Abstract
Supplemental Digital Content is available in the text
Multiple myeloma (MM), the second most common hematological malignancy, is characterized by clonal proliferation of malignant plasma cells in the bone marrow (BM).1 Inhibition of the ubiquitin proteasome system (UPS) has shown to be particularly useful for the treatment of MM.2 UPS is a protein degradation system that maintains homeostasis of intracellular proteins. Inhibition of the UPS results in the accumulation of polyubiquitinated proteins that triggers different types of cellular stress responses, followed by growth arrest and apoptosis.3 Bortezomib (Velcade®) is the first-in-class proteasome inhibitor available for the treatment of MM and mantle cell lymphoma (MCL).4 Despite encouraging clinical results, the use of bortezomib has been limited due to off-target adverse effects that lead to serious toxicities such as peripheral neuropathy. Furthermore, bortezomib has a poor pharmacokinetic profile, that is, large volume of distribution and rapid blood clearance, minimizing its therapeutic window.4 Moreover, most patients become refractory to bortezomib.4,5
A drug delivery system could improve the safety and efficacy of bortezomib. Encapsulation of drugs into nanomedicines can substantially improve their pharmacokinetic-pharmacodynamic profiles and reduce toxicity, thereby widening the therapeutic window.1,6–9 Since, MM causes increased microvessel density in its bone marrow (BM) lesions,10 we anticipated that as a consequence, nanomedicines like liposomes can locally accumulate and deliver bortezomib through the enhanced permeability and retention effect.
In the present study, we evaluated circulation kinetics, biodistribution, and therapeutic efficacy of long circulating liposomal bortezomib (LCL-BORT) using a human BM-like scaffold (huBMsc) xenograft mouse model of MM in which a human-bone mimic is created by osteogenic differentiation of human mesenchymal stromal cells on calcium phosphate scaffolds (Fig. 1A). Using this model, which besides allowing engraftment of primary MM has previously proven its translational potential with Daratumumab,11,12 we show that liposomal packaging of bortezomib improves the circulation kinetics and biodistribution, resulting in a striking anti-MM efficacy.
Figure 1.
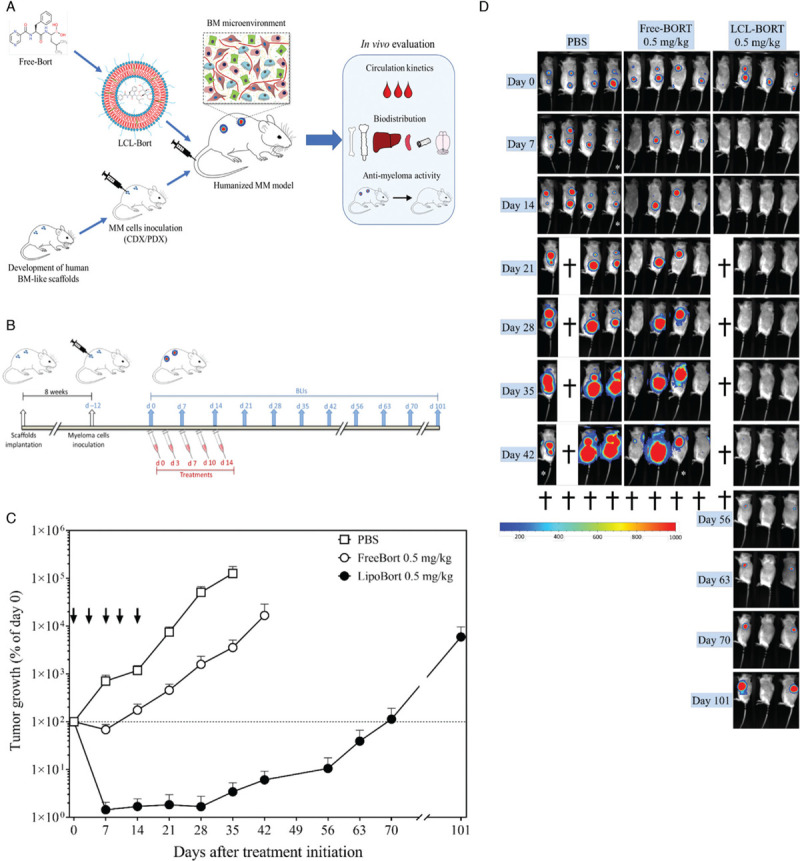
In vivo efficacy of liposomal bortezomib in MM.1S tumor bearing mice. (A) Flow-chart summarizing the experimental set-up. (B) Experimental design. Twelve days after MM.1S cell inoculations into 4 separate scaffolds per mouse, animals were randomized based on baseline BLI signals on day 0. Animals were treated twice weekly for a total of 5 injections (ie, day 0, 3, 7, 10, and 14). BLI was performed on the day of first injection (day 0) followed by once weekly or as indicated up to day 101 after treatment initiation. (C) Tumor growth curve. BLI images were analyzed to obtain luminescence intensity as counts per min/square centimeters (cpm/cm2). Percentage tumor growth was calculated relative to day 0. Statistical analysis was performed using nonlinear regression using exponential growth equation. Free and liposomal bortezomib showed tumor growth reduction at treatment regimen of 0.5 mg/kg. Liposomal bortezomib showed a substantial tumor growth delay as compared to all other treatment groups. The PBS-line stops at day 35, because day 42 includes only two animals (out of four). One animal was sacrificed on day 21 due to humane end point, and one showed less BLI signal due to improper luciferin injection. Arrows represent treatment days. Data is presented as mean ± SEM. (D) Bioluminescence images. Bioluminescence imaging (BLI) was performed on indicated days. Mouse 1 from LCL-BORT 0.5 mg/kg group was found dead in the cage on day 21. This doesn’t seem to be due to dose related toxicity as there was no sign of systemic toxicity on day 14 (∼3.8% body weight gain on day 14 compared to day 10 in this animal).
