Abstract
The emerging market of frozen meat emphasizes the need to better understand beef surface discoloration and the ideal parameters of freezing beef to retain an acceptable color. The objectives of this study were to determine the impacts of myoglobin oxygenation level prior to freezing and frozen storage duration on frozen beef color. USDA Choice strip loins (n = 36) were aged for 4 d or 20 d. Steaks were randomly assigned to a myoglobin oxygenation level [deoxygenated (DeOxy; immediately packaged after cutting), oxygenated (Oxy; oxygenated in air for 30 min), or highly oxygenated (HiOxy; packaged for 24 h in 80% O2)]. Steaks were then vacuum packaged in oxygen permeable or impermeable film and immediately frozen (−5 °C). Following either 0, 2, 4, or 6 mo of frozen storage, steaks were removed from the packaging and immediately analyzed for instrumental color (L*, a*, and b*), percent oxymyoglobin, metmyoglobin, and deoxymyoglobin, delta E, redness ratio, a*:b* ratio, hue angle, subjective discoloration, and lipid oxidation. The HiOxy steaks had greater oxygen penetration and the greatest a* values compared with DeOxy and Oxy steaks, regardless of packaging (P < 0.0005). With 4 d of aging, HiOxy steaks had greater a* values than DeOxy and Oxy at all storage times (P = 0.0118). The HiOxy steaks aged for 20 d and frozen for 6 mo had significantly higher delta E values than all other myoglobin oxygenation levels and postmortem aging periods (P < 0.0001). Redness and percent oxymyoglobin were highest for HiOxy steaks within each storage period (P < 0.0002). The HiOxy steaks had the highest percent oxymyoglobin and DeOxy had the lowest percent oxymyoglobin within each aging and storage period (P < 0.01). Conversely, DeOxy steaks had the highest percent metmyoglobin and HiOxy had the lowest percent metmyoglobin when packaged in impermeable film (P < 0.0001). The HiOxy steaks from 20 d of aging had the highest discoloration compared with 4 d aging and more discoloration than all other myoglobin treatments at 6 mo of storage (P < 0.0001). The HiOxy 20 d aged steaks exhibited the highest lipid oxidation values at 2, 4, and 6 mo (P = 0.0224) and HiOxy steaks exhibited a brighter and deeper cherry red color compared with the DeOxy steaks. The HiOxy steaks were greater in redness or similar when compared with Oxy steaks, but experienced more detrimental effects when frozen storage was extended.
Keywords: discoloration, frozen storage, oxymyoglobin
Introduction
Color is often viewed as the number one factor consumers use when purchasing beef other than cost (Hood, 1980; Kropf, 1980; Carpenter et al., 2001). Therefore, maintaining a bright cherry red color is a common concern for the meat industry in attracting consumers. It has been reported that 15% of retail beef is discounted due to discoloration resulting in an economic loss of $1 billion annually (Smith et al., 2000; Mancini and Hunt, 2005). Discoloration is caused by an accumulation of metmyoglobin on the surface of beef due to the oxidation of oxymyoglobin and deoxymyoglobin. Typically, metmyoglobin can be reduced back to deoxymyoglobin and oxygenated to oxymyoglobin (bright cherry red color); however, this is no longer possible when reducing components of the metmyoglobin reducing activity (MRA) are depleted. Meat color, specifically the reduction and oxidation of myoglobin and the bound ligand, depends on many intrinsic (pH, species, breed, sex, animal maturity, muscle to muscle variation, oxygen consumption, MRA, and lipid oxidation) and extrinsic factors (diet, electrical stimulation, postmortem aging, temperature, frozen storage, and packaging) (Hood, 1980; Renerre, 1990; Mancini and Hunt, 2005; Suman and Joseph, 2013).
While beef is typically packaged and sold fresh, the market of frozen beef has started to grow. The global meat export industry is worth over $13 billion and freezing plays an essential role in that industry, helping to ensure the safety of meat being supplied all over the world (Leygonie et al., 2012). The extended shelf-life of frozen beef allows the industry to export beef on an international basis when compared to fresh beef. One emerging market for frozen beef products is meal delivery service companies and the opportunity for increased revenue of beef products due to the increased shelf life. Recently, meal-kit delivery has a market of $4.65 billion in 2017 and is expected to grow to $11.6 billion by 2022 (Progressive Grocer, 2017). With the opportunity for increased product sales, it is essential that a better understanding is developed of how different myoglobin oxygenation levels retain color through frozen storage. Therefore, the objectives of this study were to determine the impacts of myoglobin oxygenation level prior to frozen storage and frozen storage duration on frozen beef color, and to evaluate the effects of oxygen permeability of packaging and aging time on meat color through frozen storage.
Materials and Methods
Sample collection
Thirty-six USDA Choice strip loins (A-maturity) were vacuumed packaged and obtained from Greater Omaha Packaging, Omaha, NE prior to being transported to the Loeffel Meat Lab at the University of Nebraska-Lincoln. All animals used in this study were slaughtered under the humane slaughter act and under USDA guidelines. Prior to fabrication loins were aged for either 4 or 20 d postharvest (2 ± 5 °C) under dark storage.
Sample fabrication
For each aging period, 4 or 20 d, 18 loins were fabricated into 1.27 cm steaks anterior to posterior after a 0.318-cm steak was removed and discarded at both the anterior and posterior ends to expose fresh surfaces prior to slicing. Loins were split in half into loin segments and then randomly assigned to a frozen storage period of 0 (frozen for 1 d), 2, 4, or 6 mo (n = 9 per frozen storage period) prior to fabrication. Each loin contained only two frozen storage periods forming the incomplete block design. Each half-loin was cut into six 1.27 cm steaks with a slicer for each myoglobin oxygenation level and packaging film combination for a total of 432 steaks in the study. The steaks were randomly preassigned to a myoglobin oxygenation level and packaging film prior to slicing and freezing. Myoglobin oxygenation level was described as deoxygenated (DeOxy), oxygenated (Oxy), and highly oxygenated (HiOxy). The DeOxy steaks were immediately measured for instrumental color, vacuum sealed, and placed in the freezer within 3 min after slicing. The Oxy steaks were sliced, and oxygenated in air at atmospheric pressure for 30 min, measured for instrumental color, vacuum sealed, and frozen. The HiOxy steaks were sliced and packaged for 24 h in a modified atmosphere packaging (MAP) mixture of 80% O2 and 20% CO2 before being measured for instrumental color, repackaged in an oxygen permeable or impermeable film, and frozen. A KOCH tray sealer with a MAP option (Koch Equipment LLC, Kansas City, MO) was used with the 80% O2 and 20% CO2 mixture. Steaks were randomly assigned to a packaging film and vacuum sealed with a Multivac Packaging machine (Multivac C500, Multivac, Kansas City, MO). Packaging films consisted of oxygen permeable (Cryovac Shrink Bags, 10K Oxygen Transmission Rate Fresh Fish Non-Barrier Straight End Seal, Sealed Air, Charlotte, NC) and oxygen impermeable film (3 mil standard (STD) barrier, Ultra Source, Kansas City, MO). The oxygen permeable film had an oxygen transmission rate (OTR) of at least 10,000 cc/m2/24 h at standard temperature and pressure (STP; 0 °C and 105 Pa), classifying it as a high transmitter of oxygen (Calvert, 1990). The oxygen impermeable film had an OTR of 6.77 cc/m2/24 h at STP, making it a high barrier film. Steaks were then frozen at −10 ± 3 °C on flat trays in a single layer for 3 h. After the initial freezing, steaks were then transferred into boxes and stored in the dark at –5 ± 3 °C for either 0, 2, 4, or 6 mo. Following frozen storage, steaks were removed from the packaging and immediately analyzed (while frozen) for oxygen penetration, instrumental color, and subjective color. All steaks were then repacked (3 mil STD barrier, Ultra Source), vacuum sealed, and frozen for further analysis (−80 °C) of lipid oxidation. Lipid oxidation was measured 1 mo following the completion of the last frozen storage period. Later, samples were trimmed of subcutaneous fat, frozen in liquid nitrogen, and then powdered in a metal cup blender (Model 51BL32, Waring Commercial, Torrington, CT). Powdered samples were stored at −80 °C for ~2 wk prior to lipid oxidation analysis.
Oxygen penetration
After frozen storage, steaks were removed from packaging film and a cut was made 2.54 cm from the lateral end of the steak with a sharp knife. A Westward caliper (6″/150 mm Elec Value Caliper, Grainger International, Inc., Lake Forest, IL) was used to quickly measure the oxygen penetration depth (bright red band) of the sample. Two measurements were taken for each steak, 2.54 cm from ventral and dorsal end. The measurement was taken from the edge of the surface exposed to oxygen to the edge of the oxymyoglobin band within the steak. The mean of the two measurements was used for statistical analysis.
Instrumental color (colorimeter)
Instrumental color was assessed after each frozen storage period once steaks were removed from packaging film, prior to oxygen penetration measurements. Instrumental color measurements were collected using the L*, a*, and b* scales with a Minolta CR-400 colorimeter (Minolta, Osaka, Japan) set with a D65 illuminant, 2° observer. The 8 mm opening of the handheld colorimeter was covered with an oxygen permeable film (Prime Source PSM 18 #75003815, Bunzl Processors Division, North Kansas City, MO). The colorimeter was set to record and print the mean of six readings per steak. Calibration was done through the oxygen permeable film prior to each day of use and with the white ceramic tile (Calibration Plate, Serial No. 14933058, Konica Minolta, Japan) and the D65 settings were set as follows: Y = 93.13, x = 0.3164, and y = 0.330. Once calibrated, the color space was selected to be the L*, a*, and b* scale, where L* is a measure of darkness to lightness, a* is a measure of greenness to redness, and b* is a measure of blueness to yellowness.
Delta E measures the magnitude of difference in L*, a*, and b* color space. Delta E was calculated for all frozen storage periods using the initial color readings taken during fabrication and the final readings when steaks were removed from the freezer in the frozen state for each individual steak. Delta E was then calculated using the equation ∆E = [(∆L*)2 + (∆a*)2 + (∆b*)2]1/2 from the values obtained by the colorimeter (Hunt et al., 2012). The a*:b* ratio was calculated using the equation provided by the American Meat Science Association Meat Color Measurement Guidelines (Hunt et al., 2012) with the values obtained from the colorimeter. The a*:b* ratio was calculated using the a* and b* values from the final readings when steaks were removed from the freezer in the frozen state. The a*:b* ratio was calculated by using the equation a*/b*. Hue angle (HA) was calculated based on the equation provided in the American Meat Science Association Meat Color Measurement Guidelines (Hunt et al., 2012) with the values obtained from the colorimeter. HA was calculated based on the a* and b* values, once steaks were removed from the freezer while still frozen. HA was calculated as HA = [arctangent (b*/a*)] (Hunt et al., 2012).
