals (6.3 % of the patients’ group and 3.2 % of the total) were deceased which was compliant with the death rate, previously reported for the COVID-19 (Epidemiology Working Group for Ncip Epidemic Response, C.C.F.D.C. and Prevention, 2020; Team, 2020). Important changes in vitamin D and ACE concentrations as well as the NLR have shown in our study to be among important parameters associated with the severity of the COVID-19. There is more than a century of evidence for the effects of vitamin D in remedying various pathogens’ effects (Lang et al., 2013). Extended animal studies support the regulatory effects of vitamin D on innate and adaptive immunity (Hewison, 2011). Vitamin D deficiency has been shown to be a risk factor in more severe courses of infection among critically ill patients. It has been observed to be inversely related to infections with various pathogens of the lower, as well as the upper respiratory tract (Ginde et al., 2009). In a 3.5 month follow-up study of a healthy cohort, a 2-fold less viral respiratory tract infections in individuals with >95 nmol/L of circulating 25(OH)VitD was observed (Sabetta et al., 2010). A recent study using UK Biobank samples aimed to assess the association of blood 25(OH)VitD concentration with COVID-19 risk. The results of that study did not find important link between blood vitamin D concentrations with COVID-19 risk, nor suggested the usefulness of vitamin D measurement in clinical practice to assess the risk of COVID-19 infection (Hastie et al., 2020). In our study, the insufficient concentrations of vitamin D were associated with the hospitalization of COVID-19 patients (Fig. 1). Less than 16 ng/mL values of the serum vitamin D have been reported to be possibly associated with increased risk of sepsis in critically ill patients (Moromizato et al., 2014). An optimal range for 25(OH)VitD is reported as 25−80 ng/mL, and the definition of vitamin D insufficiency is sometimes reported as <30 ng/mL. Concentrations lower than 10 ng/mL for vitamin D are reported as severe vitamin D deficiency (Kennel et al., 2010). The status of vitamin D in the four individuals with COVID-19 who deceased in the course of this study was lower than 10 ng/mL. On admission, those individuals had severely low vitamin D levels, significantly less than both the control group and the patients’ group, as depicted in Fig. 1a. Vitamin D is a steroid hormone that controls a broad range of metabolic and cell regulatory functions. It circulates in the blood as 25(OH) D and its concentration defines the vitamin D status of the body. Diabetes and other comorbidities such as hypertension, obesity and ethnicity have been reported as significant predictors of morbidity and mortality in patients with COVID-19 (Garg et al., 2020). Although it’s association with ethnicity was not supported by certain other results (Hastie et al., 2020). Vitamin D is associated with many diseases through manipulating the innate and adaptive immune system pathways (Prietl et al., 2013). Multiple cells in the immune system possess the vitamin D receptor (VDR) and, are capable of converting 25(OH)VitD to 1,25(OH)2VitD. Many other cell types than kidney cells can produce 1,25(OH)2VitD by the action of cytochrome p450 family member CYP27B1, with the assistance of TLRs or alternate PRRs. Endocrine or intracrine stimulating effect of 1,25(OH)2VitD on the expression of CYP27B1 enhances the epithelial cell expression of the antimicrobial peptide Cathelicidin LL-37 and beta-defensin (Gombart, 2009; Adams and Hewison, 2012). This mechanism has been shown to induce the chemotaxis of immune cells and prevent neutrophil apoptosis that increases their lifespan and consequently modulate the respiratory immune response to viral pathogens such as RSV and influenza (Nagaoka et al., 2012; Ahmed et al., 2019).
The NLR has been introduced as a useful indicator of systemic inflammation and tested as a guide for the prognosis of various diseases, including sepsis and cancer (Martins et al., 2019). It is a routine simple measure and not costly examination in hospitals. Association of the NLR increase has also been demonstrated in ARDS and ALI (Zhang et al., 2019). Meta‐analysis investigations support that NLR and LCR (lymphocyte to C-reactive protein ratio) values can help predict clinical severity in patients with COVID‐19 (Lagunas-Rangel, 2020). In the current study, significant decrease in lymphocyte along with increase in neutrophil count was demonstrated in patients (supplementary Fig. 1). Consequently, we have shown the NLR increase in COVID-19 patients compared to control group, with significantly higher values in those patients for whom the disease was fatal (Table 1 and Fig. 2). Considering that the blood samples of participants in this study was analyzed on admission, one possibility could be that the decreased lymphocyte count might be due to the IFN-I dependent transient lymphopenia which is observed in many viral infections (Kamphuis et al., 2006). It has not been demonstrated whether or not the direct viral infection through spike receptors in T-lymphocyte could contribute to lymphopenia (Wang et al., 2020). In a study with a Time-Lymphocyte percent model in patients with COVID-19, the importance of lymphopenia, with the lymphocyte count of less than 20 percent has been demonstrated as a decisive point to predict the disease severity (Tan et al., 2020). In our study, a significant lymphopenia was observed in COVID-19 patients, as previously reported by other studies (Lagunas-Rangel, 2020; Tan et al., 2020). However, the blood lymphocyte in none of those deceased individuals was lower than 20 percent on admission. Therefore, we rather suggest that the cost-effective NLR to be considered as a marker to aid complication predictions or poor prognosis in COVID-19.