Liposomes with an average diameter of 118 ± 1 nm with a polydispersity index (PDI) 0.08 ± 0.04 were prepared and loaded with bortezomib by a remote loading method using mannitol and meglumine as entrapping agents.13 The amount of encapsulated bortezomib was 137.3 ± 4.2 μg/mL with a near complete encapsulation efficiency of 91.5 ± 2.8%.
The in vitro cytotoxic efficacy, evaluated by a luminescence-based cytotoxicity assays, showed there was a comparable and concentration dependent cytotoxicity for both Free- and LCL-BORT with IC50 values of Free-BORT of 2.1 nM and 4.7 nM and IC50 values for LCL-BORT of 0.42 nM and 6.0 nM for the MM.1S and UM9 cell line respectively. Next, we assessed and compared the circulation times and biodistribution of LCL-BORT to Free-BORT in the huBMsc xenograft model engrafted with the MM.1S myeloma cell line. Bortezomib concentrations in plasma samples collected at different time points after a single i.v. injection of Free- or LCL-BORT were determined by LC–MS/MS. The results show marginally increased retention of the drug in plasma by liposomal encapsulation (Supplementary Fig. 1A). Nevertheless, the maximum plasma concentration (Cmax) and the plasma levels of bortezomib over time (area under the curve; AUC) were substantially higher in LCL-BORT treated mice. In fact, the Cmax of bortezomib was 8-fold higher (6048 ng/mL compared to 802 ng/mL) and the AUC0-t increased 7-fold (3886 ng/mL compared to 547 ng/ml) for the liposomal formulation. Volume of distribution (Vd) was 104.2 and 30.8 L/kg for Free-BORT and LCL-BORT, respectively, showing a substantial three-fold reduction (Supplementary Fig. 1B).
Previously, we have shown a long circulatory half-life for PEGylated liposomes in the huBMsc model, which was in agreement with the long circulating property of comparable liposomal formulations in other murine tumor models. Approximately 35% of the injected dose could still be found 24 hours post injection.14 In the present study, in Free- as well as in LCL-BORT group, the bortezomib concentrations were considerably lower already 1 hour after injection. These results are in line with previously reported data on a liposomal formulation of bortezomib for the treatment of chronic myeloid leukemia that shows similar pharmacokinetic profiles to our formulation.15
To evaluate the biodistribution of bortezomib, drug concentrations were measured in a panel of tissue homogenates including tumor bearing scaffolds and organs, that is, femur, sternum, liver, spleen, and brain by LC–MS/MS (Supplementary Fig. 1C and 1D). In MM.1S tumor-bearing scaffolds, bortezomib concentrations were slightly higher in case of LCL-BORT compared to the free drug. Relatively low bortezomib concentrations were detected in femur and sternum, which decreased over time in both Free- and LCL-BORT groups. Interestingly, drug concentrations were higher in the scaffolds than in femur and sternum indicating that more drugs accumulated at the tumor sites.
