Abstract
A randomized complete block design experiment with 32 yearling crossbred steers (average body weight [BW] = 442 ± 17.0 kg) fed a steam-flaked corn-based diet was used to evaluate the effects of dietary Zn (KemTRACE Zn propionate 27; Kemin Industries, Inc., Des Moines, IA) supplementation on live growth performance, skeletal muscle fiber, and beta-adrenergic receptor (β-AR) characteristics during the finishing phase. Steers were blocked by BW (n = 4 blocks; 8 steers/block), assigned to pens (n = 4 steers/pen), and randomly assigned to the following treatments: control (CON; 0.0 g/[head (hd) · d] of additional Zn) or additional dietary Zn (ZnP; 1.0 g/[hd · d] additional Zn). The basal diet contained Zn (60 ppm dry matter basis) from ZnSO4; additional Zn was top-dressed at feeding. Ractopamine hydrochloride (RH; Optaflexx: Elanco Animal Health, Greenfield, IN) was included at 300 mg/(hd · d) for the final 28 d of the 111-d feeding period. Longissimus muscle biopsy samples, BW, and blood were obtained on days 0, 42, 79, and 107. Final BW was collected prior to shipping on day 111. Biopsy samples were used for immunohistochemical (IHC), mRNA, and protein analysis. Serum urea nitrogen (SUN) and nonesterified fatty acid (NEFA) concentrations were measured. Steers fed ZnP had a greater average daily gain (P = 0.02) and gain to feed ratio (G:F; P = 0.03) during the RH feeding period compared with CON. There were no differences (P > 0.05) in other growth performance variables, carcass traits, mRNA abundance, or relative protein concentration for fiber type and β-AR. Fiber types I and IIA had no differences in the cross-sectional area; however, the IIX area was greater for CON (P < 0.04) compared with ZnP and increased (P < 0.02) over time. There were no differences between treatments for the β1-AR density (P > 0.05) in skeletal muscle tissue throughout the study. A treatment × day interaction was observed in β2-AR density (P = 0.02) and β3-AR density (P = 0.02) during the RH feeding period, where the abundance of the receptors increased with ZnP but did not change in CON. Compared with CON, ZnP had greater (P < 0.01) mean NEFA concentrations. Mean SUN concentrations did increase by day (P < 0.01). Additional dietary Zn, supplied as Zn propionate, upregulates β2-AR and β3-AR and improves growth performance in feedlot steers during the RH feeding period, likely through a shift of resource utilization from lipogenesis to muscle maintenance and hypertrophy.
Keywords: beef, beta-adrenergic agonist, trace mineral, zinc
Introduction
Increasing trace mineral supplementation has become increasingly more popular in feedlot diets to improve health and maximize growth potential. Among these trace minerals, Zn has been one of the most heavily researched, as it is an intracellular signaling molecule, which required in a multitude of cellular functions (Fukada et al., 2011). According to the 2016 nutrient requirements for beef cattle, the Zn requirement for beef cattle is 30 mg Zn/kg dry matter (DM) (NASEM, 2016). However, Zn is not readily mobilized from tissue, because it is bound intracellularly. Therefore, an increase in dietary Zn may improve the Zn availability to the animal (Miao et al., 2013). Several studies have reported improvements in average daily gain (ADG) and feed efficiency, as well as heavier body weights (BWs) in steers, heifers, and lambs with supplementation of Zn beyond the level of requirement (Spears, 1989; Nunnery et al., 2007). Previous research has also concluded that Zn is intricately involved in protein synthesis and regulatory functions during transcription and has increased the bioavailability when supplied in an organic form, rather than an inorganic form (Brinkhaus et al., 1998; NASEM, 2016). The effects of Zn propionate on skeletal muscle growth and development have been minimally investigated; however, it was hypothesized that Zn propionate may alter muscle development and growth metabolism. Therefore, a study was designed to determine the effects of dietary zinc (KemTRACE Zn propionate 27: Kemin Industries, Inc., Des Moines, IA) supplementation on feedlot growth performance, carcass characteristics, blood metabolites, and β-adrenergic receptor (β-AR) agonists during the finishing phase of feedlot steers.
Materials and Methods
Animal management and treatments
All procedures were approved by the Texas Tech University Institutional Animal Care and Use Committee (protocol number: 15016-02). Bos taurus crossbred steers (n = 32; 442 ± 17 kg) were obtained from a single source and processed upon arrival at the Burnett Center of Texas Tech University, Idalou, TX. Steers received ear tags for identification, were treated with a topical anthelmintic (Dectomax Pour-On solution, Zoetis USA, Florham Park, NJ), and vaccinated (Vista 5 SQ, Merck Animal Health, Summit, NJ; Vision CD, Merck Animal Health; Myco-Bac B, Texas Vet Lab, Inc., San Angelo, TX) in accordance with Texas Tech University health protocols. Steers were blocked by initial BW and organized in a randomized complete block design (four steers/pen; eight pens total; four pens/treatment). Pens within a block were randomly assigned one of two treatments: 1) control (CON; 0.0 g/[head (hd) · d] of additional zinc) or 2) zinc-added (ZnP; KemTRACE Zn to provide 1.0 g/[hd · d] additional dietary zinc; Kemin Industries, Inc., Des Moines, IA). Steers had ad libitum access to feed, using clean bunk management, with a 90% concentrate finishing diet designed to meet National Research Council (NRC, 1996) requirements for growing-finishing beef cattle (Table 1) that contained 14% crude protein (CP; DM basis), NRC (1996) recommended levels of supplemental vitamins and minerals, monensin (Rumensin: Elanco Animal Health, Greenfield, IN; 30 g/ton of DM), and tylosin tartrate (Tylan: Elanco Animal Health; 10 g/ton of DM) were used. All steers were fed ractopamine hydrochloride (RH; Optaflexx: Elanco Animal Health) at 300 mg/(hd · d) during the final 32 d of the feeding period. Animal health and welfare were monitored daily via visual assessment by an approved animal handler. The basal diet contained Zn (60 ppm DM basis) from ZnSO4. The Zn propionate, incorporated into a ground corn carrier, was added as a daily top-dress in each ZnP pen [0.45 kg/(pen · d)]. Daily feed deliveries were recorded, and orts were weighed weekly to calculate the feed intake.
Table 1.
Description of experimental diets (DM basis)1
| Ingredients, % DM2 | Value |
|---|---|
| Steam-flaked corn | 75.10 |
| Alfalfa hay, chopped | 10.38 |
| Cottonseed meal | 4.97 |
| Molasses | 2.94 |
| Supplement | 2.30 |
| Fat (yellow grease) | 2.43 |
| Urea | 0.97 |
| Calcium carbonate | 0.91 |
| Chemical composition, DM3 | |
| DM, % | 81.3 |
| CP, % | 14.9 |
| ADF, % | 8.7 |
| NDF, % | 14.4 |
| Ash, % | 4.4 |
| Total digestible nutrients, % | 89.9 |
| NEm, Mcal/kg | 2.24 |
| NEg, Mcal/kg | 1.55 |
| Zn content, mg/kg4 | |
| CON diet | 92.6 |
| ZnP diet | 200.1 |
| ZnP supplement | 8,888.9 |
1Diets were formulated to meet or exceed NRC (2000) requirements for growing–finishing beef cattle.