Another factor demonstrated in this study was the significant increase of circulating ACE in the COVID-19 patients (Fig. 1B). The renin angiotensin system (RAAS) was primarily thought to be responsible for the regulation of blood pressure and sodium and water homeostasis. However, it has been revealed that RAAS could be closely associated with the lung injury (Chen et al., 2013). In a regular way, juxtaglomerular cells within the kidneys release renin following a blood pressure drop, which hydrolyzes circulating angiotensinogen to produce Ang I, which is then cleaved by ACE and converted to biologically active octapeptide Ang II. Ang II is the most important effector peptide of the RAAS that preferentially binds to and stimulates the Ang II type 1 receptor (AT1R), inducing vasoconstriction, inflammation, oxidative stress, and cell proliferation (Schalekamp and Danser, 2013). When metabolized by ACE2 to form Ang-(1-7), Ang II can induce the G-protein coupled MasR axis and subsequently oppose the vasoconstrictor effects of Ang II, aldosterone secretion and counteract the AT1R downstream effects. Inhibition of the Ang II signaling pathway and/or RAAS has protective effects on lung injury (Yu et al., 2016). Clear shreds of evidence show that RAAS activation contributes to pulmonary arterial hypertension through actions of Ang II and particularly aldosterone (Maron and Leopold, 2014). An increase in ACE can potentially overdrive the Ang II generation and promote the detrimental effects of the AT1R classical axis. In addition to elevated ACE, we have also observed an association between increased ACE level and vitamin D insufficiency in COVID-19 patients (Fig. 1D). These results are in line with the fact that RAAS can provide feedback to vitamin D signaling and block the act of vitamin D as a transcription factor in renin gene suppression whereby it exerts a negative endocrine regulator activity on RAAS(Shroff et al., 2012). Lung is a major source of ACE and therefore a major site of systemic Ang II synthesis and RAAS action. It is thought that ACE2 activity is upregulated by Increased Ang II levels. SARS coronavirus has been suggested as a predisposing factor for ARDS. RAAS components including ACE, Ang II and the Ang II type 1a receptor (AT1a) exacerbate, while ACE2 can protect, from the disease outcomes including lung edema and impaired lung function (Imai et al., 2005). Pulmonary ACE2 appears to regulate the balance between the levels of circulating Ang II and Ang-(1–7). Transcriptome analysis for ACE2 expression in the lungs of patients with comorbidities has shown high expressions in patients with severe COVID-19, compared to control individuals (Pinto et al., 2020). In the case of diabetes, as the expression of ACE2 depends on the progression of the disease, adverse outcomes might be reduces through patient management strategies, rigorous glucose monitoring and careful consideration of drug interactions (Hussain et al., 2020b). Collectively, it seems that SARS-CoV-2 in the same way as SARS-CoV infection, could shift the balance of ACE/Ang II/AT1R axis over the ACE2/Ang (1–7)/MasR axis in the lung, resulting in acute lung injury (Kuba et al., 2005). High expression of VDR in the lung and interaction with vitamin D can prevent lung injury through blocking the RAAS (Kong et al., 2013). Altogether, sufficient vitamin D can have a modulating effect on the consequences of SARS-CoV-2 infection through interference with the RAAS and immune system elements functions through VDR which is a ligand-activated transcription factor.
CRediT authorship contribution statement
R. Mardani: Conceptualization, Methodology. A. Alamdary: Visualization, Formal analysis. S.D. Mousavi Nasab: Validation, Writing - review & editing. R. Gholami: Investigation, Writing - review & editing. N. Ahmadi: Writing - review & editing, Funding acquisition. A. Gholami: Project administration, Supervision, Writing - original draft, Writing - review & editing.
Acknowledgement
Authors would like to thank Behpooyan clinic for generous collaboration. This research was supported with the grant number 23824 from Vice-Chancellor for Research at Shahid Beheshti University of Medical Sciences .