Next, we evaluated the therapeutic efficacy of liposomal bortezomib formulation in the huBMsc xenograft model. Twelve days after inoculation with MM.1S tumor cells, huBMsc-mice received five injections of either Free-, LCL-BORT or PBS as a control (twice weekly, i.v.) on day 0, 3, 7, 10, and 14 (day 0 considered to be the first day of injections) (Fig. 1B). Bioluminescence imaging (BLI) was used to monitor tumor growth over time. As expected, significant tumor growth inhibition was seen in the Free-BORT 0.5 mg/kg treated animals. However, only one of the four animals showed a complete tumor regression 7 days after the start of treatment. Interestingly, despite a marginal improvement in the pharmacokinetic profile, complete tumor regression was seen in all mice of the LCL-BORT 0.5 mg/kg treatment group already after two injections, that is, 7 days after treatment initiation (Fig. 1C, 1D, and Supplementary Fig. 2). These results are also reflected by the tumor doubling time (TDT) analyzed by non-linear regression. TDT was 3.4 days (95% CI = 3.3–3.6) for the PBS treated group, 6.1 days (95% CI = 5.6–7.3) for the Free-BORT 0.5 mg/kg group, and could not be calculated for the LCL-BORT group as the tumors experienced complete regressions (Fig. 1C). Two animals of the LCL-BORT group relapsed on day 42 (ie, 4 weeks after treatments cessation), reached the baseline on day 70 (approximately 113% tumor growth compared to day 0) and could be monitored till day 101, the point where the tumor burden reached its humane endpoint. Humane endpoints in all other groups were reached on or before day 42. These observations underline a striking improvement in therapeutic efficacy of liposomal encapsulation of bortezomib. Moreover, this increase in affectivity was not accompanied by an obvious increase in toxicity as both treatment groups showed approximately a 10% body weight reduction. This reduction was, however, reversible after cessation of the treatments (Supplementary Fig. 3).
Finally, we compared Free- with LCL-BORT using a patient derived xenograft (PDX) of the huBMsc-model. As indicated (Fig. 2A), 6 weeks after cells inoculation mice were treated with PBS, Free- or LCL-BORT at 0.5 mg/kg twice weekly (day 0, 3, 7, 10, and 14). Similar to the MM.1S xenografts, both Free-BORT and LCL-BORT resulted in a significant inhibition of tumor growth (Fig. 2B). Although the Free-BORT group in this PDX model also resulted in complete tumor regression in 3 out of 4 animals, they were not long-lasting with relapses starting at 28 days after treatment was started. In contrast, the LCL-BORT treatment showed complete tumor regression in all mice (Fig. 2B, 2C and Supplementary Fig. 4) that did not relapse till the end of the experiment. Again, the increase of affectivity was not accompanied with an increase in toxicity, since comparable reductions in weight of the mice was observed, which was partially reversible after treatments were stopped (Supplementary Fig. 5).
Figure 2.