2Supplement composition (DM basis): 67.755% cottonseed meal, 15.000% NaCl, 10.000% KCl, 3.760% urea, 0.986% Zn sulfate, 0.750% Rumensin 90 (Elanco, Greenfield, IN), 0.506 Tylan 40 (Elanco), 0.500% ENDOX (Kemin Industries, Des Moines, IA), 0.196% copper sulfate, 0.167% manganese oxide, 0.157% vitamin E (500 IU/g), 0.125% selenium premix (0.2% Se), 0.083% iron sulfate, 0.010% vitamin A (1,000,000 IU/g), 0.003% ethylenediamine dihydroiodide, and 0.002% cobalt carbonate.
3Values as measured by proximate analysis of bunk samples on a DM basis except Diet DM (ServiTech Laboratories, Amarillo, TX).
4Tabular values were used to estimate dietary Zn based on the Zn sulfate and Zn propionate content of each diet. Basal only contained Zn sulfate. ZnP diet contained Zn sulfate at the basal level and the ZnP supplement provided at 0.45kg/4 hd pen.
Sample collection
BWs for all cattle were measured prior to the time of feeding on days 0, 42, 79, 107, and 111. Skeletal muscle tissue and blood samples were acquired on days 0, 42, 79, and 107 from a subset of black-hided steers that were closest to the mean pen BW (2 hd/pen; n = 16).
Skeletal muscle samples were obtained via biopsy from the longissimus muscle (LM) to evaluate biochemical and physiological changes. During the biopsy procedure, steers were restrained using a hydraulic squeeze chute. The area surrounding the incision site was shaved using a disposable razor, then sanitized using a solution of water, 7.5% povidone iodine surgical scrub (Betadine, Purdue Products, L.P., Stamford, CT), and 70% ethanol. A local anesthetic (lidocaine HCl, 20 mg/mL, 8 mL per biopsy) was administered subcutaneously in a 6-cm2 rhombus-shaped pattern (four injection sites, 2 mL lidocaine HCl per site) no less than 5 min prior to the biopsy incision. The area was sterilized using 70% ethanol and sterile gauze post-lidocaine administration, then a 1-cm-long incision was made using a sterile scalpel. A sterile 6-mm diameter Bergstrom biopsy needle was used to collect approximately 2 g of LM tissue. The sample was placed on sterile gauze in a covered plastic container to be taken to the sample preparation area. The incision was closed using veterinary tissue adhesive (VetBond, 3M Animal Care Products, St. Paul, MN) and coated with a protective aerosol bandage (AluShield, Neogen Corp, Lexington, KY) to aid in infection prevention during the healing process. The initial biopsy punctured the LM between the 12th and 13th rib, and the subsequent consecutive biopsies alternated laterally across the spine. Subsequent alternate biopsies progressed anteriorly along the spine to avoid previous surgery sites.
In the sample preparation area, the tissue was divided into portions for three purposes: immunohistochemical (IHC) analysis, ribonucleic acid (RNA) analysis, and protein analysis. The IHC sample was analyzed under a magnifying glass, and muscle fibers were identified. The fibers were placed parallel to each other on a 1 × 1.5-cm piece of corkboard and frozen in Premium Frozen Section Compound (VWR International, West Chester, PA) using isopentane cooled with dry ice. The samples on cork pieces were wrapped in foil, placed in sample bags (Whirl-Pak, NASCO, Fort Atkinson, WI), and stored on dry ice until transport back to Texas Tech University, Lubbock, TX. The portions for RNA analysis and protein analysis were sealed in sample bags, frozen in liquid N, and stored on dry ice until transport back to Texas Tech University. All skeletal muscle samples were stored at −80 °C until further processing.
Blood samples were collected via jugular venipuncture using silicone-coated glass vacuum blood collection tubes containing no additives (Vacutainer, BD Diagnostics, Franklin Lakes, NJ). Tubes were stored at 4 °C overnight to allow for clotting, then centrifuged at 1,200 × g for 15 min to separate serum. Serum samples were then stored at −20 °C until laboratory analysis.
Steers were transported 288 km to a commercial abattoir for harvest on day 111 after undergoing a 4-d observational period to allow for withdrawal from lidocaine. Carcass data were collected by West Texas A&M University Beef Carcass Research Center personnel and included hot carcass weight (HCW), 12th rib fat thickness (FT), LM area, percent kidney/pelvic/heart fat, yield grade, and marbling score.
Sample processing
LM biopsy sample processing and analysis for immunohistochemistry, RNA, and protein were adapted from the standard operating procedures in the Texas Tech University laboratory, previously established by Hergenreder et al. (2016).
IHC analysis
Embedded LM samples for IHC staining and analysis were moved from 80 °C to −20 °C to thaw for 24 h. Samples were removed from the cork, cut into 10-µm-thick cryosections with a cross-sectional orientation at −20 °C using a Leica CM1950 cryostat (Leica Biosystems, Buffalo Grove, IL), and affixed to positively charged glass slides (four slides per sample and five cryosections per slide; Superfrost Plus, VWR International). Cryosections were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS; Thermo Fisher Scientific) for 10 min at room temperature, then rinsed briefly twice in PBS followed by one 10-min bath in PBS at room temperature. A blocking solution consisting of 2% bovine serum albumin (MD Biomedicals, Solon, OH), 5% horse serum (Invitrogen), and 0.2% Triton X-100 (Thermo Fisher Scientific) in PBS was then applied to the fixed cryosections and allowed to incubate for 30 min at room temperature to prevent nonspecific antibody binding. The cryosections were incubated with primary antibodies in blocking solution for 1 h at room temperature. Slide 1 was evaluated using the following primary antibodies: 1:100 α-dystrophin, rabbit, Immunoglobulin G (IgG) (Thermo Fisher Scientific); 1:100 supernatant anti-myosin heavy chain (MHC)-I, mouse, IgG2b (BA-D5; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); and supernatant anti-MHC all except type IIX, mouse, IgG1 (BF-35, Developmental Studies Hybridoma Bank). For slide 2, 1:750 α-β-1 AR, rabbit, IgG (Abcam); 1:750 α-β-2 AR, chicken, IgY (Abcam); 1:500 α-β-3 AR, goat, IgG (Abcam) were used. The cryosections were rinsed in PBS for 5 min at room temperature three times. Secondary antibodies in the blocking solution were then applied to the cryosections for 30 min at room temperature in the dark. Slide 1 was treated with 1:1,000 goat α-rabbit, IgG, Alexa Fluor 488 (Invitrogen); 1:1,000 goat α-mouse, IgG1, Alexa Fluor 546 (Invitrogen); and 1:1,000 goat α-mouse, IgG2b, Alexa Fluor 633 (Invitrogen). Slide 2 was treated with 1:1,000 goat α-chicken, IgY, H & L, Alexa Fluor 488 (Abcam); 1:1,000 donkey α-rabbit, IgG, Alexa Fluor 546 (Invitrogen); and 1:1,000 donkey α-goat, IgG, Alexa Fluor 633 (Invitrogen). Following the incubation period, the slides were rinsed three times with PBS for 5 min at room temperature. Muscle fiber type and area slides were then cover-slipped using ProLong Gold with 4′6-diamidino-2-phenylindole mounting media (Life Technologies) and thin glass coverslips (VWR International). Slides were then left to cure in the dark for 36 h at room temperature. Slides used for β-AR density were cover-slipped with Aqua-Mount mounting media (Thermo Fisher Scientific) and stored at 4 °C for 24 h to cure.