Instrumental color (spectrophotometer)
Instrumental color for the spectrophotometer measurements was assessed after each frozen storage period and once steaks were removed from packaging film, prior to oxygen penetration measurements. Color was evaluated on steaks while frozen under a plastic oxygen permeable film (Prime Source PSM 18 #75003815, Bunzl Processors Division, North Kansas City, MO). Six readings were taken on each steak using a portable Quality Spec Trek (Malvern Panalytical, Longmont, CO) with a spectral range of 350 to 2,500 nm and a wavelength accuracy of ±1.0 nm after calibration with a white tile and oxygen permeable film. The portable spectrophotometer utilized a quartz tungsten halogen bulb and a numerical aperture setting of 0.22 giving a filed-of-view of 25° full conical angle combined through a 1.2 mm diameter opening. Reflectance values at 473, 525, 572, and 730 nm were obtained and used to calculate percent Oxy, DeOxy, and metmyoglobin for each reading using the equations provided by the American Meat Science Association Meat Color Measurement Guidelines on page 58 (Krzywicki, 1979; Hunt et al., 2012). Statistical analyses were calculated using the mean of the six myoglobin state calculations obtained from each reflectance values reading.
| (1) |
| (2) |
| (3) |
| (4) |
Redness ratio was assessed after each frozen storage period and once steaks were removed from packaging film, prior to oxygen penetration measurements. Redness ratio was obtained using the Quality Spec Trek (Malvern Panalytical, Longmont, CO) as previously described. Reflectance values of 580 and 630 nm were used to calculate a redness ratio. The equation 630 nm/580 nm was used to calculate the redness ratio (Hunt et al., 2012). The mean of the six readings per steak was used for statistical analysis of redness ratio.
Subjective color (visual discoloration)
Visual discoloration was assessed after each frozen storage period and once steaks were removed from packaging film, prior to oxygen penetration measurements. Steaks were assessed with a trained five-person panel composed of graduate students from the University of Nebraska-Lincoln. Students were trained prior to evaluating steaks with a color guide that pictured varying discoloration percentages that were also used as a reference when evaluating individual steaks. A percentage scale was used were 0% meant no discoloration and 100% meant complete surface discoloration. The mean of five panelist ratings was used for the statistical analysis.
Lipid oxidation
Lipid oxidation was determined using the 2-thiobarbituric acid reactive substances (TBARS) method for all steaks following all frozen storage periods, according to Ahn et al. (1998). Five grams of powdered meat from the entire trimmed steak sample were placed into a 50-mL conical tube to which 14 mL of distilled deionized water and 1 mL of butylated hydroxyanisole (BHA) solution (10% BHA: 90% ethanol) were added. Samples were homogenized using a Polytron homogenizer (POLYTRON Kinimatica CH-6010, Switzerland) for 15 s at medium-to-high speed. The samples were centrifuged (2,000 × g for 5 min at 10 °C) and 1 mL of supernatant was transferred into a 15-mL conical tube with 2 mL of 2-thiobarbuturic acid/trichloracetic acid solution (15% trichloracetic acid and 20 mM 2-thiobarbuturic acid in deionized distilled water). Tubes were then placed in a 70 °C water bath for 30 min. Tubes were cooled for at least 10 min in a water bath (22 °C) and centrifuged (2,000 × g for 15 min at 10 °C). Two hundred microliters of supernatant were transferred to a 96-well plate in duplicate. Absorbance values were then read at 540 nm using a microplate spectrophotometer (Model Epoch, Biotek, Winooski, VT). Results were expressed in mg of malondialdehyde per kilogram of tissue.
Statistical analysis
Statistical analyses were conducted with SAS (version 9.4, Cary, NC). Data were analyzed as a split–split plot design with aging time as the whole-plot, frozen storage as the split-plot and a 3 × 2 factorial of oxygenation level and packaging film as the split–split plot. Frozen storage period was analyzed as an incomplete block design with each loin containing two random storage periods. Data were analyzed using the PROC GLIMMIX procedure of SAS and loin was the experimental unit. All means were separated with the LS MEANS statement with statistical significance determined at P < 0.05. Interactions with frozen storage as a significant term were analyzed using the slice function in SAS.
Results and Discussion
Tables are presented with least square means based on statistical interactions. Table 1 contains the ANOVA table and P-values with all main effects and possible interactions. Table 2 displays the means for the selected interactions of aging time, packaging films, and frozen storage duration for the different myoglobin oxygenation levels. Means for the interaction of aging time and packaging films are displayed in Table 3. Table 4 contains the means for the interactions of aging time, packaging films, and frozen storage periods. In Table 5, the interaction of aging time, frozen storage, and myoglobin oxygenation level are displayed. Finally, Table 6 contains the interactions for myoglobin oxygenation level, packaging film, aging time, and frozen storage. Figures follow the order in which they are presented in the results.
Table 1.
Analysis of variance P-values for each analysis and possible interactions including: aging time (4 d or 20 d), packaging films (impermeable or permeable), frozen storage (0, 2, 4, or 6 mo), and myoglobin oxygenation levels (DeOxy, Oxy, or HiOxy1).
| Analysis | Oxygen Penetration | L* | a* | b* | Oxy-myoglobin, % | Deoxy-myoglobin, % | Met-myoglobin, % | Delta E | Redness ratio2 | a*: b* ratio | HA | Discolor-ation | Lipid oxidation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aging time | 0.2241 | 0.1887 | <0.0001 | <0.0001 | 0.0143 | 0.0059 | 0.5976 | 0.0001 | <0.0001 | 0.0002 | 0.0004 | 0.0788 | 0.0183 |
| Frozen storage | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0240 | 0.0169 |
| Aging time*frozen storage | <0.0001 | <0.0001 | 0.1695 | 0.3623 | 0.2296 | 0.0009 | 0.4598 | 0.0217 | <.0001 | 0.3692 | 0.5605 | 0.0267 | 0.3029 |
| Myoglobin oxygenation level | <0.0001 | 0.0005 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0366 | 0.0918 | 0.0041 | <0.0001 |
| Aging time* myoglobin oxygenation level | 0.9457 | 0.0416 | 0.0105 | 0.0299 | 0.0073 | 0.2136 | 0.0003 | <0.0001 | 0.0188 | 0.2779 | 0.2257 | 0.0047 | 0.0164 |
| Frozen storage* myoglobin oxygenation level | <0.0001 | 0.0684 | <0.0001 | <0.0001 | 0.0002 | <0.0001 | 0.0095 | <0.0001 | <.0001 | 0.0244 | 0.1436 | <0.0001 | 0.0232 |
| Aging time*frozen storage* myoglobin oxygenation level | <0.0001 | 0.5891 | 0.0118 | 0.0833 | 0.0686 | 0.4519 | 0.0188 | 0.0057 | 0.7083 | 0.3134 | 0.1427 | <0.0001 | 0.0224 |
| Packaging film | <0.0001 | 0.1940 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.9919 | <0.0001 | 0.8655 | 0.3633 | 0.2239 | 0.0809 |
| Aging time* packaging film | 0.3755 | 0.1145 | 0.0065 | 0.0197 | 0.0130 | 0.0622 | 0.0250 | 0.0559 | 0.3045 | 0.7581 | 0.7983 | 0.2365 | 0.7441 |
| Frozen storage* packaging film | 0.0619 | 0.6141 | 0.6053 | 0.2105 | 0.9767 | 0.0012 | <0.0001 | 0.0047 | 0.4721 | <0.0001 | 0.0001 | 0.2177 | 0.5205 |
| Aging time*frozen storage* packaging film | 0.9294 | 0.9719 | 0.7482 | 0.6373 | 0.6210 | 0.4275 | 0.6541 | 0.0718 | 0.8872 | 0.8537 | 0.7974 | 0.2399 | 0.9398 |
| Myoglobin oxygenation level*Pack-aging film | 0.0004 | 0.1602 | <0.0001 | 0.0022 | <0.0001 | <0.0001 | <0.0001 | 0.1048 | 0.0280 | 0.0670 | 0.1359 | 0.2444 | 0.7800 |
| Aging time* myoglobin oxygenation level*pack-aging film | 0.6338 | 0.2556 | 0.5508 | 0.8639 | 0.1624 | 0.3489 | 0.0814 | 0.0787 | 0.0485 | 0.5761 | 0.5372 | 0.2636 | 0.9163 |
| Frozen storage* myoglobin oxygenation level*pack-aging film | 0.1899 | 0.4553 | 0.0882 | 0.0859 | 0.1104 | 0.0025 | 0.2899 | 0.5859 | 0.5406 | 0.0442 | 0.2592 | 0.2042 | 0.5899 |
| Aging time* frozen storage* myoglobin oxygenation level*pack-aging film | 0.5679 | 0.8708 | 0.8683 | 0.8675 | 0.6511 | 0.9515 | 0.3181 | 0.8133 | 0.6896 | 0.7802 | 0.7405 | 0.2351 | 0.5272 |
1Deoxygenated = DeOxy, Oxygenated = Oxy, and highly oxygenated = HiOxy.
2Redness ratio—reflectance values for 630 nm ÷ 580 nm.
Table 2.
The effects of aging (4 d or 20 d), packaging films (impermeable or permeable), and frozen storage (0, 2, 4, or 6 mo) for different myoglobin oxygenation levels (DeOxy, Oxy, or HiOxy1) on color variables of steaks (n = 72; n = 72; n = 36)
| Age | Myoglobin oxygenation level | |||||
|---|---|---|---|---|---|---|
| Variable | Variable | DeOxy | Oxy | HiOxy | P-value | SEM |
| L* | 4 | 46.43bc | 46.28bc | 47.31a | 0.0416 | 0.2848 |
| 20 | 45.85c | 46.38bc | 46.45b | |||
| b* | 4 | 6.87b | 7.02b | 8.06a | 0.0299 | 0.1534 |
| 20 | 5.40d | 6.09c | 7.04b | |||
| Oxymyoglobin, % | 4 | 65.17d | 69.15bc | 73.57a | 0.0073 | 0.5757 |
| 20 | 63.72d | 68.88c | 70.45b | |||
| Packaging | ||||||
| Oxygen penetration (mm) | Impermeable | .04d | .06c | .11a | 0.0004 | 0.0027 |
| Permeable | .07b | .07b | .11a | |||
| a* | Impermeable | 11.45e | 12.38d | 15.28a | <0.0001 | 0.1921 |
| Permeable | 13.21c | 13.74b | 15.48a | |||
| b* | Impermeable | 5.68d | 6.27c | 7.47a | 0.0022 | 0.1320 |
| Permeable | 6.59b | 6.83b | 7.63a | |||
| Oxymyoglobin, % | Impermeable | 58.58d | 66.20c | 71.48ab | <0.0001 | 0.5184 |
| Permeable | 70.31b | 71.82a | 72.54a | |||
| Metmyoglobin, % | Impermeable | 31.98a | 31.01b | 29.32c | <0.0001 | 0.2650 |
| Permeable | 28.77cd | 28.56d | 28.80cd | |||
| Frozen storage | ||||||
| b* 3 | 0 | 6.37c | 7.51b | 9.01a | <0.0001 | 0.1828 |
| 2 | 5.66b | 5.77b | 7.01a | |||
| 4 | 5.79b | 5.92b | 6.89a | |||
| 6 | 6.71bc | 7.01ab | 7.31a | |||
| Oxymyoglobin, %3 | 0 | 68.12c | 75.47b | 78.89a | 0.0002 | 0.7482 |
| 2 | 65.29c | 69.43b | 73.47a | |||
| 4 | 62.53c | 66.54b | 68.95a | |||
| 6 | 61.83c | 64.60b | 66.73a | |||
| Redness ratio2,3 | 0 | 2.52c | 2.82b | 3.63a | <0.0001 | 0.0520 |
| 2 | 2.39b | 2.41b | 2.78a | |||
| 4 | 1.99b | 2.05b | 2.29a | |||
| 6 | 2.15b | 2.15b | 2.29a |
1Deoxygenated = DeOxy, Oxygenated = Oxy, and highly oxygenated = HiOxy.