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.virusres.2020.198148.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adams J.S., Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012;523(1):95–102. doi: 10.1016/j.abb.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A. Human antimicrobial peptides as therapeutics for viral infections. Viruses. 2019;11(8) doi: 10.3390/v11080704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.N. Dysregulated renin-angiotensin system contributes to acute lung injury caused by hind-limb ischemia-reperfusion in mice. Shock. 2013;40(5):420–429. doi: 10.1097/SHK.0b013e3182a6953e. [DOI] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of, V The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arienzoa M., Coniglio A. Assessment of the SARS-CoV-2 basic reproduction number, R0, based on the early phase of COVID-19 outbreak in Italy. J. Biosaf. Health Educ. 2020 doi: 10.1016/j.bsheal.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epidemiology Working Group for Ncip Epidemic Response, C.C.F.D.C. and Prevention [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- Foley R.N., Parfrey P.S., Sarnak M.J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 1998;32(5 Suppl 3):S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- Garg M. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North-supports vitamin D as a factor determining severity. Authors’ reply. Aliment. Pharmacol. Ther. 2020;51(12):1438–1439. doi: 10.1111/apt.15796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginde A.A., Mansbach J.M., Camargo C.A., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009;169(4):384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart A.F. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie C.E. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. 2020;14(4):561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewison M. Vitamin D and innate and adaptive immunity. Vitam. Horm. 2011;86:23–62. doi: 10.1016/B978-0-12-386960-9.00002-2. [DOI] [PubMed] [Google Scholar]
- Holick M.F. Vitamin D status: measurement, interpretation, and clinical application. Ann. Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M. Structural variations in human ACE2 may influence its binding with SARS-CoV-2 spike protein. J. Med. Virol. 2020 doi: 10.1002/jmv.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A., Bhowmik B., do Vale Moreira N.C. COVID-19 and diabetes: knowledge in progress. Diabetes Res. Clin. Pract. 2020;162:108142. doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis E. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108(10):3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- Kennel K.A., Drake M.T., Hurley D.L. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin. Proc. 2010;85(8):752–757. doi: 10.4065/mcp.2010.0138. quiz 757-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol. Endocrinol. 2013;27(12):2116–2125. doi: 10.1210/me.2013-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.O. How important is vitamin D in preventing infections? Osteoporos. Int. 2013;24(5):1537–1553. doi: 10.1007/s00198-012-2204-6. [DOI] [PubMed] [Google Scholar]
- Mardani R. Laboratory parameters in detection of COVID-19 patients with positive RT-PCR; a diagnostic accuracy study. Arch. Acad. Emerg. Med. 2020;8(1):e43. [PMC free article] [PubMed] [Google Scholar]
- Maron B.A., Leopold J.A. The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension (2013 Grover Conference series) Pulm. Circ. 2014;4(2):200–210. doi: 10.1086/675984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau A.R. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins E.C. Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an intensive care unit: a case-control study. Rev. Bras. Ter. Intensiva. 2019;31(1):64–70. doi: 10.5935/0103-507X.20190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moromizato T. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit. Care Med. 2014;42(1):97–107. doi: 10.1097/CCM.0b013e31829eb7af. [DOI] [PubMed] [Google Scholar]
- Muniz-Rodriguez K. Severe acute respiratory syndrome coronavirus 2 transmission potential, Iran, 2020. Emerg Infect Dis. 2020;26(8) doi: 10.3201/eid2608.200536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka I. Modulation of neutrophil apoptosis by antimicrobial peptides. ISRN Microbiol. 2012;2012:345791. doi: 10.5402/2012/345791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto B.G. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. medRxiv. 2020 doi: 10.1093/infdis/jiaa332. p. 2020.03.21.20040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prietl B. Vitamin D and immune function. Nutrients. 2013;5(7):2502–2521. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabetta J.R. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5(6):e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalekamp M.A., Danser A.H. How does the angiotensin II type 1 receptor ‘trump’ the type 2 receptor in blood pressure control? J. Hypertens. 2013;31(4):705–712. doi: 10.1097/HJH.0b013e32835d6d11. [DOI] [PubMed] [Google Scholar]
- Shenoy V. The angiotensin-converting enzyme 2/angiogenesis-(1-7)/Mas axis confers cardiopulmonary protection against lung fibrosis and pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2010;182(8):1065–1072. doi: 10.1164/rccm.200912-1840OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff R., Wan M., Rees L. Can vitamin D slow down the progression of chronic kidney disease? Pediatr. Nephrol. 2012;27(12):2167–2173. doi: 10.1007/s00467-011-2071-y. [DOI] [PubMed] [Google Scholar]
- Tan L. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target. Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team C.C.-R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis C., Thomas M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012;2012:256294. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell. Mol. Immunol. 2020 doi: 10.1038/s41423-020-0498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int. J. Infect. Dis. 2020;93:264–267. doi: 10.1016/j.ijid.2020.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q.H. Captopril pretreatment protects the lung against severe acute pancreatitis induced injury via inhibiting angiotensin II production and suppressing Rho/ROCK pathway. Kaohsiung J. Med. Sci. 2016;32(9):439–445. doi: 10.1016/j.kjms.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Neutrophil-lymphocyte ratio as an early new marker in AIV-H7N9-infected patients: a retrospective study. Ther. Clin. Risk Manag. 2019;15:911–919. doi: 10.2147/TCRM.S206930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.Y. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.