Slides underwent 200× magnification with an inverted fluorescence microscope (Nikon Eclipse, Ti-E; Nikon Instruments Inc., Melville, NY) using a UV light source (Nikon Intensilight; C-HGFIE), and images were obtained using a CoolSnap ES2 monochrome camera. Five images of the LM IHC sections were randomly captured from each slide. Images were artificially colored and analyzed with NIS-Elements imaging software (Nikon Instruments Inc.). Muscle fibers in each image were identified and reported as a percentage of the total number of muscle fibers. The cross-sectional area of each fiber in each image was measured using NIS-Elements imaging software and reported on a square micrometer (µm) basis. β-ARs were identified on the slide, respectively, stained, counted, and densities are reported on a square millimeter (mm) basis.
RNA isolation and real-time quantitative reverse transcription-polymerase chain reaction
RNA from LM tissue was isolated with cold buffer containing TRI Reagent (Sigma, St. Louis, MO). Approximately, 1.5 g of frozen tissue was homogenized with TRI Reagent at a ratio of 0.5:1 g of tissue to milliliter reagent. The homogenate was pipetted into two microcentrifuge tubes (1 mL sample per tube), and 200 μL chloroform was added to each tube, vortexed for 30 s, and incubated for 5 min. The sample was then centrifuged at 15,000 × g for 15 min. The most superficial supernatant layer was pipetted into new microcentrifuge tubes. Cold isopropyl alcohol (250 μL) was added to the supernatant, mixed, and incubated for 10 min on ice. The samples were then centrifuged at 15,000 × g for 10 min. The supernatant was decanted, and the RNA pellets at the bottom of each tube were allowed to dry. Then, 500 μL of 75% ethanol was added to each tube to rinse and suspend the RNA pellets. Samples were then placed in a −80 °C freezer. Upon further analysis, samples were removed from the freezer and thawed on ice. Samples were then centrifuged at 15,000 × RPM for 10 min, ethanol was poured off, and the pellet was air-dried. Nuclease-free water (30 μL) was then added to each sample to dissolve the RNA pellets. The concentration of RNA was determined with a spectrophotometer at an absorbance of 260 nm using a NanoDrop 1000 (NanoDrop Products, Wilmington, DE). Samples were then treated with DNAse to remove any DNA contaminants using a DNA-free kit (Life Technologies, Grand Island, NY). The RNA then underwent reverse transcription to synthesize cDNA. The cDNA was used for real-time quantitative reverse transcription-PCR (RT-qPCR) to measure the abundance of MHC-I, MHC-IIA, and MHC-IIX mRNA as a fraction of total RNA isolated from muscle tissue. Bovine primers and probes for MHC-I, MHC-IIA, and MHC-IIX were the same as those presented in Winterholler et al. (2007). Assays were performed in the GeneAmp 7900HT Sequence Detection System (Applied Biosystems, Life Technologies) using thermal cycling parameters recommended by the manufacturer (40 cycles of 15 s at 95 °C and 1 min at 60 °C).
Protein extraction, Western blots, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Protein from the LM was isolated with whole muscle extraction buffer (WMEB; 2 % sodium dodecyl sulfate, 10 mM phosphate, pH 7.0). The samples were centrifuged at 15,000 × g for 15 min. The middle supernatant of the three layers was pipetted off and placed into microcentrifuge tubes. The protein samples were then diluted with WMEB to determine protein concentration using the Pierce BCA protein assay (Thermo Fisher Scientific, Fairlawn, NJ). Protein concentration was determined using a NanoDrop 1000 spectrophotometer at 562 nm. All samples were then diluted to the same concentration. Modified Wang’s tracking dye was added to Western blot samples, and MHC tracking dye was added to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) samples. Samples were denatured with β-mercaptoethanol and incubated for 2 min at 95 °C.
Samples for Western blots were loaded onto Novex 4–12% Bis-Tris gels (Invitrogen, Grand Island, NY), and protein was separated by gel electrophoresis. The gels were run for approximately 35 min at 165 V. Proteins were transferred onto a nitrocellulose membrane (Invitrogen) for 7 min. Following the transfer, the membrane was incubated with nonfat dry milk (BIO-RAD, Hercules, CA) in 1× tris-buffered saline (TBS) for 1 h at room temperature to block nonspecific antibody binding. The blocking solution was then removed from the membrane. The appropriate primary antibodies 1:1,000 α-β-1 AR, rabbit, IgG (Abcam, Cambridge, MA); 1:1,000 α-β-2 AR, goat, IgG (Abcam); and 1:1,000 α-β-3 AR, goat, IgG (Abcam) were mixed into 1× TBS-Tween (0.04%) solution, added to the membrane, and incubated for 2 h (β 1-AR) or 1 h (β 2-AR and β 3-AR) at room temperature. The membrane was then rinsed three times for 10 min in TBS-Tween. Alexa fluorescent antibodies, goat α-rabbit, IgG, Alexa Fluor 633 (Invitrogen), and donkey α-goat, IgG, Alexa Fluor 633 (Invitrogen), were then added at a dilution of 1:2,000 in TBS-Tween to the membrane and incubated for 1 h at room temperature in darkness. The membranes were then rinsed three times for 10 min in TBS-Tween in unlit conditions. The membranes were dried and visualized using the Imager Scanner II and ImageQuant TL programs. Densitometry measurements were made on the bands corresponding to β 1-AR, β 2-AR, and β 3-AR using a molecular weight standard for reference (Precision Plus Protein All Blue Standards; BIO-RAD).