2Redness ratio—reflectance values for 630 nm ÷ 580 nm.
3Means within each row with different superscripts are different (P < 0.05).
a–e Means within the same variable with different superscripts are different (P < 0.05).
Table 3.
The effects of aging time (4 d or 20 d) for different packaging films (impermeable or permeable) on color variables of steaks (n = 108).
| Packaging film | |||||
|---|---|---|---|---|---|
| Variable | Age | Impermeable | Permeable | P-Value | SEM |
| a* | 4 | 13.59b | 15.06a | 0.0065 | 0.1914 |
| 20 | 12.49c | 13.23b | |||
| b* | 4 | 6.94b | 7.69a | 0.0197 | 0.1406 |
| 20 | 6.01d | 6.35c | |||
| Oxymyoglobin, % | 4 | 65.77c | 72.72a | 0.0130 | 0.5125 |
| 20 | 65.08c | 70.28b | |||
| Metmyoglobin, % | 4 | 30.90a | 28.42b | 0.0250 | 0.2633 |
| 20 | 30.64a | 29.01b |
a–d Means within the same variable with different superscripts are different (P < 0.05).
Table 4.
The effects of aging time (4 d or 20 d) and packaging films (impermeable or permeable), for different frozen storage periods (0, 2, 4, or 6 mo) on color variables of steaks (n = 54)
| Frozen Storage | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Age | 0 | 2 | 4 | 6 | P-Value | SEM |
| L* | 4 | 46.29a | 46.35b | 46.78a | 47.27a | <0.0001 | 0.3372 |
| 20 | 44.89b | 47.99a | 47.51a | 44.51b | |||
| Deoxymyoglobin, % | 4 | .37a | 1.18a | 1.72b | .93b | 0.0009 | 0.5102 |
| 20 | .31a | 1.81a | 3.71a | 4.13a | |||
| Redness ratio1 | 4 | 3.41a | 2.45b | 2.36a | 2.46a | <0.0001 | 0.0474 |
| 20 | 2.57b | 5.60a | 1.86b | 1.94b | |||
| Packaging | |||||||
| Metmyoglobin, % | Impermeable | 25.53a | 30.06a | 32.59a | 34.91a | <0.0001 | 0.3417 |
| Permeable | 25.16a | 28.16b | 30.18b | 31.34b | |||
| Delta E | Impermeable | 4.06b | 5.92a | 6.44a | 6.90a | 0.0047 | 0.2993 |
| Permeable | 5.11a | 5.95a | 5.85a | 6.42a | |||
| HA | Impermeable | .42b | .45a | .47a | .51a | 0.0001 | 0.0050 |
| Permeable | .43a | .45a | .46a | .50b |
1Redness ratio—reflectance values for 630 nm ÷ 580 nm.
a–e Means within each column for each variable with different superscripts are different (P < 0.05).
Table 5.
The effects of aging time (4 d or 20 d) by frozen storage (0, 2, 4, or 6 mo) by myoglobin oxygenation level (DeOxy, Oxy, or HiOxy1) on color variables of steaks (n = 18)
| 4 d | 20 d | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Frozen Storage | DeOxy | Oxy | HiOxy | DeOxy | Oxy | HiOxy | P-Value | SEM |
| Oxygen penetration (mm) | 0 | 0.05cd | 0.07b | 0.14a | 0.05d | 0.07bc | 0.13a | <0.0001 | 0.0037 |
| 2 | 0.05c | 0.05c | 0.08b | 0.07b | 0.07b | 0.11a | |||
| 4 | 0.08b | 0.08b | 0.13a | 0.06c | 0.07bc | 0.13a | |||
| 6 | 0.02e | 0.04de | 0.10a | 0.05cd | 0.06bc | 0.07b | |||
| a* | 0 | 15.38d | 17.17c | 20.40a | 13.11e | 15.15d | 18.79b | 0.0118 | 0.3939 |
| 2 | 12.75b | 12.35bc | 14.92a | 10.37d | 11.30c | 14.20a | |||
| 4 | 12.12b | 12.12b | 13.80a | 10.57c | 11.04bc | 14.01a | |||
| 6 | 13.17b | 12.94b | 14.78a | 11.17c | 12.44b | 12.17bc | |||
| Metmyoglobin, % | 0 | 24.94a | 24.86a | 24.92a | 25.80a | 25.37a | 26.17a | 0.0188 | 0.5520 |
| 2 | 29.71ab | 30.04a | 28.12c | 29.79a | 28.81ab | 28.20bc | |||
| 4 | 32.06ab | 31.79ab | 30.20c | 32.90a | 31.10bc | 30.27bc | |||
| 6 | 34.11a | 34.16a | 30.97b | 33.71a | 32.18b | 33.64a | |||
| Delta E | 0 | 5.40a | 3.77c | 3.88bc | 5.24ab | 4.32abc | 4.92abc | 0.0057 | 0.4923 |
| 2 | 4.83c | 5.60bc | 6.45ab | 4.76c | 7.13a | 6.83ab | |||
| 4 | 4.95b | 4.73b | 7.10a | 4.79b | 7.30a | 8.01a | |||
| 6 | 5.42cd | 4.81d | 6.15bc | 5.75cd | 7.03b | 10.79a | |||
| Discoloration | 0 | 0b | 0b | 0b | 0b | 0b | 0b | <0.0001 | 1.7994 |
| 2 | 0b | 0b | 0b | 0b | 0b | 0b | |||
| 4 | 0b | 0b | 0b | 0b | 0b | 0b | |||
| 6 | 0b | 0b | 0b | .13b | .13b | 18.80a | |||
| Lipid oxidation | 0 | 0.50ab | 0.44b | 0.76a | 0.70ab | 0.72ab | 0.88ab | 0.0224 | 0.1643 |
| 2 | 0.50c | 0.50c | 10.03ab | 0.88bc | 0.73bc | 10.49a | |||
| 4 | 0.59b | 0.55b | 0.84b | 0.96b | 0.95b | 10.42a | |||
| 6 | 0.59b | 0.66b | 0.87b | 0.89b | 0.87b | 20.00a |
1Deoxygenated = DeOxy, Oxygenated = Oxy, and highly oxygenated = HiOxy.
a–e Means within each row for each variable with different superscripts are different (P < 0.05).
Table 6.
The effects of myoglobin oxygenation level (DeOxy, Oxy, or HiOxy1) by packaging films (impermeable or permeable) by either aging (4 d or 20 d) or frozen storage (0, 2, 4, or 6 mo) on color variables of steaks (n = 72; n = 36)
| Impermeable | Permeable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Age | DeOxy | Oxy | HiOxy | DeOxy | Oxy | HiOxy | P-Value | SEM |
| Redness rratio2 | 4 | 2.37e | 2.44de | 2.90a | 2.66bc | 2.74b | 2.92a | 0.0485 | 0.0520 |
| 20 | 1.90g | 2.09f | 2.52cd | 2.13f | 2.16f | 2.65bc | |||
| Storage | |||||||||
| Deoxymyoglobin, %3 | 0 | 12.62a | 1.59b | −4.58c | .09b | −3.07c | −4.60c | 0.0025 | 0.6998 |
| 2 | 9.33a | 2.99b | −1.51d | .60c | −.70cd | −1.74d | |||
| 4 | 8.40a | 3.63b | 1.39c | 1.79c | .62c | .47c | |||
| 6 | 7.39a | 2.94b | 1.50bc | 1.22bc | 1.62bc | .53c | |||
| a*/b* ratio3 | 0 | 2.42a | 2.21b | 2.22b | 2.17b | 2.17b | 2.18b | 0.0442 | 0.0352 |
| 2 | 2.06a | 2.03a | 2.11a | 2.09a | 2.09a | 2.09a | |||
| 4 | 1.94b | 1.95ab | 2.02ab | 2.00ab | 1.99ab | 2.03a | |||
| 6 | 1.81ab | 1.77b | 1.84ab | 1.85ab | 1.87a | 1.86a |
1Deoxygenated = DeOxy, Oxygenated = Oxy, and highly oxygenated = HiOxy.
2Redness ratio—reflectance values for 630 nm ÷ 580 nm.
3Means within each row with different superscripts are different (P < 0.05).
a–e Means within the same variable with different superscripts are different (P < 0.05)
Oxygen penetration
Oxygen penetration had a packaging by myoglobin oxygenation level interaction (P = 0.0004; Table 2). Oxygen penetration or the depth of the bright red cherry color, was significantly greater for the HiOxy steaks regardless of packaging (P < 0.0001; Figure 1). As expected, HiOxy steaks had a higher oxygen penetration depth since they were packaged in a high-oxygen MAP prior to frozen storage. The higher oxygen pressure environment allowed oxygen to penetrate deeper into the meat leading to a thicker Oxy layer prior to freezing. It is well established in the literature that high-oxygen MAP produces a redder fresh meat product than meat that is exposed to atmospheric oxygen (Kim et al., 2010, 2012; Li et al., 2012). The Oxy and DeOxy treatments packaged in the permeable film had higher amounts of oxygen penetration than steaks packaged in the impermeable film (P < 0.005). The increase in oxygen penetration between the two packaging films can be attributed to the oxygen permeable film allowing oxygen to penetrate into the meat during freezing and frozen storage, creating a greater depth of oxygen penetration.