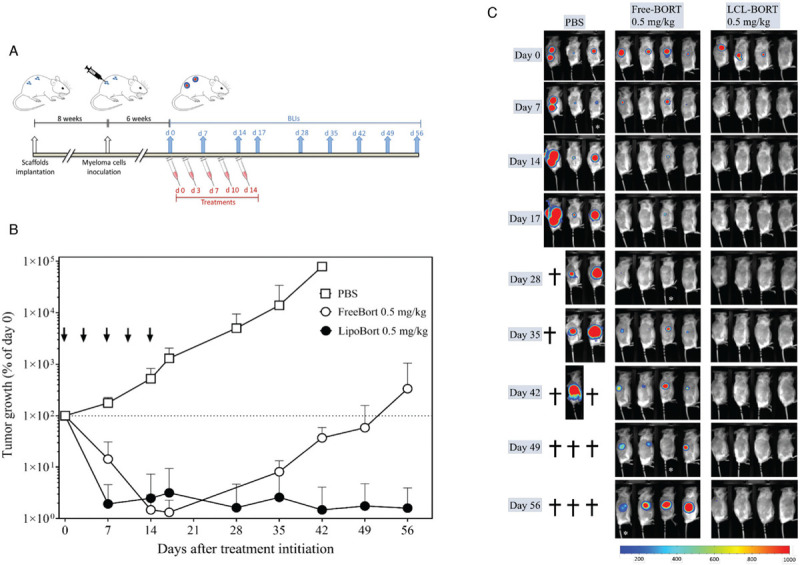
In vivo efficacy of liposomal bortezomib in mice bearing pMM patient-derived xenografts. (A) Experimental design. Six weeks after patient cells inoculations into 4 separate scaffolds per mouse, animals were randomized based on baseline BLI signals on day 0. Animals were treated twice weekly for a total of 5 injections (ie, day 0, 3, 7, 10, and 14). BLI was performed on the day of first injection (day 0) followed by once weekly up to 8 weeks after treatment initiation. (B) Tumor growth curve. BLI images were analyzed to obtain luminescence intensity as counts per min/square centimeters (cpm/cm2). Percentage tumor growth was calculated relative to day 0. Statistical analysis was performed using nonlinear regression using exponential growth equation. Free and liposomal bortezomib showed tumor growth reduction at treatment regimen of 0.5 mg/kg. Liposomal bortezomib showed a great difference in tumor growth delay compared to free drug and all other treatment groups. Arrows represent treatment days. Data is presented as mean ± SEM. (C) Bioluminescence images. Mice were inoculated with luciferase marked patient derived cells into the human bone containing scaffolds. Six weeks after tumor cells inoculation, animals were treated with PBS, free bortezomib and liposomal bortezomib 0.5 mg/kg. All treatments were given twice weekly via the tail vein, total of 5 injections. Bioluminescence imaging (BLI) was performed weekly. BLI images on the right side of the animals are shown.
Taken together, liposomal bortezomib outperformed free drug in both xenograft models tested by remarkably improving therapeutic efficacy and enhancing the survival of animals. Although only a marginal improvement in drug accumulation was seen in tumor-bearing scaffolds and bones, an overall higher drug exposure and bioavailability was noted in case of liposomal bortezomib (increased Cmax and AUC) as compared to free bortezomib, resulting in a 7-fold higher plasma concentration up to 24 hours. This might be key to the difference in the efficacy that we have observed here. Importantly, in clinical practice bortezomib is currently given subcutaneously and not intravenously in order to reduce toxicity, mainly peripheral neuropathy, while maintaining efficacy. This reduction in toxicity has been attributed to the lower Cmax achieved by the subcutaneous route.16,17 In this current preclinical study, the higher Cmax for liposomal bortezomib, however, should not be interpreted as indicative for an increased potential of peripheral toxicity. The Cmax of the liposomal formulation is a result of total drug in the circulation, that is, free bortezomib plus liposomal-encapsulated bortezomib. At the time of the Cmax the majority of circulating drug is liposomal-encapsulated and, therefore, not being exposed to healthy tissues, reducing the chance of systemic toxicity, but also prevented from rapid clearance, hence, the higher Cmax.
To our knowledge this is the first time that complete tumor regression is shown by liposomal bortezomib therapy in a clinically relevant mouse model of multiple myeloma. Moreover, the results show that liposomal encapsulation of bortezomib exerts a striking therapeutic efficacy compared to the free drug. Liposomal bortezomib could be further exploited in clinical settings for MM treatment and has potential to be implemented to the existing treatment regimens, especially for the frail patients.
Sources of Funding
This work was partly funded by Netherlands Organization for Scientific Research (NWO) High Tech Systems & Materials grant number 13312.