For SDS-PAGE, 6% acrylamide separating gels with 4% acrylamide stacking gels were prepared and allowed to polymerize at 4 °C for 24 h. Gels were loaded with samples and an internal reference standard used for quantification, and protein was separated by gel electrophoresis, approximately 72 h at 100 V. Gels were placed in 300 mL Coomassie Fluor Orange (Life Technologies) for 30 min at room temperature in a light-protected container. The Coomassie Fluor Orange was drained off each gel, then the gel was briefly rinsed in 7.5% acetic acid followed by 18-ω filtered water. The gels were then visualized using the Imager Scanner II and ImageQuant TL programs. Densitometry measurements were made on the bands corresponding to MHC I and II. Due to similar molecular weights, MHC IIA and IIX were quantified as total MHC II.
Sera nonesterified fatty acids and urea-N
Blood serum samples were analyzed for nonesterified fatty acid (NEFA) content using the NEFA-HR(2) kit (Wako Diagnostics, Richmond, VA), glucose concentrations using an enzymatic Autokit Glucose kit (Wako Diagnostics), insulin levels using a bovine-specific insulin ELISA (Alpco Diagnostics, Salem, NH), and serum urea nitrogen (SUN) using a Urea Nitrogen Liqui-UV kit (Stanbio Laboratory, Boerne, TX).
Statistical analyses
Growth performance and carcass data continuous in nature were analyzed as a randomized complete block design using the GLIMMIX procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC), considering pen as the experimental unit. The statistical model included the fixed effect of treatment, while the block was considered a random effect. Least squares means were generated using the LSMEANS statement of SAS. Data were separated and denoted to be different using the pairwise comparisons PDIFF and LINES option of SAS when a significant preliminary F-test was detected. An α level of 0.05 was used to determine the significance, with tendencies discussed at P-values between 0.05 and 0.15.
IHC and sera metabolite data were analyzed as a randomized complete block design with repeated measures over time, using the MIXED procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC). Pen was considered the experimental unit for β-AR and sera metabolite data. The statistical model for the effect of treatment and days on feed for β-AR and sera metabolites included the fixed effect of implant, day, and the interaction of implant × day. Block was included as a random effect. A repeated statement was used and included day as the repeated variable. The covariance structure with the lowest Akaike information criterion was used. All results are reported as least-squares means. Data were separated using the PDIFF option of SAS if a significant preliminary F-test was detected. The Kenward–Roger adjustment was utilized to correct the degrees of freedom. An α-level of 0.05 was used to determine the significance, with tendencies discussed at P-values between 0.05 and 0.15.
Results and Discussion
Effects of Zn propionate on growth performance and carcass characteristics
Steers fed ZnP had greater ADG (P = 0.02; Table 2) and gain to feed ratio (G:F; P = 0.03) during the beta-agonist feeding period compared with CON, but other growth performance variables did not differ (P > 0.05). Numerically, the mean final BW for ZnP was 13 kg heavier than CON. Spears (1989) reported improved ADG and G:F in lambs dosed orally with 300 mg of Zn and greater BW in heifers supplemented with 25 mg/kg of dietary Zn as ZnO and Zn-methionine (ZnMet). A tendency for improved feed efficiency for ZnP, compared with CON, occurred during the day 43 to 79 interim period (P = 0.15) and tended to be preserved in the analysis of the total study, day 0 to 111 (P = 0.13). Nunnery et al. (2007) reported an improvement in feed efficiency during a 168-d finishing period in heifers supplemented with 75 mg/kg of dietary Zn. Supplementation of ZnP resulted in a 6.8% increase in ADG and a 5.1% improvement in feed efficiency compared with non-supplemented heifers (Nunnery et al., 2007). However, another study detected no differences for BW, DMI, ADG, or G:F in Holstein steers fed ZnMet (an additional 720 mg Zn/[hd · d] compared with control) and zilpaterol hydrochloride (Hergenreder et al., 2016).
Table 2.
Effects of Zn propionate supplementation in combination with RH on feedlot growth performance
| Treatment1 | ||||
|---|---|---|---|---|
| Item | CON | ZnP | SEM | P-value |
| Initial BW2, kg | 422 | 420 | 1.98 | 0.32 |
| Final BW3, kg | 577 | 590 | 9.95 | 0.28 |
| Day 0 to 424 | ||||
| ADG, kg | 1.7 | 1.8 | 0.12 | 0.48 |
| DMI, kg | 9.4 | 9.3 | 0.15 | 0.50 |
| G:F | 0.183 | 0.197 | 0.0110 | 0.33 |
| Day 43 to 79 | ||||
| ADG, kg | 1.7 | 1.8 | 0.08 | 0.19 |
| DMI, kg | 9.8 | 9.9 | 0.08 | 0.75 |
| G:F | 0.179 | 0.192 | 0.0070 | 0.15 |
| Day 80 to 111 | ||||
| ADG, kg | 0.7a | 0.9b | 0.04 | 0.02 |
| DMI, kg | 8.8 | 9.0 | 0.09 | 0.20 |
| G:F | 0.087a | 0.107b | 0.0050 | 0.03 |
| Day 0 to 111 | ||||
| ADG, kg | 1.4 | 1.5 | 0.08 | 0.16 |
| DMI, kg | 9.3 | 9.3 | 0.06 | 0.77 |
| G:F | 0.157 | 0.172 | 0.0070 | 0.13 |
1Con, control, 0.0 g/[hd·d] of additional Zn; ZnP, zinc propionate, 1.0 g/[hd·d] additional Zn.
2No shrink applied to initial BW.
3Final BW with a 2% shrink.
4To adjust for fill, a 4% shrink was applied to interim BW for growth performance calculations.
a,bMeans within the same row with different superscripts are statistically different.
No differences (P > 0.05; Table 3) in HCW, ribeye area (REA), FT, yield grade, marbling score, or dressing percentage observed between ZnP and CON. There was a tendency (P = 0.14) for ZnP cattle to have a greater percentage of the kidney, pelvic, and heart fat (KPH). Recently, studies supplementing 300 mg Zn/kg diet DM, as ZnSO4 and ZnO, with and without beta-adrenergic agonist (β-AA), in steers and heifers showed various carcass traits indicating an increase in fat deposition as a result of Zn (Van Bibber-Krueger et al., 2017a, 2017b). Steers supplemented with 300 mg Zn/kg diet DM, with and without zilpaterol hydrochloride, had a tendency to have greater KPH and grade United States Department of Agriculture (USDA) over steers receiving 60 mg (Van Bibber-Krueger et al., 2017b). Current study results indicate that there is improved live weight gain during the RH feeding period and possibly an increase in fat development in animals fed greater levels of Zn but no effect on carcass characteristics.
Table 3.
Effects of Zn propionate supplementation in combination with RH on carcass characteristics
| Treatment1 | ||||
|---|---|---|---|---|
| Item | CON | ZnP | SEM | P-value |
| HCW, kg | 377 | 387 | 6.72 | 0.27 |
| REA, cm3 | 91.2 | 92.0 | 2.06 | 0.74 |
| FT, cm | 1.24 | 1.09 | 0.130 | 0.28 |
| KPH, % | 1.8 | 1.9 | 0.53 | 0.14 |
| USDA yield grade | 2.8 | 2.6 | 0.08 | 0.18 |
| Marbling score2 | 460 | 435 | 18.8 | 0.28 |
| Dressing percentage, %3 | 65.3 | 65.6 | 0.45 | 0.88 |
1Con, control, 0.0 g/[hd·d] of additional Zn; ZnP, zinc propionate, 1.0 g/[hd·d] additional Zn.