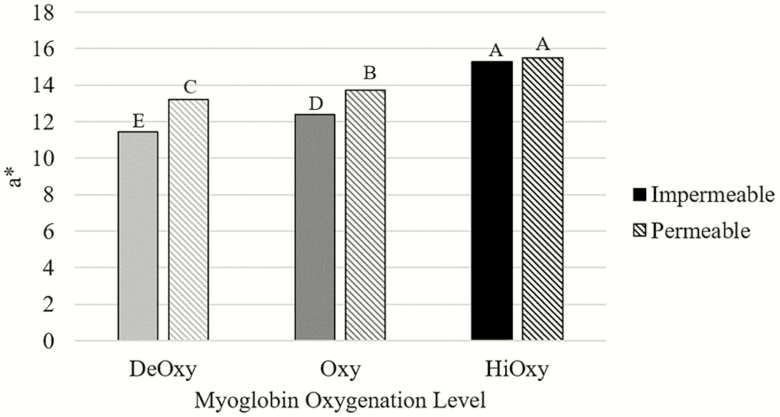
Figure 1.
Oxygen penetration values of steaks in either a deoxygenated, oxygenated, or high oxygenated state and impermeable or permeable packaging (n = 72; SEM = 0.0027).
a–dDifferent superscripts indicated differences among treatments (P < 0.05).
A three-way interaction between myoglobin oxygenation level, aging time, and frozen storage period was also observed for oxygen penetration depth (P < 0.0001; Table 5). The HiOxy steaks, regardless of aging time, had the greatest oxygen penetration depth compared with Oxy and DeOxy at 0 and 4 mo frozen storage (P < 0.0001). However, at 2 mo the HiOxy 20 d steaks had the greatest depth of oxygen penetration and at 6 mo the HiOxy steaks from beef aged 4 d had the greatest depth (P < 0.001). The DeOxy and Oxy steaks had lower amounts of oxygen penetration most likely due to the limited oxygen exposure prior to freezing. Researchers have reported that an increase in aging improves the initial color intensity by minimizing the competition for oxygen between myoglobin and mitochondria, allowing the steaks to have a higher blooming capacity (MacDougall, 1982; Bekhit and Faustman, 2005). The greater depth of oxygen penetration at 2 mo of frozen storage for the HiOxy 20 d aged steaks, compared with HiOxy 4 d aged steaks, could likely be attributed to the meat being aged, leading to a larger capacity to bloom due to the decrease in oxygen consumption with postmortem aging. Based on our results, the HiOxy steaks had greater oxygen penetration values and within myoglobin oxygenation levels the permeable packaging aided in obtaining a thick oxymyoglobin layer. It is important to note that oxygen penetration depth values were minimal and more precise methods may need to be developed for greater accuracy.
Instrumental color (L*, a*, and b*)
Significant interactions for L* included aging time by myoglobin oxygenation level (P = 0.0416; Table 2) and aging time by frozen storage period (P < 0.0001; Table 4). For the interaction of aging time by myoglobin oxygenation level, the HiOxy steaks aged for 4 d were lighter than all other treatments (P < 0.05). The HiOxy steaks aged for 20 d were lighter than the DeOxy steaks aged for 20 d (P = 0.0301). There was an interaction between aging time and frozen storage period. Steaks aged for 4 d were lighter than steaks aged for 20 d after 0 and 6 mo of frozen storage. However, at 2 mo, steaks aged for 20 d were lighter than steaks aged for 4 d. Typically, meat that has been aged expresses a higher L* value which MacDougall (1982) attributed to the structural changes in the muscle proteins during aging of fresh meat. Vitale et al. (2014) found similar results in fresh meat aged for just 3 d, which had higher L*, a*, and b* values than unaged meat at the initial start of retail display. Research has well established that high oxygen MAP causes higher L* values compared samples that are vacuum packed (Kim et al., 2011, 2012; Li et al., 2012; Bonny et al., 2017). Kim et al. (2011) attributed the increase in lightness to an elevated oxygenation of myoglobin under an oxygen-rich condition which was observed with the HiOxy 4 d steaks. According to McKenna et al. (2005), L* plays a minimal role in predicting color stability since muscles of high color stability were on opposite ends of the L* spectrum in their study.
When comparing the a* values for different treatments, significant interactions between packaging and aging time (P = 0.0065; Table 3) and myoglobin oxygenation level by packaging (P < 0.0001; Table 2) were observed. There was also a significant three-way interaction between myoglobin oxygenation level, aging time, and frozen storage (P = 0.0118; Table 5). The interaction of packaging film and aging time showed that steaks aged for 4 d and frozen in the permeable film had the highest a* values while steaks aged for 4 d, frozen in impermeable film and steaks aged 20 d and frozen in permeable film were intermediate while steaks that were packaged in the impermeable film and aged for 20 d had the lowest a* values (P < 0.05). Postmortem aging has an impact on meat color, such that aged beef has a higher blooming capacity than nonaged beef and that aged beef discolors at a faster rate than nonaged beef (Hood, 1980; MacDougall, 1982; Mancini and Ramanathan, 2014; Vitale et al., 2014; English et al., 2016). The high blooming capacity of aged meat can be attributed to the diminished mitochondrial activity and reduced oxygen consumption with longer aging times. Conflicting results have been documented in the literature with several researchers finding no color differences with varying postmortem aging periods (Ledward, 1970; Ledward, 1971). On the contrary, Vitale et al. (2014) found that the initial a* values in fresh beef were higher for meat that was aged compared with unaged meat. English et al. (2016) found steaks packaged in polyvinyl chloride film and high oxygen MAP and aged for 42 and 62 d had lower a* values at the start of retail display than 21-d aged steaks. Our results align with English et al., especially at 0 mo of frozen storage where steaks aged for 4 d had greater a* values than steaks aged 20 d within each myoglobin oxygenation level (P < 0.05). With an increase in frozen storage though, the difference of a* values between aging periods decreased. The difference in our results could be related to the postmortem aging time that was selected. Aging for 4 d postmortem could have provided enough of a reduction in oxygen consumption that allowed for optimal blooming conditions and high a* values.
Regardless of packaging, for the myoglobin oxygenation level and packaging film interaction, the HiOxy steaks had higher a* values than the other treatments, shown in Figure 2 (P < 0.0001). While packaging did not impact the a* values for HiOxy steaks, the Oxy and DeOxy steaks exhibited higher a* values when packaged in the permeable film (P < 0.05) when compared with the impermeable film. The DeOxy steaks that were packaged in the impermeable film had the lowest a* values (P < 0.0001). This can be attributed to the limited oxygen exposure prior to packaging and freezing along with the fact that the impermeable packaging prohibited oxygen from coming in contact with the steaks.
Figure 2.
Instrumental color values for a* of steaks in either a deoxygenated, oxygenated, or high oxygenated state and impermeable or permeable packaging (n = 72; SEM = 0.1921).
a–e Different superscripts indicated differences among treatments (P < 0.05).
The significant three-way interaction of myoglobin oxygenation level, frozen storage duration, and aging time displayed that the HiOxy steaks that were aged for 4 d and frozen for only 0 mo had higher a* values compared with all other myoglobin oxygenation levels, postmortem aging times, and frozen storage duration (P < 0.05). The next highest a* values were the HiOxy steaks aged for 20 d and frozen for 0 mo which was higher than all other treatments besides the HiOxy 4 d steaks at 0 mo (P < 0.05). Within 0, 2, and 4 mo of frozen storage, the HiOxy steaks, regardless of aging time, had significantly higher a* values than the other myoglobin oxygenation levels (P < 0.05). However, following 6 mo of frozen storage, the HiOxy steaks 4 d age were the reddest and the HiOxy steaks aged 20 d were similar to the other myoglobin oxygenation levels (P < 0.001). It was expected that the HiOxy steaks would have the highest a* values. It has been reported that high-oxygen MAP produces initially higher a* values compared with packaging films (Kim et al., 2010, 2012; Li et al., 2012). The HiOxy steaks were exposed to a large amount of oxygen prior to freezing compared with the other treatments, allowing the oxygen to bind, create oxymyoglobin, and produce the higher a* value and bright red cherry color. Current studies support similar findings in that freezing meat decreases the redness and color stability of frozen meat (Kim et al., 2018; Setyabrata and Kim, 2019). Holman et al. (2018) found a decrease in myoglobin content across an increase in frozen storage. With a decrease in myoglobin content, it is expected that a decrease in redness would occur, which Wanous et al. (1989), Brewer and Wu (1993), and Vieira et al. (2009) found as storage time increased, similar to our results. In summary, high a* values were easily obtained in meat that had been packaged in a high oxygen environment prior to freezing, oxygen permeable film improved the a* values for steaks with limited oxygen exposure prior to freezing, a* values decreased with frozen storage, and 4 d of aging appeared to provide optimal a* compared with 20 d of aging.
There were four significant two-way interactions for b* values including myoglobin oxygenation level by packaging (P = 0.0022; Table 2), myoglobin oxygenation level by aging time (P = 0.0299; Table 2), myoglobin oxygenation level by frozen storage duration (P < 0.0001; Table 2), and packaging by aging time (P = 0.0197; Table 3). The b* values interaction between myoglobin oxygenation level and packaging was very similar to the a* values. The HiOxy steaks regardless of film had the highest b* values (P < 0.0001). Several researchers have found similar results in fresh meat with high-oxygen MAP increasing b* values compared with other packaging systems (Li et al., 2012; Bonny et al., 2017). The impermeable film had lower b* values for both the DeOxy and Oxy states (P < 0.0005). For the interaction of myoglobin oxygenation level by aging time, the HiOxy steaks aged for 4 d had the highest b* values compared with the other treatments (P < 0.0001). The DeOxy steaks aged for 20 d had the lowest b* values compared with the other treatments (P < 0.0001). Myoglobin oxygenation level by frozen storage duration showed the HiOxy steaks frozen for only 0 mo had the highest b* values compared with all other myoglobin oxygenation levels and frozen storage periods (P < 0.05) Within the 0 mo of frozen storage the HiOxy steaks had the highest b* values followed by Oxy, and DeOxy (P < 0.0001). However, at both 2 and 4 mo, Oxy and DeOxy were no longer different and at 6 mo of frozen storage the only difference was between HiOxy and DeOxy steaks (P = 0.0049). With a significant interaction of packaging by aging time (P = 0.0197), steaks that were aged for 4 d then packaged in the permeable film had the highest b* values followed by 4 d impermeable film, 20 d permeable film, and 20 d impermeable film (P < 0.05). While differences in b* values were observed, little value has been placed on the importance of b* values in relation to meat color.