Disclosures
AKD and SNM are employed by Sun Pharma. JMM is CEO at Enceladus Pharma. JDdB and HY are stock owners of Kuros Biosciences AG and Scinus Cell Expansion BV. TM, ACMM and RWJG received research support from Takeda and Janssen Pharmaceuticals. TM also received research support from Novartis, Genmab, Onkimmune, Gadeta, and Aduro Biotech.
Supplementary Material
Footnotes
Citation: Deshantri AK, Fens MH, Ruiter RW, Metselaar JM, Storm G, Mandhane SN, Graat GH, Lentjes EG, Yuan H, de Bruijn JD, Mutis T, Martens AC, Groen RW, Schiffelers RM. Complete tumor regression by liposomal bortezomib in a humanized mouse model of multiple myeloma. HemaSphere, 2020;4:5(e463). http://dx.doi.org/10.1097/HS9.0000000000000463
References
- 1.Deshantri AK, Varela Moreira A, Ecker V, et al. Nanomedicines for the treatment of hematological malignancies. J Control Release. 2018;287:194–215. [DOI] [PubMed] [Google Scholar]
- 2.Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017;36:561–584. [DOI] [PubMed] [Google Scholar]
- 3.Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol. 2017;14:417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets. 2011;11:239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaal EA, Wu W, Jansen G, Zweegman S, Cloos J, Berkers CR. Bortezomib resistance in multiple myeloma is associated with increased serine synthesis. Cancer Metab. 2017;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshantri AK, Kooijmans SA, Kuijpers SA, et al. Liposomal prednisolone inhibits tumor growth in a spontaneous mouse mammary carcinoma model. J Control Release. 2016;243:243–249. [DOI] [PubMed] [Google Scholar]
- 7.Banciu M, Schiffelers RM, Metselaar JM, Storm G. Utility of targeted glucocorticoids in cancer therapy. J Liposome Res. 2008;18:47–57. [DOI] [PubMed] [Google Scholar]
- 8.Schiffelers RM, Banciu M, Metselaar JM, Storm G. Therapeutic application of long-circulating liposomal glucocorticoids in auto-immune diseases and cancer. J Liposome Res. 2006;16:185–194. [DOI] [PubMed] [Google Scholar]
- 9.Schiffelers RM, Metselaar JM, Fens MH, Janssen AP, Molema G, Storm G. Liposome-encapsulated prednisolone phosphate inhibits growth of established tumors in mice. Neoplasia. 2005;7:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliani N, Storti P, Bolzoni M, Palma BD, Bonomini S. Angiogenesis and multiple myeloma. Cancer Microenviron. 2011;4:325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nijhof IS, Groen RW, Noort WA, et al. Preclinical evidence for the therapeutic potential of CD38-targeted immuno-chemotherapy in multiple myeloma patients refractory to lenalidomide and bortezomib. Clin Cancer Res. 2015;21:2802–2810. [DOI] [PubMed] [Google Scholar]
- 12.Groen RW, Noort WA, Raymakers RA, et al. Reconstructing the human hematopoietic niche in immunodeficient mice: opportunities for studying primary multiple myeloma. Blood. 2012;120:e9–e16. [DOI] [PubMed] [Google Scholar]
- 13.Deshantri AK, Metselaar JM, Zagkou S, et al. Development and characterization of liposomal formulation of bortezomib. Int J Pharm X. 2019;1:100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deshantri AK, Fens MH, Ruiter RWJ, et al. Liposomal dexamethasone inhibits tumor growth in an advanced human-mouse hybrid model of multiple myeloma. J Control Release. 2019;296:232–240. [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Pang J, Shen N, et al. Liposomal bortezomib is active against chronic myeloid leukemia by disrupting the Sp1-BCR/ABL axis. Oncotarget. 2016;7:36382–36394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreau P, Karamanesht II, Domnikova N, et al. Pharmacokinetic, pharmacodynamic and covariate analysis of subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma. Clin Pharmacokinet. 2012;51:823–829. [DOI] [PubMed] [Google Scholar]
- 17.Ye Z, Chen J, Xuan Z, Yang W, Chen J. Subcutaneous bortezomib might be standard of care for patients with multiple myeloma: a systematic review and meta-analysis. Drug Des Devel Ther. 2019;13:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