2400, USDA Small00; 500, USDA Modest00.
3Calculated using HCW/Final BW with a 2% shrink.
MHC fiber type distribution, area, and relative expression
No treatment × day interactions (P > 0.05) or treatment effects (P > 0.05) were detected for percentage of muscle fiber type (Table 4). There was a tendency (P = 0.08) for a decrease in the percentage of MHC-I fibers on day 42. However, MHC IIA and IIX fibers did not differ (P > 0.05) over time. No differences in treatment or day and no treatment × day interactions (P > 0.05) were detected for the area of MHC I or IIA (P > 0.05; Table 5). There was a difference (P = 0.04) between treatments for the MHC-IIX fiber area, where control cattle averaged a greater type IIX cross-sectional area. Although CON had a greater IIX fiber area, the REA likely did not increase because the total average fiber areas were 5,985 and 5,257 µm2 for CON and ZnP on day 107, respectively. In addition to this relatively small difference, the similarities between treatments for MHC I and IIA sizes likely contributed to the REA size. The percentage of IIX fibers also increased (P = 0.02) over time, which is expected as steers mature. Hergenreder et al. (2016) observed an increase for MHC-I distribution and a decrease for MHC-IIX fibers in ZnMet compared with control, which contradicted the results of the current study. Based on the differences between the absorption and metabolism of propionate and methionine, these equivocal results are likely due to the different sources of complexed Zn. The analysis of mRNA for MHC I, IIA, and IIX revealed no differences (P > 0.05; Table 6) between ZnP and CON. Hergenreder et al. (2016) reported a decrease in MHC-IIX mRNA concentrations in ZnMet compared with control. There was no difference (P > 0.05; Table 7) in MHC I or II relative protein concentrations between treatments. The addition of Zn and RH to bovine satellite cells in a cell culture experiment did not affect MHC I, IIA, and IIX, or β 1-AR and β 2-AR, supporting the results in the current study (Hergenreder et al., 2016). The differences in study results suggest that the utilization of ZnMet and ZnP differ in the cattle, which may be due to the absorption differences between methionine and propionate, but within the current study, additional dietary Zn seems to have a minimal effect on muscle fiber development.
Table 4.
Effects of Zn propionate supplementation in combination with RH on fiber type distribution of LM biopsies of feedlot steers
| Percent fiber type | |||
|---|---|---|---|
| Treatment | Type I | Type IIA | Type IIX |
| CON | |||
| Day 0 | 25.09 | 39.27 | 35.63 |
| Day 42 | 21.46 | 39.60 | 38.94 |
| Day 79 | 26.40 | 39.20 | 34.41 |
| Day 107 | 26.93 | 37.27 | 35.80 |
| ZnP | |||
| Day 0 | 25.67 | 36.15 | 38.18 |
| Day 42 | 19.10 | 43.90 | 37.00 |
| Day 79 | 24.40 | 40.54 | 34.36 |
| Day 107 | 26.23 | 37.27 | 36.50 |
| SEM | 9.676 | 10.963 | 8.271 |
| P-values | |||
| Treatment | 0.658 | 0.831 | 0.867 |
| Day | 0.078 | 0.426 | 0.606 |
| Trt × day | 0.935 | 0.698 | 0.841 |
Table 5.
Effects of Zn propionate supplementation in combination with RH on fiber type area of LM biopsies of feedlot steers
| Area, µm2 | |||
|---|---|---|---|
| Treatment | Type I | Type IIA | Type IIX |
| CON | |||
| Day 0 | 4,018.7 | 4,177.1 | 5,752.7b |
| Day 42 | 4,186.5 | 5,133.6 | 6,811.5ab |
| Day 79 | 4,887.0 | 5,511.8 | 6,759.7ab |
| Day 107 | 4,480.2 | 5,375.3 | 7,752.0a |
| ZnP | |||
| Day 0 | 4,097.8 | 4,778.7 | 5,255.8b |
| Day 42 | 4,878.2 | 5,399.8 | 6,287.8ab |
| Day 79 | 4,073.6 | 4,739.1 | 5,719.0b |
| Day 107 | 4,795.7 | 5,162.5 | 5,684.7b |
| SEM | 63.61 | 60.03 | 74.24 |
| P-values | |||
| Treatment | 0.500 | 0.790 | 0.038 |
| Day | 0.232 | 0.186 | 0.015 |
| Trt × day | 0.250 | 0.227 | 0.294 |
a,bMeans within the same column with the same superscript are not statistically different.
Table 6.
Effects of Zn propionate supplementation in combination with RH on relative mRNA concentrations of MHC-I, MHC-IIA, and MHC-IIX genes in LM tissue
| Treatment1 | ||||
|---|---|---|---|---|
| Gene | CON | ZnP | SEM | P-value |
| MHC-I | 0.673 | 0.602 | 0.061 | 0.497 |
| MHC-IIA | 1.705 | 1.314 | 0.189 | 0.346 |
| MHC-IIX | 0.992 | 0.976 | 0.1067 | 0.894 |
1Con, control, 0.0 g/[hd·d] of additional Zn; ZnP, zinc propionate, 1.0 g/[hd·d] additional Zn.
Table 7.
Effects of Zn propionate supplementation in combination with RH on relative protein concentrations of β 1-AR, β 2-AR, β 3-AR, MHC-I, and MHC-II in LM tissue
| Treatment1 | ||||
|---|---|---|---|---|
| Item | CON | ZnP | SEM | P-value |
| Receptor | ||||
| β 1 | 8,182.7 | 8,559.2 | 290.51 | 0.42 |
| β 2 | 16,819.1 | 16,481.0 | 629.02 | 0.77 |
| β 3 | 13,496.0 | 12,860.1 | 526.28 | 0.55 |
| MHC | ||||
| I | 26,628.4 | 28,091.8 | 862.37 | 0.51 |
| II | 31,076.8 | 33,280.6 | 1,094.97 | 0.45 |
1Con, control, 0.0 g/[hd·d] of additional Zn; ZnP, zinc propionate, 1.0 g/[hd·d] additional Zn.