Instrumental color (percent oxymyoglobin, percent deoxymyoglobin, and percent metmyoglobin)
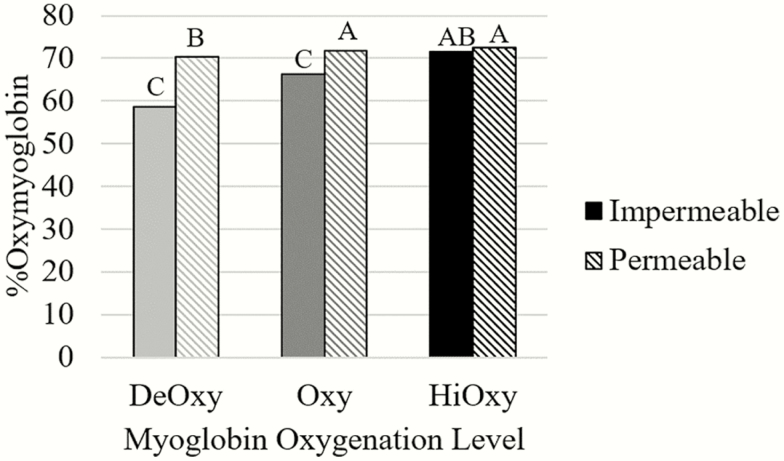
There were four significant two-way interactions for percent oxymyoglobin: myoglobin oxygenation level by packaging film (P < 0.0001; Table 2), packaging film by aging (P = 0.0130; Table 3), myoglobin oxygenation level by aging (P = 0.0073; Table 2), and myoglobin oxygenation level by frozen storage period (P = 0.0002; Table 2). In Figure 3, the DeOxy steaks that were packaged in impermeable film had the lowest percent oxymyoglobin (P < 0.0001). Within Oxy and DeOxy, steaks packaged in permeable film had significantly more percent oxymyoglobin than steaks in the impermeable film (P < 0.0001). However, when comparing the impermeable films, the HiOxy steaks had more percent oxymyoglobin than Oxy and DeOxy (P < 0.0001). The high concentration of oxygen prior to freezing for the HiOxy steaks, particularly in the impermeable packaging, allowed more oxygen to bind to myoglobin and contribute to a larger percent oxymyoglobin. The difference in packaging films demonstrates the importance of a permeable film allowing oxygen to pass through and bind to the myoglobin molecule, increasing the percentage of oxymyoglobin.
Figure 3.
Instrumental color values for percent Oxy of steaks in either a deoxygenated, oxygenated, or highly oxygenated state and impermeable or permeable packaging (n = 72; SEM = 0.5184).
a–c Different superscripts indicated differences among treatments (P < 0.05).
The interaction of aging time by packaging film showed that steaks aged for 4 d and frozen in the permeable film had the most oxymyoglobin followed by steaks aged for 20 d in the permeable film, and then steaks packaged in impermeable film, regardless of aging time (P = 0.0130). The same trend was observed for a* values which supports the finding of Mohan et al. (2010) who reported a strong relationship in fresh beef between reflectance spectrophotometric data for myoglobin redox forms and colorimetric values. The use of a completely impermeable package leads to heme pigments that are able to reduce to the DeOxy state and then become oxygenated once the package is open and exposed to air (Clydesdale and Francis, 1971; Jeremiah, 2001). However, after frozen storage, the DeOxy steaks may not have been able to bloom as well with exposure to oxygen. This could be attributed to a reduction in MRA or depletion of reducing equivalents such as NADH following frozen storage.
The interaction of myoglobin oxygenation level and aging time was also significant (P = 0.0073). The HiOxy steaks aged for 4 d had the highest percent oxymyoglobin compared to the other treatments (P < 0.05). Regardless of aging the DeOxy steaks had the lowest percent oxymyoglobin with Oxy being intermediate of the treatments (P = 0.0073). Myoglobin oxygenation level by frozen storage duration showed that the HiOxy steaks, within each frozen storage period, had the highest percent oxymyoglobin followed by Oxy and then DeOxy as seen in Figure 4 (P = 0.0002). With an increase in frozen storage, a decrease in percent oxymyoglobin for all myoglobin oxygenation levels was observed (P = 0.0002). As expected, the HiOxy steaks had high levels of percent oxymyoglobin. The literature has shown that high-oxygen MAP produces initially higher percent oxymyoglobin compared to other packaging treatments (Kim et al., 2010, 2012; Li et al., 2012). Overall, the percent oxymyoglobin values support the findings observed with the a* values in that HiOxy steaks in permeable packaging, and shorter frozen storage periods enhanced the bright red cherry color consumers desire.
Figure 4.
Instrumental color values for percent Oxy of steaks in either a deoxygenated, oxygenated, or highly oxygenated state and frozen for either 0, 2, 4, or 6 mo (n = 36; SEM = 0.7482).
a–c Different superscripts indicated differences within frozen storage period (P < 0.05).
The percent deoxymyoglobin had a significant interaction between aging and frozen storage (P =0.0009; Table 4) and myoglobin oxygenation level, packaging, and frozen storage (P = 0.0025; Table 6). The interaction of aging time and frozen storage showed that within 0 and 2 mo of frozen storage there were no statistical differences between 4 and 20 d aged steaks, but at 4 and 6 mo steaks aged for 20 d had significantly more percent deoxymyoglobin (P = 0.0009). A significant three-way interaction of myoglobin oxygenation level, packaging, and frozen storage (P = 0.0025) was also present. Regardless of frozen storage period, steaks packaged in impermeable film in the DeOxy myoglobin oxygenation level had significantly higher percent deoxymyoglobin (P = 0.0025). Since the DeOxy steaks had limited exposure to oxygen prior to packaging, it was expected that they would have the largest percent deoxymyoglobin initially at frozen storage. Li et al. (2012) also found that vacuum packaging in impermeable films yields a higher content of DeOxy compared with packaging with oxygen. This is because metmyoglobin formed at low oxygen pressure in vacuum packaging can be reduced back to DeOxy when reducing components of the MRA cycle are present. While there were statistically significant differences for percent deoxymyoglobin, it is important to note that values were obtained based on the isobestic wavelength calculations that could lead to small fluctuations in values. Based on our knowledge, this is the first study to use a portable spectrophotometer and the equations by Krzywicki (1979) on frozen steaks which may attribute to the negative values.
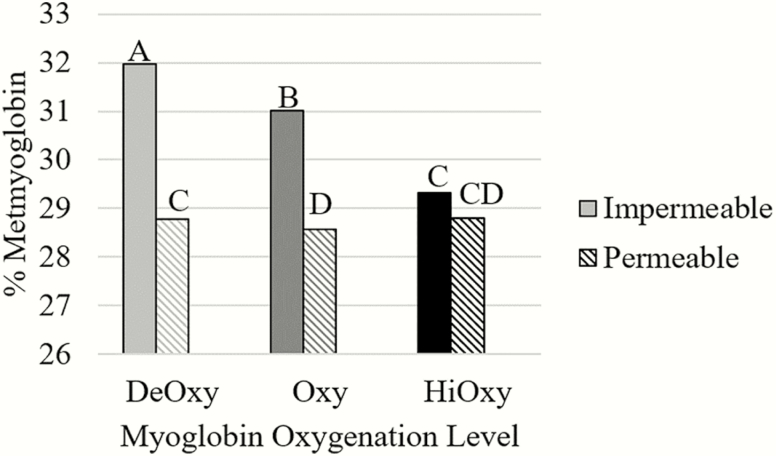
Myoglobin oxygenation level by packaging film (P < 0.0001; Table 2), packaging film by aging (P = 0.0250; Table 3), packaging film by frozen storage (P < 0.0001; Table 4), and myoglobin oxygenation level by aging by frozen storage (P = 0.0188) were all significant interactions for percent metmyoglobin. While packaged in impermeable film the DeOxy steaks had the highest amount of percent metmyoglobin followed by the Oxy steaks in the impermeable film (P < 0.0001) for the interaction of myoglobin oxygenation level by packaging film. There was no difference in metmyoglobin percent between packaging film for the HiOxy steaks as noted in Figure 5 (P < 0.05). The interaction of packaging film and aging time showed that the steaks packaged in the impermeable film had significantly higher percent metmyoglobin values, regardless of aging (P = 0.0250). Our findings contradict Greene (1969), Jeremiah (2001), and Li et al. (2012) who assert that anaerobic packaging through the use of vacuum packaging in impermeable films can prevent metmyoglobin formation and rancidity with sufficient MRA in fresh meat. However, as the permeability of the film decreases, a low partial pressure of oxygen is reached where oxidation is favored, forming metmyoglobin (Clydesdale and Francis, 1971). This may be why our percent metmyoglobin values are higher for impermeable films than permeable films in that we could have had ideal partial pressure in our packaging due to limited oxygen exposure prior to packaging and frozen storage.
Figure 5.
Instrumental color values for percent metmyoglobin of steaks in either a deoxymyoglobin, oxygenated, or high oxygenated state and impermeable or permeable packaging (n = 72; SEM = 0.2650).
a–d Different superscripts indicated differences among treatments (P < 0.05).
At 0 mo of frozen storage, no difference in the percent of metmyoglobin between packaging film was observed for frozen storage duration and packaging film. There was a significant difference between packaging films at 2, 4, and 6 mo with impermeable film having higher percent metmyoglobin values than permeable films (P < 0.0001). For the interaction of myoglobin oxygenation level, aging time, and frozen storage duration, we found that at 0 mo of frozen storage there were no significant differences among myoglobin oxygenation level and aging (P < 0.05). Yet, the difference in myoglobin oxygenation level and aging exacerbated metmyoglobin differences over time (P = 0.0188). Throughout frozen storage at 2,4, and 6 mo the HiOxy steaks aged for 4 d had significantly less metmyoglobin percent than the DeOxy steaks, regardless of aging time (P = 0.0188). The increase in percent metmyoglobin can most likely be attributed to the decrease in metmyoglobin reducing enzymes that have been well documented with extended frozen storage (Wanous et al., 1989; Farouk and Swan, 1998; Vieira et al., 2009). Discoloration is the accumulation of metmyoglobin on the surface of meat. It has been estimated that meat departments lose 5.4% of sales each year due to fresh meat discoloration (Bekhit and Faustman, 2005). Therefore, it is important to note that myoglobin oxygenation level, aging, and packaging impact the percent metmyoglobin; frozen storage duration is detrimental and leads to the accumulation of metmyoglobin.
Delta E
Delta E was measured as the change in color space over time, therefore, a large delta E indicates a greater change in color over time. There was a significant interaction between packaging and frozen storage duration (P = 0.0047; Table 4), as well as myoglobin oxygenation level, aging time, and frozen storage duration (P = 0.0057; Table 5) for delta E. With increasing frozen storage, a larger delta E value was observed for steaks frozen in impermeable film and was similar for permeable film steaks (P = 0.0047). This can be attributed to steaks having a lower MRA as longer frozen storage causes more discoloration and greater change in color over time. The interaction of myoglobin oxygenation level, aging time, and frozen storage duration showed steaks that were packaged in impermeable film for 0 mo had the lowest delta E values compared with all other frozen storage periods (P < 0.005). The main difference when comparing steaks at different frozen storage durations, aging periods, and myoglobin oxygenation level was that the HiOxy steaks aged for 20 d and frozen for 6 mo had significantly higher delta E values than all other treatments (P < 0.0001). Ledward et al. (1986) concluded that delta E values over 3.0 in fresh meat were very obvious to most observers. Based on our results, regardless of myoglobin oxygenation level, aging time, packaging film, and frozen storage period, all of the mean values would be over the 3.0 value, especially the HiOxy 20 d aged steaks which had over a threefold value of what consumers observe as an obvious change. With extended frozen storage, it was expected that meat would change color over time largely due to the association of frozen storage with an increase in discoloration. Setyabrata and Kim (2019) attribute the decrease in color stability to the denaturation of myoglobin and reduced myoglobin redox system caused by ice crystal damage. While freezing meat has been noted to alter the color (Ramsbottom and Koonz, 1941; Kiani and Sun, 2011; Kim et al., 2018), freezing for a short duration still had a profound effect on meat color. With such a short duration and large change in delta E, it is evident that packaging and freezing play a large role in meat color.
Redness ratio
Redness ratio had two significant two-way interactions between myoglobin oxygenation level and frozen storage period (P < 0.0001; Table 2) and aging time and frozen storage period (P < 0.0001; Table 4). There was also a significant three-way interaction between myoglobin oxygenation level, packaging film, and aging time (P = 0.0485; Table 6). The ratio of 630/580 nm was established to indicate the change in meat color due to the formation of metmyoglobin. Therefore, it can be used to evaluate surface color stability (Hunt, 1980; Joseph et al., 2012; Li et al., 2017). Larger ratios indicate a larger amount redness due to oxymyoglobin and therefore, a ratio of 1.0 would essentially be 100% metmyoglobin (Strange et al., 1974; Hunt et al., 2012; Joseph et al., 2012; Li et al., 2017).
At 0 mo of storage, there was a significant difference between all myoglobin oxygenation levels with HiOxy having the highest redness ratio followed by Oxy and then DeOxy (P < 0.0001). Nonetheless, at 2, 4, and 6 mo, the redness ratio for HiOxy steaks was significantly higher than Oxy and DeOxy that were similar with extended frozen storage (P < 0.05). The results for redness ratio are similar to the results for percent oxymyoglobin and a* values, reinforcing that HiOxy steaks have greater redness compared with steaks minimally exposed to oxygen throughout frozen storage. Gatellier et al. (2005) found a high correlation between beef redness when comparing a* values and the ratio of 630/580. However, Khliji et al. (2010) found that a* values were more strongly related to consumer perception of redness than the 630/580 ratio.
The interaction of aging time and frozen storage duration showed steaks that were aged for 4 d had significantly higher redness ratios than the 20 d aged steaks for 0, 4, or 6 mo (P < 0.0001). Yet, at 2 mo of frozen storage, the steaks that were aged for 20 d had a higher redness ratio than 4 d (P = 0.0358). The significant three-way interaction between myoglobin oxygenation level, packaging film, and aging time demonstrated that steaks that were aged for 4 d and in the HiOxy myoglobin oxygenation level treatment had the highest redness ratio regardless of packaging (P < 0.05). The lowest redness ratio was the DeOxy steaks packaged in impermeable film and aged for 20 d prior to freezing (P < 0.0001). However, when comparing within an aging period, HiOxy steaks were superior (greater redness), regardless of packaging, for both 4 and 20 d of aging (P < 0.0001). Within 4 d of aging for both Oxy and DeOxy, the permeable packaging film gave higher redness ratios than the impermeable packaging (P < 0.05). Typically, impermeable films can lead to less discoloration, but they tend to yield darker steaks (Mancini et al., 2009) which is similar to our results where 4 d of aging and impermeable packaging had a lower redness ratio and a* values. Morrissey et al. (2008) reported that when the ratio fell below 3.5, consumers thought the color was more brown than red and unacceptable, but Khliji et al. (2010) found the threshold to be 3.3 or lower and Jacob et al. (2007) found it to be below 3.0. Redness ratio is an indicator of brown and the discoloration values observed in our study were fairly low, suggesting that steaks have an acceptable red color even at a threshold of 3.0. However, the varying results may be attributed to our steaks being frozen compared with the studies conducted on fresh beef.
a*:b* ratio
Aging had a significant main effect on the a*:b* ratio (P = 0.0002). The mean a*:b* ratio for steaks aged 4 d was 1.97 compared with steaks aged 20 d at 2.09. A frozen storage by myoglobin oxygenation level by packaging interaction was also observed for the a*:b* ratio (P = 0.0442; Table 6). During frozen storage, DeOxy steaks that were stored for 0 mo in the impermeable film had a higher a*:b* ratio than all other treatments at 0 mo (P < 0.0001). However, at 2, 4, and 6 mo of frozen storage, there was little significant difference among treatments. Setser (1984) and Hunt et al. (2012) indicated that larger ratios of a*:b* indicate more redness and less discoloration. This supports our findings of having higher a*:b* values with short frozen storage because meat that has been stored for shorter periods of time will be redder and less discolored. Research has shown that storage duration can impact the quality of meat (Wanous et al., 1989; Brewer and Wu, 1993; Farouk and Swan, 1998; Vieira et al., 2009). However, it is interesting to note that a higher a*:b* values were observed for meat aged for 20 d (P = 0.0002). While a* values were greater for 4 d so were the b* values resulting in a lower a*:b* ratio. The slight decrease in a*:b* value highlights that meat loss redness and started to discolor with extended storage.
Hue angle
HA had a significant aging main effect (P = 0.0004). The mean HA for 4 d aged steaks was 0.47 and 0.45 for the 20 d steaks, respectively. There was also a packaging by frozen storage duration interaction for HA (P = 0.0001; Table 4). Steaks packaged in impermeable film at 6 mo had a greater HA than the permeable film packaged steaks (P < 0.05). Steaks that were frozen in the impermeable film for 0 mo of frozen storage was lower than the permeable film (P = 0.0001). Tapp et al. (2011) found HA to represent the change of color from red to yellow with large angles indicating less red meat and an increase in discoloration over time. The increase in HA supports the notion that frozen storage duration leads to an increase in discoloration and decrease in redness also evident in oxygen penetration, a* values, percent Oxy, and redness ratio.
Subjective color (visual discoloration)
Discoloration is caused by an accumulation of metmyoglobin on the surface of beef due to the oxidation of oxymyoglobin and deoxymyoglobin. Packaging film had no effect on discoloration for frozen steaks. However, a three-way interaction between myoglobin oxygenation level, aging, and storage period was found to be significant for discoloration (P < 0.0001; Table 5). Discoloration was low (<1%) for all treatments throughout frozen storage expect at 6 mo of frozen storage for the HiOxy 20 d aged steaks exhibited a mean of 18.80% discoloration. According to Hood and Riordan (1973), a level of 20% discoloration during retail display, reduced beef sales by 50%. Even at lower levels of discoloration, consumer discrimination against discolored meat was observed. It has been reported that 15% of retail beef is discounted due to discoloration resulting in an economic loss of $1 billion annually (Mancini and Hunt, 2005). Typically, metmyoglobin can be reduced back to deoxymyoglobin and oxygenated to oxymyoglobin (bright cherry red color); however, this is no longer possible when reducing components of the MRA are depleted. Many factors influence MRA, but in particular Hood (1980) found that aged beef discolors at a quicker rate compared with fresh beef due to the decay of mitochondrial activity and depletion of MRA. While this provides an explanation for our discoloration values in frozen steaks it does not support our values for percent metmyoglobin that do not follow as close to our discoloration results as one would expect. This could be attributed to very low discoloration values that were subjectively measured by the trained panel.
Kim et al. (2010) found that myoglobin oxidation can occur in high-oxygen MAP leading to the formation of metmyoglobin on the surface of the meat. Research has also demonstrated that high-oxygen MAP promotes oxidative changes in meat affecting both the color stability and discoloration more than other packaging methods (Jayasingh et al., 2002; Grobbel et al., 2008a, b; Zakrys et al., 2008; Mancini et al., 2009; Kim et al., 2010). Lipid oxidation is one of the main causes of quality loss in meat during storage and processing leading to rancidity and discoloration (Renerre, 1999).
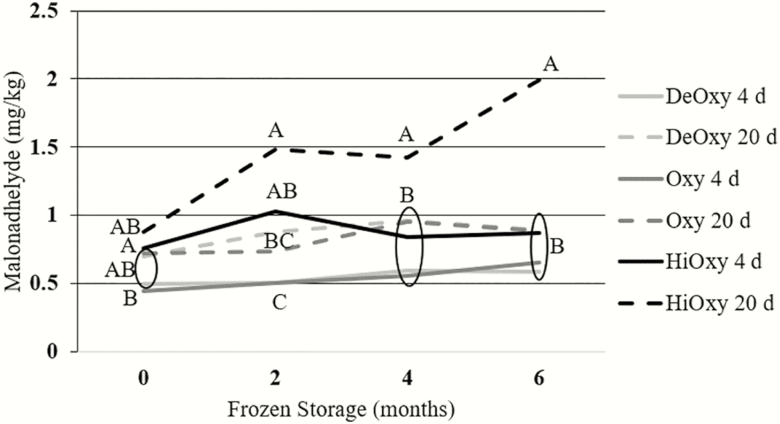
Lipid oxidation
Lipid oxidation had a significant three-way interaction between myoglobin oxygenation level, aging time, and frozen storage period (P = 0.0224; Table 5). At both 4 and 6 mo of frozen storage, the HiOxy steaks aged for 20 d had significantly higher TBARS values than all other treatments (P < 0.05). At 2 mo of storage, the HiOxy steaks aged for 20 d had higher TBARS values than all treatments expect the HiOxy 4 d steaks (P < 0.0001). Over the 6 mo of frozen storage, HiOxy steaks aged for 20 d had the greatest increase in malondialdehyde values (Figure 6). According to Campo et al. (2006), consumers were able to detect a rancid flavor that overpowered the beef flavor in beef steaks at 2.28 mg malondialdehyde/kg tissue using the TBARS method which is higher than any treatment at any frozen storage period. Therefore, even with the larger TBARS value for HiOxy 20 d aged steaks, oxidation in this study would not be a large concern for consumer acceptance since the values fell under the 2.28 mg malondialdehyde/kg tissue threshold amount.
Figure 6.
TBARS values of steaks in either a deoxygenated, oxygenated, or highly oxygenated state, aged for 4 or 20 d and frozen for 0, 2, 4, or 6 mo (n = 18; SEM = 0.0224).
a–c Different superscripts indicated differences within frozen storage period (P < 0.05).