Influence of Zn propionate on β-AR density and expression
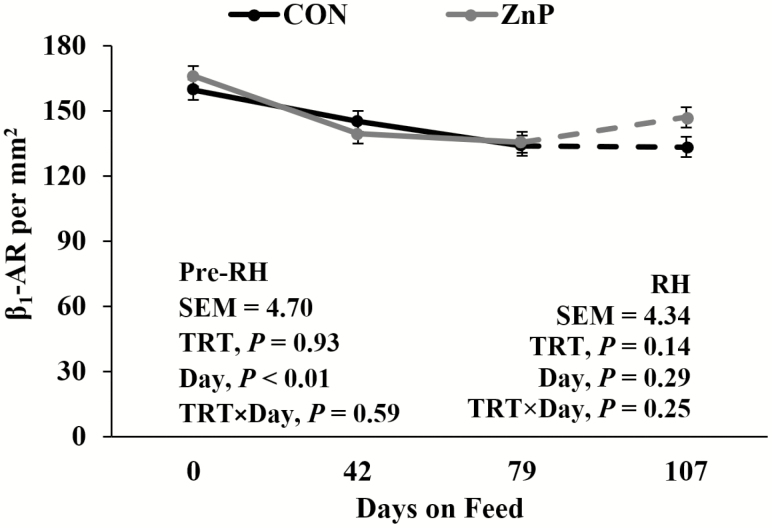
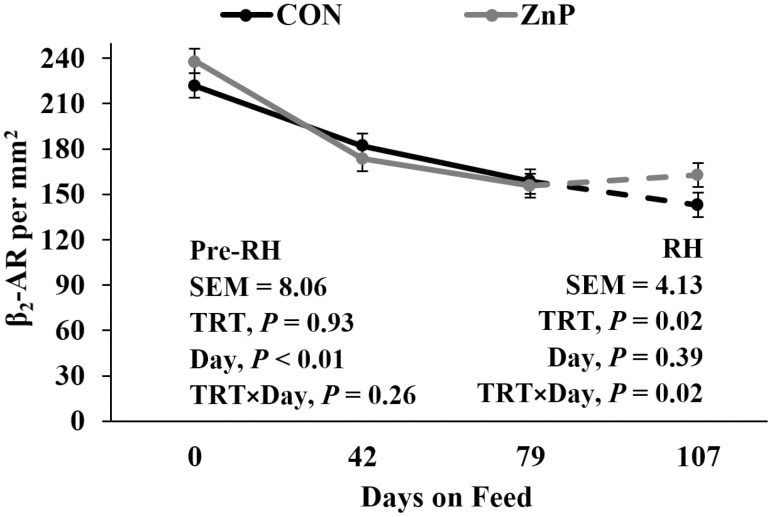
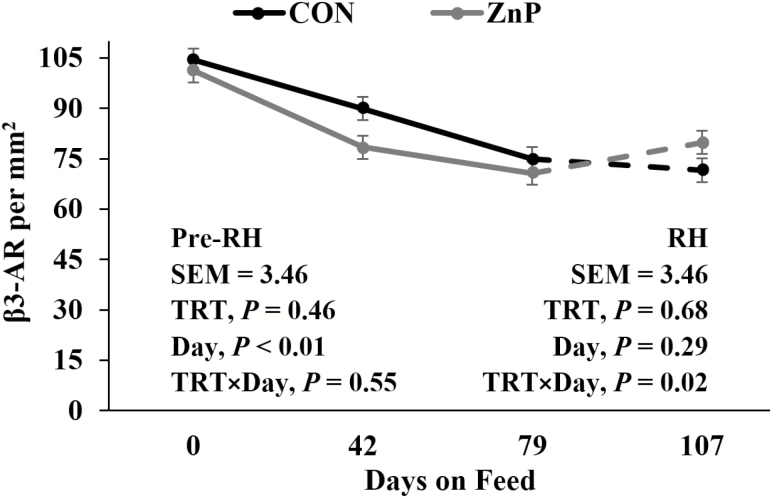
Western blotting of β 1-AR, β 2-AR, and β 3-AR revealed no differences (P > 0.05; Table 7) in β 1-AR, β 2-AR, and β 3-AR relative protein concentrations between treatments. As determined by IHC staining (Figure 1), there were no interactions or differences between treatments for β 1-AR (P > 0.05; Figure 2) in LM tissue. A decrease in β 1-AR has been shown in response to ZnMet (Hergenreder et al., 2016). There was a tendency for a treatment × day interaction for β 2-AR (P = 0.15; Figure 3) as well as β 3-AR (P = 0.09; Figure 4) throughout the RH phase. A treatment × day interaction was observed in β 2-AR (P = 0.02) and β 3-AR (P = 0.02) during the RH feeding period, where the abundance of the receptors increased with ZnP but did not change in CON.
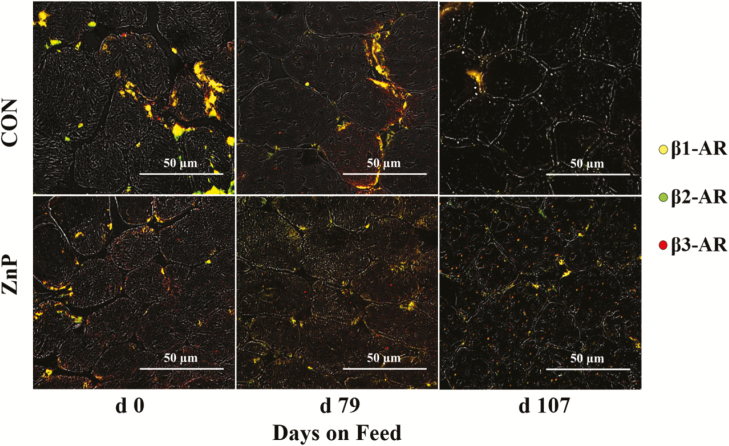
Figure 1.
Example of IHC staining of cross-sectional cryosections of skeletal muscle fibers for β-AR in Zn propionate-supplemented and control finishing steers pre- and post-RH feeding. An inverted fluorescence microscope was used at 200× magnification for imaging, and five images of the LM IHC sections were randomly captured from each slide. Then the receptors were identified, counted, and reported as a density on an mm2 basis. For visualization purposes, these images were selected as an example with a scale bar of 50 µm for reference.
Figure 2.
Effects of zinc supplementation on β 1-AR density prior to and during the RH feeding period (day 79 to 107). Mean receptor density for β 1-AR declined (P < 0.01; pooled standard error of the mean [SEM] = 4.70) prior to RH but did not change (P = 0.29) during the RH. For the last 28 d, a numerical difference between treatments was seen, but no interaction was detected (P = 0.25).
Figure 3.
Effects of zinc supplementation on β 2-AR density prior to and during the RH feeding period (day 79 to 107). Mean receptor density was different between over time for β 2-AR declined (P < 0.01; pooled standard error of the mean [SEM] = 8.06) prior to RH. An interaction (P = 0.02) was detected during RH.
Figure 4.
Effects of zinc supplementation on β 3-AR density prior to and during the RH feeding period (day 79 to 107). Mean receptor density for β 3-AR declined (P < 0.01; pooled standard error of the mean [SEM] = 3.46) prior to RH. An interaction (P = 0.02) was detected during RH.