Lipid oxidation has been noted as one of the largest factors impacting color and flavor and is a main cause of quality loss in meat during storage and processing, leading to rancidity and discoloration (Renerre, 1999; Faustman et al., 2010). Postmortem aging has been documented to increase lipid oxidation (Faustman and Cassens, 1990; Epley, 1992; Jakobsen and Bertelsen, 2000; Joseph et al., 2012; Bonny et al., 2017). While not always significant, aging steaks tended to cause higher TBARS values within the myoglobin treatments within this study. Faustman and Cassens (1990) attribute the increase in lipid oxidation from postmortem aging to a decrease in the endogenous antioxidant activity that aids in limiting oxidation. English et al. (2016) found that aging decreased the MRA, increased lipid oxidation and had a detrimental effect on color stability. A relationship between lipid oxidation and myoglobin oxidation has been well documented (Greene, 1969; Wanous et al., 1989; Akamittath et al., 1990; Faustman and Cassens, 1990; Faustman et al., 2010; Bonny et al., 2017). However, in this study while discoloration was higher at 6 mo of frozen storage for the HiOxy steaks aged 20 d similar to lipid oxidation, the same trend was not evident at 2 and 4 mo for discoloration as seen in lipid oxidation. The lipid oxidation results, while a bit more divergent than discoloration, showed that the HiOxy steaks aged for 20 d at 6 mo of frozen storage exhibited the largest discoloration and lipid oxidation. With the similar patterns between discoloration and lipid oxidation, it reasserts the relationship between lipid and myoglobin oxidation.
As expected, an upward trend of TBARS values was noted with an increase in frozen storage duration. Other researchers have found that as extended frozen storage increases, an increase in lipid oxidation, odor intensity, tenderness, and a decrease in metmyoglobin reducing enzymes have been noted (Wanous et al., 1989; Farouk and Swan, 1998; Vieira et al., 2009). Soyer et al. (2010) and Setyabrata and Kim (2019) attribute this to the structural changes that are induced by ice crystallization, which increases meat susceptibility to oxidative damage due to ruptured cell membranes releasing prooxidant compounds into the muscle, accelerating oxidation.
Steaks that have been packaged in a highly oxygenated environment have been found to have an increase in oxidation (Jayasingh et al., 2001; Zakrys et al., 2008; Kim et al., 2010; Bonny et al., 2017). Researchers have shown that high-oxygen MAP causes greater lipid oxidation compared with other packaging treatments (Jayasingh et al., 2002; Zakrys et al., 2008; Kim et al., 2010; Bonny et al., 2017). The increase in oxidation from high-oxygen MAP can lead to the development of off-flavors and beef flavors that consumers find unappealing (Grobbel et al., 2008b; Zakrys et al., 2008; Kim et al., 2010). Since the HiOxy steaks were packaged in a high-oxygen MAP for 24 h, it is not surprising that they typically had higher TBARS values than the other myoglobin level treatments. Overall, there is little difference in lipid oxidation expect for the HiOxy steaks aged for 20 d with extended frozen storage.
The HiOxy steaks exhibited the greatest oxygen penetration depth, a* vales, and percent oxymyoglobin while in the frozen state compared to the other myoglobin treatments. Conversely, the HiOxy steaks had lower percent metmyoglobin compared with the other myoglobin oxygenation levels demonstrating the superior red color. Delta E, a*:b* ratios, and discoloration were highest for the HiOxy 20 d aged steaks at 6 mo of frozen storage representing the impact that postmortem aging has on meat color stability. Throughout the study, it was observed that a*, redness ratio, and percent oxymyoglobin decreased with extended frozen storage for all myoglobin oxygenation levels compared to delta E and percent metmyoglobin increased with frozen storage duration. Our results showed that with an extended storage a decrease in color stability and appeal is observed regardless of packaging, aging, and myoglobin oxygenation level.
Conclusion
The HiOxy steaks exhibited a brighter and deeper cherry red color compared with the DeOxy steaks. The HiOxy steaks were superior or similar when compared with Oxy steaks, but displayed more detrimental effects when frozen storage was extended beyond 4 mo. Steaks aged for 4 d typically displayed a redder color than 20 d steaks. Permeable packaging allowed oxygen to pass through the packaging, leading to a bright cherry red color of the frozen meat compared with impermeable packaging. Extended frozen storage had a negative impact on meat color and meat quality. Based on the results, HiOxy steaks aged for 4 d give a superior red color for frozen storage up to 4 mo with few unfavorable color effects. However, it is not advised to freeze deoxygenated steaks and expect a cherry red color through frozen storage.
Acknowledgments
The author would like to thank Malvern Panalytical for the use of their Quality Spec Trek. Without them and the use of their instruments this research would not have been possible.
Glossary
Abbreviations
- BHA
butylated hydroxyanisole
- DeOxy
deoxygenated
- HA
hue angle
- HiOxy
highly oxygenated
- MAP
modified atmosphere packaging
- MMB
metmyoglobin
- MRA
metmyoglobin reducing activity
- OMB
oxymyoglobin
- OTR
oxygen transmission rate
- Oxy
oxygenated
- STD
standard
- STP
standard time and pressure
- TBARS
thiobarbituric acid reactive substances
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Ahn D. U., Olson D. G., Jo C., Chen X., Wu C., and Lee J. I.. . 1998. Effect of muscle type, packaging, and irradiation on lipid oxidation, volatile production, and color in raw pork patties. Meat Sci. 49:27–39. doi: 10.1016/s0309-1740(97)00101-0. [DOI] [PubMed] [Google Scholar]
- Akamittath J. G., Brekke C. J., and Schanus E. G.. . 1990. Lipid oxidation and color stability in restructured meat systems during frozen storage. J. Food Sci. 55:1513–1517. doi: 10.1111/j.1365-2621.1990.tb03557.x. [DOI] [Google Scholar]
- Bekhit A. E. D., and Faustman C.. . 2005. Metmyoglobin reducing activity. Meat Sci. 71:407–439. doi: 10.1016/j.meatsci.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Bonny D. S. S., Li X., Li Z., Li M., Du M., Gao L., and Zhang D.. . 2017. Colour stability and lipid oxidation of beef longissimus lumborum under different packaging conditions. Polish J. Food Nutr. Sci. 67:275–281. doi: 10.1515/pjfns-2017-0016. [DOI] [Google Scholar]
- Brewer M. S., and Wu S. Y.. . 1993. Display, packaging, and meat block location effects on color and lipid oxidation of frozen lean ground beef. J. Food Sci. 58:1219–1236. doi: 10.1111/j.1365-2621.1993.tb06152.x. [DOI] [Google Scholar]
- Calvert J. G. 1990. Glossary of atmospheric chemistry terms. In: International Union of Pure and Applied Chemistry (IUPAC). Pure and applied chemistry. Vol. 62 Boulder, CO: Atmospheric Chemistry Division; p. 2167–2219. [Google Scholar]
- Campo M. M., Nute G. R., Hughes S. I., Enser M., Wood J. D., and Richardson R. I.. . 2006. Flavour perception of oxidation in beef. Meat Sci. 72:303–311. doi: 10.1016/j.meatsci.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Carpenter C. E., Cornforth D. P., and Whittier D.. . 2001. Consumer preferences for beef color and packaging did not affect eating satisfaction. Meat Sci. 57:359–363. doi: 10.1016/s0309-1740(00)00111-x. [DOI] [PubMed] [Google Scholar]
- Clydesdale F. M., and Francis F. J.. . 1971. The chemistry of meat color. Food Prod. Dev. 5:81–90. [Google Scholar]
- English A. R., Mafi G. G., VanOverbeke D. L., and Ramanathan R.. . 2016. Effects of extended aging and modified atmospheric packaging on beef top loin steak color. J. Anim. Sci. 94:1727–1737. doi: 10.2527/jas.2015-0149. [DOI] [PubMed] [Google Scholar]
- Epley R. J. 1992. Aging beef. Minnesota Ext. Serv. AG-FS-5968:1–2. [Google Scholar]
- Farouk M. M., and Swan J. E.. . 1998. Effect of muscle condition before freezing and simulated chemical changes during frozen storage on the pH and colour of beef. Meat Sci. 50:245–256. doi: 10.1016/s0309-1740(98)00036-9. [DOI] [PubMed] [Google Scholar]
- Faustman C., and Cassens R. G.. . 1990. The biochemical basis for discoloration in fresh meat: a review. J. Muscle Foods. 1:217–243. doi: 10.1111/j.1745-4573.1990.tb00366.x. [DOI] [Google Scholar]
- Faustman C., Sun Q., Mancini R., and Suman S. P.. . 2010. Myoglobin and lipid oxidation interactions: mechanistic bases and control. Meat Sci. 86:86–94. doi: 10.1016/j.meatsci.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Gatellier P., Mercier Y., Juin H., and Renerre M.. . 2005. Effect of finishing mode (pasture- or mixed-diet) on lipid composition, colour stability and lipid oxidation in meat from Charolais cattle. Meat Sci. 69:175–186. doi: 10.1016/j.meatsci.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Greene B. E. 1969. Lipid oxidation and pigment changes in raw beef. J. Food Sci. 34:110–113. doi: 10.1017/CBO9781107415324.004. [DOI] [Google Scholar]
- Grobbel J. P., Dikeman M. E., Hunt M. C., and Milliken G. A.. . 2008a. Effects of different packaging atmospheres and injection-enhancement on beef tenderness, sensory attributes, desmin degradation, and display color. J. Anim. Sci. 86:2697–2710. doi: 10.2527/jas.2007-0824. [DOI] [PubMed] [Google Scholar]
- Grobbel J. P., Dikeman M. E., Hunt M. C., and Milliken G. A.. . 2008b. Effects of packaging atmospheres on beef instrumental tenderness, fresh color stability, and internal cooked color. J. Anim. Sci. 86:1191–1199. doi: 10.2527/jas.2007-0479. [DOI] [PubMed] [Google Scholar]
- Holman B. W. B., Coombs C. E. O., Morris S., Bailes K., and Hopkins D. L.. . 2018. Effect of long term chilled (up to 5 weeks) then frozen (up to 12 months) storage at two different sub-zero holding temperatures on beef: 2. Lipid oxidation and fatty acid profiles. Meat Sci. 136:9–15. doi: 10.1016/j.meatsci.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Hood D. E. 1980. Factors affecting the rate of metmyoglobin accumulation in pre-packaged beef. Meat Sci. 4:247–265. doi: 10.1016/0309-1740(80)90026-1. [DOI] [PubMed] [Google Scholar]
- Hood D. E., and Riordan E. B.. . 1973. Discolouration in pre-packaged beef: measurement by reflectance spectrophotometry and shopper discrimination. Int. J. Food Sci. Technol. 8:333–343. doi: 10.1111/j.1365-2621.1973.tb01721.x. [DOI] [Google Scholar]
- Hunt M. C. 1980. Meat color measurements. In: American Meat Science Association & National Livestock and Meat Board. 33rd Reciprocal Meat Conference. Vol. 33 Kearney, MO:American Meat Science Association; p. 41–46. [Google Scholar]
- Hunt M. C., King A., Barbut S., Clause J., Cornforth D. P., Hanson D., Lindahl G., Mancini R. A., Milkowski A., and Mohan A.. . 2012. AMSA meat color measurement guidelines. Champaign, IL:American Meat Science Association. [Google Scholar]
- Jacob R. H., D’Antuono M. F., Smith G. M., Pethick D. W., and Warner R. D.. . 2007. Effect of lamb age and electrical stimulation on the colour stability of fresh lamb meat. Aust. J. Agric. Res. 58:374–382. doi: 10.1071/AR06126. [DOI] [Google Scholar]
- Jakobsen M., and Bertelsen G.. . 2000. Colour stability and lipid oxidation of fresh beef. Development of a response surface model for predicting the effects of temperature, storage time, and modified atmosphere composition. Meat Sci. 54:49–57. doi: 10.1016/s0309-1740(99)00069-8. [DOI] [PubMed] [Google Scholar]
- Jayasingh P., Cornforth D. P., Carpenter C. E., and Whittier D.. . 2001. Evaluation of carbon monoxide treatment in modified atmosphere packaging or vacuum packaging to increase color stability of fresh beef. Meat Sci. 59:317–324. doi: 10.1016/s0309-1740(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Jayasingh P., Cornforth D. P., Brennand C. P., Carpenter C. E., and Whittier D. R.. . 2002. Sensory evaluation of ground beef stored in high-oxygen modified atmosphere packaging. J. Food Sci. 67:3493–3496. doi: 10.1111/j.1365-2621.2002.tb09611.x. [DOI] [Google Scholar]
- Jeremiah L. E. 2001. Packaging alternatives to deliver fresh meats using short- or long-term distribution. Food Res. Int. 34:749–772. doi: 10.1016/S0963-9969(01)00096-5. [DOI] [Google Scholar]
- Joseph P., Suman S. P., Rentfrow G., Li S., and Beach C. M.. . 2012. Proteomics of muscle-specific beef color stability. J. Agric. Food Chem. 60:3196–3203. doi: 10.1021/jf204188v. [DOI] [PubMed] [Google Scholar]
- Khliji S., van de Ven R., Lamb T. A., Lanza M., and Hopkins D. L.. . 2010. Relationship between consumer ranking of lamb colour and objective measures of colour. Meat Sci. 85:224–229. doi: 10.1016/j.meatsci.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Kiani H., and Sun D. W.. . 2011. Water crystallization and its importance to freezing of foods: a review. Trends Food Sci. Technol. 22:407–426. doi: 10.1016/j.jpgs.2011.04.011. [DOI] [Google Scholar]
- Kim Y. H., Huff-Lonergan E., Sebranek J. G., and Lonergan S. M.. . 2010. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 85:759–767. doi: 10.1016/j.meatsci.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kim Y. H., Frandsen M., and Rosenvold K.. . 2011. Effect of ageing prior to freezing on colour stability of ovine longissimus muscle. Meat Sci. 88:332–337. doi: 10.1016/j.meatsci.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Kim J. H., Seo J. K., Setyabrata D., and Kim Y. H. B.. . 2018. Effects of aging/freezing sequence and freezing rate on meat quality and oxidative stability of pork loins. Meat Sci. 139:162–170. doi: 10.1016/j.meatsci.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Kim Y. H., Stuart A., Black C., and Rosenvold K.. . 2012. Effect of lamb age and retail packaging types on the quality of long-term chilled lamb loins. Meat Sci. 90:962–966. doi: 10.1016/j.meatsci.2011.11.040. [DOI] [PubMed] [Google Scholar]
- Kropf D. H. 1980. Effects of retail display conditions on meat color. In: Proceedings of 33rd Reciprocal Meat Conference. Vol. 33 Kearney, MO:American Meat Science Association; p. 15–32. [Google Scholar]
- Krzywicki K. 1979. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci. 3:1–10. doi: 10.1016/0309-1740(79)90019-6. [DOI] [PubMed] [Google Scholar]
- Ledward D. A. 1970. Metmyoglobin formation in beef stored in carbon dioxide enriched and oxygen depleted atmospheres. J. Food Sci. 35:33–37. doi: 10.1111/j.1365-2621.1970.tb12362.x. [DOI] [Google Scholar]
- Ledward D. A. 1971. Metmyoglobin formation in beef muscles as influenced by water content and anatomical location. J. Food Sci. 36:138–140. doi: 10.1111/j.1365-2621.1971.tb02055.x. [DOI] [Google Scholar]
- Ledward D. A., Dickinson R. F., Powell V. H., and Shorthose W. R.. . 1986. The colour and colour stability of beef Longissimus dorsi and Semimembranosus muscles after effective electrical stimulation. Meat Sci. 16:245–265. doi: 10.1016/0309-1740(86)90037-9. [DOI] [PubMed] [Google Scholar]
- Leygonie C., Britz T. J., and Hoffman L. C.. . 2012. Impact of freezing and thawing on the quality of meat : review. Meat Sci. 91:93–98. doi: 10.1016/j.meatsci.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Li X., Lindahl G., Zamaratskaia G., and Lundström K.. . 2012. Influence of vacuum skin packaging on color stability of beef longissimus lumborum compared with vacuum and high-oxygen modified atmosphere packaging. Meat Sci. 92:604–609. doi: 10.1016/j.meatsci.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang Y., Li Z., Li M., Liu Y., and Zhang D.. . 2017. The effect of temperature in the range of -0.8 to 4°C on lamb meat color stability. Meat Sci. 134:28–33. doi: 10.1016/j.meatsci.2017.07.010. [DOI] [PubMed] [Google Scholar]
- MacDougall D. B. 1982. Changes in the colour and opacity of meat. Food Chem. 9:75–88. doi: 10.1016/0308-8146(82)90070-X. [DOI] [Google Scholar]
- Mancini R. A., and Hunt M. C.. . 2005. Current research in meat color. Meat Sci. 71:100–121. doi: 10.1016/j.meatsci.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Mancini R. A., and Ramanathan R.. . 2014. Effects of postmortem storage time on color and mitochondria in beef. Meat Sci. 98:65–70. doi: 10.1016/j.meatsci.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Mancini R. A., Suman S. P., Konda M. K., and Ramanathan R.. . 2009. Effect of carbon monoxide packaging and lactate enhancement on the color stability of beef steaks stored at 1°C for 9 days. Meat Sci. 81:71–76. doi: 10.1016/j.meatsci.2008.06.021. [DOI] [PubMed] [Google Scholar]
- McKenna D. R., Mies P. D., Baird B. E., Pfeiffer K. D., Ellebracht J. W., and Savell J. W.. . 2005. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 70:665–682. doi: 10.1016/j.meatsci.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Mohan A., Hunt M. C., Barstow T. J., Houser T. A., and Hueber D. M.. . 2010. Near-infrared oximetry of three post-rigor skeletal muscles for following myoglobin redox forms. Food Chem. 123:456–464. doi: 10.1016/j.foodchem.2010.04.068. [DOI] [Google Scholar]
- Morrissey E. R., Jacob R. H., and Pluske J. M.. . 2008. Perception of red brown colour by consumers. In: 54th International Congress of Meat Science and Technology. Helsinki, Finland:International Congress of Meat Science and Technology; p. 10–15. [Google Scholar]
- Progressive Grocer. 2017. Value of the fresh-food meal-kit delivery service market in the United States from 2016 to 2022 (in billion U.S. dollars). Statista. Available from https://www.statista.com/statistics/761621/meal-kitdelivery-service-market-value/
- Ramsbottom J. M., and Koonz C. H.. . 1941. Freezer storage temperature as related to drip and to color in frozen-defrosted beef. J. Food Sci. 6:571–580. doi: 10.1111/j.1365-2621.1941.tb16315.x. [DOI] [Google Scholar]
- Renerre M. 1990. Review: factors involved in the discolouration of beef. Int. J. Food Sci. Technol. 25:613–630. doi:org/ 10.1111/j.1365-2621.1990.tb01123.x [DOI] [Google Scholar]
- Renerre M. 1999. Biochemical basis of fresh meat colour. In: 45th International Congress of Meat Science and Technology. Helsinki, Finland: International Congress of Meat Science and Technology; p. 344–353. [Google Scholar]
- Setser C. S. 1984. Color: reflections and transmissions. J. Food Qual. 6:183–197. doi:org/ 10.1111/j.1745-4557.1984.tb00824.x [DOI] [Google Scholar]
- Setyabrata D., and Kim Y. H. B.. . 2019. Impacts of aging/freezing sequence on microstructure, protein degradation and physico-chemical properties of beef muscles. Meat Sci. 151:64–74. doi: 10.1016/j.meatsci.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Smith G. C., Belk K. E., Sofos J. N., Tatum J. D., & Williams S. N.. . 2000. Economic implications of improved color stability in beef. In Decker E. A., Faustman C., and Lopez-Bote C. J., editors, Antioxidants in muscle foods: nutritional strategies to improve quality. New York: Wiley Interscience; p. 397–426. [Google Scholar]
- Soyer A., Özalp B., Dalmiş Ü., and Bilgin V.. . 2010. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 120:1025–1030. doi: 10.1016/j.foodchem.2009.11.042. [DOI] [Google Scholar]
- Strange E. D., Benedict R. C., Gugger R. E., Metzger V. G., and Swift C. E.. . 1974. Simplified methodology for measuring meat color. J. Food Sci. 39:988–992. doi: 10.1111/j.1365-2621.1974.tb07293.x. [DOI] [Google Scholar]
- Suman S. P., and Joseph P.. . 2013. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 4:79–99. doi: 10.1146/annurev-food-030212-182623. [DOI] [PubMed] [Google Scholar]
- Tapp W. N. 3rd, Yancey J. W., and Apple J. K.. . 2011. How is the instrumental color of meat measured? Meat Sci. 89:1–5. doi: 10.1016/j.meatsci.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Vieira C., Diaz M. T., Martínez B., and García-Cachán M. D.. . 2009. Effect of frozen storage conditions (temperature and length of storage) on microbiological and sensory quality of rustic crossbred beef at different states of ageing. Meat Sci. 83:398–404. doi: 10.1016/j.meatsci.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Vitale M., Pérez-Juan M., Lloret E., Arnau J., and Realini C. E.. . 2014. Effect of aging time in vacuum on tenderness, and color and lipid stability of beef from mature cows during display in high oxygen atmosphere package. Meat Sci. 96:270–277. doi: 10.1016/j.meatsci.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Wanous M. P., Olson D. G., and Kraft A. A.. . 1989. Pallet location and freezing rate effects on the oxidation of lipids and myoglobin in commercial fresh pork sausage. J. Food Sci. 54:549–552. doi: 10.1111/j.1365-2621.1989.tb04647.x. [DOI] [Google Scholar]
- Zakrys P. I., Hogan S. A., O’Sullivan M. G., Allen P., and Kerry J. P.. . 2008. Effects of oxygen concentration on the sensory evaluation and quality indicators of beef muscle packed under modified atmosphere. Meat Sci. 79:648–655. doi: 10.1016/j.meatsci.2007.10.030. [DOI] [PubMed] [Google Scholar]