It has been shown that multiple allosteric-binding sites for zinc are available on β 2-AR (Mersmann, 1998; Swaminath et al., 2002). These allosteric sites affect both agonist affinity and the ability of an antagonist to bind (Swaminath et al., 2003). Avendaño-Reyes et al. (2006) discussed the desensitization of β-AR as a result of prolonged administration of β-adrenergic agonists. Sissom et al. (2007) observed a tendency for RH to increase β2-AR mRNA and a numerical increase of β 3-AR mRNA associated with RH in conjunction with an implant by RH interaction. These data suggest that an upregulation of β 2-AR and β 3-AR occurs in feedlot steers in response to ZnP supplementation during the RH feeding period, which may prevent the desensitization of β-AR during the beta agonist-feeding period. The desensitization due to the conformational shift of the receptor post-ligand binding may be combatted by the ability of Zn to allosterically stabilize the protein shape, which would allow the continued presence of receptor for ligand binding over time. This rebound of β 2-AR and β 3-AR density in LM seen via IHC staining in ZnP indicates agonist affinity maintenance through the allosteric effect of zinc.
Alterations of sera concentrations of NEFAs and urea-N
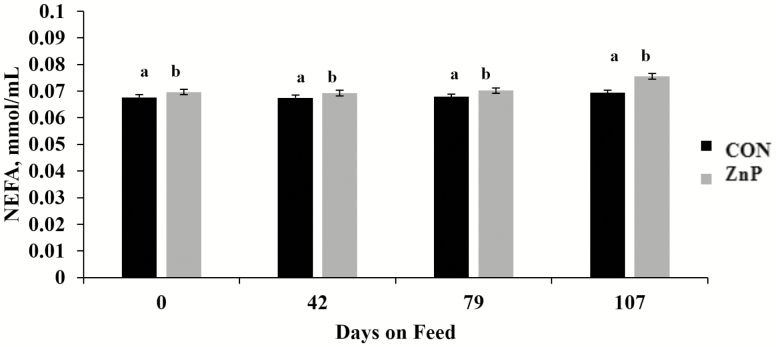
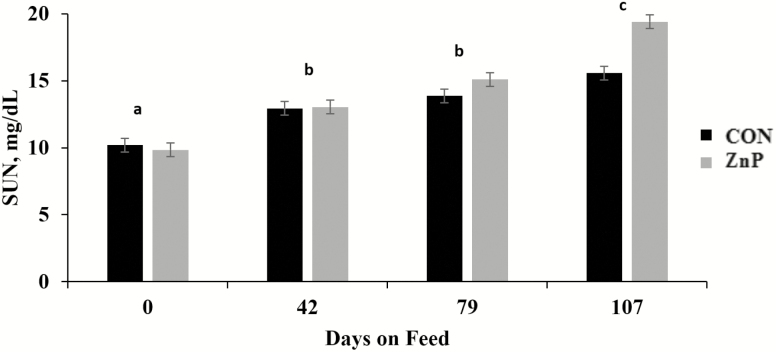
There was no treatment × day interaction (P > 0.05) for either NEFA or SUN, while ZnP-fed cattle had greater (P < 0.01) NEFA compared with CON (Figure 5). Such a pattern was not observed (P = 0.26) for SUN concentrations between treatments. Regardless of dietary treatment, SUN concentrations increased by day (P < 0.01; Figure 6), while NEFA tended (P = 0.13) to shift over time, with day 107 increasing relative to samples taken prior.
Figure 5.
Effects of zinc supplementation on serum NEFA on days 0, 42, 79, and 107. Mean concentrations of NEFA were different between treatments (CON = 0.068 mmol/mL and ZnP = 0.071 mmol/mL; P = 0.001; pooled standard error of the mean [SEM] = 0.001). Treatments with the same superscript (a and b) are not different.
Figure 6.
Effects of Zn propionate supplementation on SUN concentration on days 0, 42, 79, and 107. There was no difference in mean SUN concentrations between treatments (P = 0.263). However, mean SUN concentrations did increase by day (P < 0.001). Days with the same superscript (a-c) are not different. The error bar denotes the pooled standard error of the mean (SEM = 0.513).
In the current study, a numerical greater mean NEFA values were observed for ZnP from day 0, which indicated that group variation not associated with treatment. However, the tendency for all steers’ NEFA concentrations to increase during the β-AA feeding period indicated an expected metabolic shift from lipogenesis to the mobilization of fatty acids to support the energetic demands of protein accretion (Abney et al., 2007). Parr et al. (2014) reported a temporary increase in NEFA concentrations during the zilpaterol hydrochloride feeding period and explained this as an increase in the muscular demand for energy. However, Bryant et al. (2010) observed no effect on NEFA concentrations in steers fed RH. In both studies, the use of a β-AA resulted in decreased SUN concentrations, which was attributed to the anabolic state caused by β-AA (Bryant et al., 2010; Parr et al., 2014). A more recent study by Carmichael et al. (2018) showed that steers supplemented with a Zn–amino acid complex (145 mg Zn/kg DM) or fed RH had increased nitrogen retention. As β-AA affected animals metabolically, they retained nitrogen for muscle-protein accretion. However, the cause of the nitrogen retention for the Zn–amino acid-fed cattle was not determined. Despite the conflicting results of the previous studies, as animals mature, dietary protein is utilized less for lean muscle accretion and N is left in circulation, supporting the increased SUN over time in this study.
Conclusions
Zinc supplementation at 1.0 g/(hd · d) from Zn propionate improved the growth performance of finishing steers during the beta-agonist feeding period. Results indicated that ZnP supplementation modified fat metabolism but did not mitigate the increased NEFA levels during RH feeding. Although the muscle fiber type data did not elucidate how ZnP affects protein deposition or muscle fiber development, the decrease in cross-sectional area for type IIX fibers in treatment cattle indicates further investigation would be beneficial. The ZnP supplementation may aid the prevention of β-AR desensitization through the upregulation of β 2-AR and β 3-AR occurring in feedlot steers during the RH feeding period. Further investigation of the effect of ZnP, and its interaction with β-adrenergic agonists, is warranted.
Glossary
Abbreviations
- AA
adrenergic agonist
- ADG
average daily gain
- AR
adrenergic receptor
- BW
body weight
- CP
crude protein
- DM
dry matter
- DMI
dry matter intake
- FT
fat thickness
- G:F
gain to feed ratio
- HCW
hot carcass weight
- IHC
immunohistochemical
- KPH
kidney
- pelvic
heart fat
- LM
longissimus muscle
- MHC
myosin heavy chain
- NEFA
nonesterified fatty acid
- PBS
phosphate-buffered saline
- REA
ribeye area
- RH
ractopamine hydrochloride
- RNA
ribonucleic acid
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SUN
serum urea nitrogen
- TBS
tris-buffered saline
- WMEB
whole muscle extraction buffer
Conflict of interest statement
W.R. and J.E.H. are employed by Kemin Animal Nutrition and Health North America, the developing company for the investigated product. B.C.B. is currently employed by Zoetis, but at the time when this research was conducted, he was employed by Texas Tech University. Other authors have no conflicts of interest to declare.
Literature Cited
- Abney C. S., Vasconcelos J. T., McMeniman J. P., Keyser S. A., Wilson K. R., Vogel G. J., and Galyean M. L.. . 2007. Effects of ractopamine hydrochloride on performance, rate and variation in feed intake, and acid-base balance in feedlot cattle. J. Anim. Sci. 85:3090–3098. doi: 10.2527/jas.2007-0263 [DOI] [PubMed] [Google Scholar]
- Avendaño-Reyes L., Torres-Rodríguez V., Meraz-Murillo F., Pérez-Linares C., Figueroa-Saavedra F., and Robinson P.. . 2006. Effects of two β-adrenergic agonists on finishing performance, carcass characteristics, and meat quality of feedlot steers. J. Anim. Sci. 84(12):3259–3265. doi: 10.2527/jas.2006-173 [DOI] [PubMed] [Google Scholar]
- Brinkhaus F., Mann J., Zorich C., and Greaves J. A.. . 1998. Bioavailability of zinc propionate in dogs. J. Nutr. 128(12 Suppl):2596S–2597S. doi: 10.1093/jn/128.12.2596S [DOI] [PubMed] [Google Scholar]
- Bryant T. C., Engle T. E., Galyean M. L., Wagner J. J., Tatum J. D., Anthony R. V., and Laudert S. B.. . 2010. Effects of ractopamine and trenbolone acetate implants with or without estradiol on growth performance, carcass characteristics, adipogenic enzyme activity, and blood metabolites in feedlot steers and heifers. J. Anim. Sci. 88:4102–4119. doi: 10.2527/jas.2010-2901 [DOI] [PubMed] [Google Scholar]
- Carmichael R. N., Genther-Schroeder O. N., Blank C. P., Deters E. L., Hartman S. J., Niedermayer E. K., and Hansen S. L.. . 2018. The influence of supplemental zinc and ractopamine hydrochloride on trace mineral and nitrogen retention of beef steers. J. Anim. Sci. 96:2939–2948. doi: 10.1093/jas/sky177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada T., Yamasaki S., Nishida K., Murakami M., and Hirano T.. . 2011. Zinc homeostasis and signaling in health and diseases. J. Biol. Inorg. Chem. 16(7):1123–1134. doi: 10.1007/s00775-011-0797-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergenreder J. E., Legako J. F., Dinh T. T. N., Spivey K. S., Baggerman J. O., Broadway P. R., Beckett J. L., Branine M. E., and Johnson B. J.. . 2016. Zinc methionine supplementation impacts gene and protein expression in calf-fed Holstein steers with minimal impact on feedlot performance. Biol. Trace Elem. Res. 171:315–327. doi: 10.1007/s12011-015-0521-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersmann H. J. 1998. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J. Anim. Sci. 76:160–172. doi: 10.2527/1998.761160x [DOI] [PubMed] [Google Scholar]
- Miao X., Sun W., Fu Y., Miao L., and Cai L.. . 2013. Zinc homeostasis in the metabolic syndrome and diabetes. Front. Med. 7:31–52. doi: 10.1007/s11684-013-0251-9 [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC). 1996. Nutrient requirements of beef cattle.
- National Academies of Sciences, Engineering, and Medicine (NASEM) 2016. Nutrient requirements of beef cattle. 8th rev. ed. Washington (DC): The National Academies Press. [Google Scholar]
- Nunnery G. A., Vasconcelos J. T., Parsons C. H., Salyer G. B., Defoor P. J., Valdez F. R., and Galyean M. L.. . 2007. Effects of source of supplemental zinc on performance and humoral immunity in beef heifers. J. Anim. Sci. 85:2304–2313. doi: 10.2527/jas.2007-0167 [DOI] [PubMed] [Google Scholar]
- Parr S. L., Brown T. R., Ribeiro F. R., Chung K. Y., Hutcheson J. P., Blackwell B. R., Smith P. N., and Johnson B. J.. . 2014. Biological responses of beef steers to steroidal implants and zilpaterol hydrochloride. J. Anim. Sci. 92:3348–3363. doi: 10.2527/jas.2013-7221 [DOI] [PubMed] [Google Scholar]
- Sissom E. K., Reinhardt C. D., Hutcheson J. P., Nichols W. T., Yates D. A., Swingle R. S., and Johnson B. J.. . 2007. Response to ractopamine-HCl in heifers is altered by implant strategy across days on feed. J. Anim. Sci. 85:2125–2132. doi: 10.2527/jas.2006-660 [DOI] [PubMed] [Google Scholar]
- Spears J. W. 1989. Zinc methionine for ruminants: relative bioavailability of zinc in lambs and effects of growth and performance of growing heifers. J. Anim. Sci. 67:835–843. doi: 10.2527/jas1989.673835x [DOI] [PubMed] [Google Scholar]
- Swaminath G., Lee T. W., and Kobilka B.. . 2003. Identification of an allosteric binding site for Zn2+ on the β2 adrenergic receptor. J. Biol. Chem. 278(1):352–356. doi: 10.1074/jbc.M206424200 [DOI] [PubMed] [Google Scholar]
- Swaminath G., Steenhuis J., Kobilka B., and Lee T. W.. . 2002. Allosteric modulation of beta2-adrenergic receptor by Zn(2+). Mol. Pharmacol. 61:65–72. doi: 10.1124/mol.61.1.65 [DOI] [PubMed] [Google Scholar]
- Van Bibber-Krueger C. L., Miller K. A., Amachawadi R. G., Scott H. M., Gonzalez J. M., and Drouillard J. S.. . 2017a. Interaction between supplemental zinc oxide and zilpaterol hydrochloride on growth performance, carcass traits, and blood metabolites in feedlot steers. J. Anim. Sci. 95:5573–5583. doi: 10.2527/jas2017.1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bibber-Krueger C. L., Miller K. A., Amachawadi R. G., Scott H. M., Gonzalez J. M., and Drouillard J. S.. . 2017b. Interaction between supplemental zinc oxide and zilpaterol hydrochloride on growth performance, carcass traits, and blood metabolites in feedlot steers. J. Anim. Sci. 95:5573–5583. doi: 10.2527/jas2017.1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterholler S. J., Parsons G. L., Reinhardt C. D., Hutcheson J. P., Nichols W. T., Yates D. A., Swingle R. S., and Johnson B. J.. . 2007. Response to ractopamine-hydrogen chloride is similar in yearling steers across days on feed. J. Anim. Sci. 85:413–419. doi: 10.2527/jas.2006-555 [DOI] [PubMed] [Google Scholar]