Abstract
Context
Sex differences exist in heart failure (HF) phenotypes, but there is limited research on the role of sex hormones in HF and its subtypes.
Objective
To examine the associations of total testosterone, dehydroepiandrosterone sulfate (DHEA-S), and sex hormone-binding globulin (SHBG) with incident HF, HF with preserved ejection fraction (HFpEF), and HF with reduced ejection fraction (HFrEF).
Design
Atherosclerosis Risk in Communities (ARIC) study (prospective cohort study). Median follow-up is 19.2 years.
Setting
General community.
Participants
4107 men and 4839 postmenopausal women, with mean age of 63.2 (standard deviation [SD] 5.7) and 62.8 (5.5) years, respectively.
Exposure
Plasma sex hormone levels were measured at visit 4 (1996-1998).
Main Outcome Measures
Incident HF events were identified through hospital discharge codes and death certificates.
Results
The Hazard Ratios for HF associated with 1 SD decrease in log-transformed total testosterone, DHEA-S, and SHBG were 1.10 (95% confidence interval 1.03, 1.17), 1.07 (1.00, 1.15), and 1.04 (0.96, 1.11) in men, and 1.05 (0.99, 1.13), 1.17 (1.09, 1.24), and 0.93 (0.85, 1.01) in women, respectively. The associations between sex hormones with subtypes of HF had similar patterns but were attenuated and became statistically insignificant.
Conclusion
In this prospective cohort, lower levels of endogenous testosterone and DHEA-S in men and DHEA-S in postmenopausal women were associated with the development of HF. Similar directions of association in both sexes and both HF subtypes suggest that sex hormones play a role in the development of HF through common pathways regardless of sex.
Keywords: testosterone, DHEA-S, SHBG, sex hormone, heart failure, HFpEF, HFrEF
Clinical phenotypes of heart failure (HF) patients differ markedly by sex. Up to 60% of patients with HF with preserved ejection fraction (HFpEF) are women, in contrast to only 40% of patients with HF with reduced ejection fraction (HFrEF) (1, 2). Compared with men, women develop HF at a more advanced age (3), are more likely to have nonischemic cardiomyopathy and eccentric myocardial left ventricular (LV) remodeling, and have better prognosis and reduced mortality in response to treatment (4).
The biological mechanisms underlying these sex differences are uncertain, but it has been hypothesized that they may be related to variations in endogenous sex hormone levels. In men, higher plasma testosterone levels are associated with lower risk of cardiovascular disease (CVD) and mortality (5-7). In women, higher plasma testosterone levels have generally been associated with an increased risk of CVD and HF (8, 9), although the evidence has been inconsistent across studies (10, 11). Most studies, however, have evaluated the association of sex hormone levels with overall CVD, and there is a paucity of research on the role of sex hormones in HF and its subtypes. Additionally, most studies have focused on testosterone. Understanding the role of sex hormones in the etiology of HF is particularly important for HFpEF, given its high and escalating prevalence, female predominance, excessive mortality burden, and lack of effective treatment.
To address this gap, we examined the associations of sex hormones (testosterone, dehydroepiandrosterone sulfate [DHEA-S], and sex hormone binding globulin [SHBG]) with incident HF, HFrEF, and HFpEF among men and postmenopausal women in the Atherosclerosis Risk in Communities (ARIC) study, a large, community-based cohort with long-term follow-up.
Materials and Methods
Study participants
The ARIC study is a prospective cohort study of 15 792 men and women recruited in 1987-1989, when they were 45-64 years of age, from 4 US communities: Forsyth County, NC; Jackson, MS; Minneapolis suburbs, MN; and Washington County, MD (12). Participants have been followed with 6 subsequent in-person visits, annual or semi-annual follow-up phone interviews, and reviews of hospitalization records. Our baseline for analysis was visit 4 (1996-1998) when the 11 656 participants were 52-75 years of age. Among them, we excluded participants who were not Black or White (n = 31), Blacks from the Minnesota or Maryland centers due to small numbers (n = 38), participants who had HF at or prior to visit 4 (n = 641), or women who were premenopausal at visit 4 (n = 392). We further excluded 1608 participants missing information on sex hormones due to sample availability (n = 854), incident HF (n = 157), or other covariates (n = 597). The final sample included 8946 participants (4107 men and 4839 women; all supplementary material and figures are located in a digital research materials repository (13)). The institutional review boards from all centers approved the ARIC protocol and study materials and all participants provided written informed consent.
Measurement of sex hormones
Plasma levels of total testosterone, DHEA-S and SHBG were measured in samples obtained at visit 4 (1996-1998). Blood samples were collected in the morning and stored at −80°C until analyzed. Sex hormone levels were measured in 2015-2016 using a chemiluminescent microparticle immunoassay with the Architect i2000sr instrument (Abbott Laboratories, Abbott Park, IL) as previously described (14). In reliability tests with blind duplicates, the Pearson’s correlation coefficients for total testosterone, DHEA-S, and SHBG were 0.96, 0.95, and 0.90, respectively. Albumin levels were not available at visit 4 to calculate bioavailable testosterone or free testosterone. Estradiol levels were available only in a small subsample of study participants via an ancillary study and are not reported here.
Measurement of HF
Hospitalization and deaths occurring in ARIC participants were identified through annual surveillance telephone follow-up calls, review of hospital discharge diagnoses, and death certificates. Incident HF was defined as the first hospitalization or death related to HF. Prior to 2005, incident HF events were identified from hospital discharge lists or death certificates as International Classification of Diseases (ICD) 9th Revision code 428 or ICD 10th Revision code I50 (15). After January 1, 2005, hospitalized HF events were adjudicated by the ARIC HF Classification Committee into subtypes based on LV ejection fraction (LVEF) results from the most recent inpatient diagnostic test or preadmission imaging test as HFpEF (defined as HF with LVEF ≥50%) and HFrEF (defined as HF with LVEF <50%) (15, 16).
Other measurements
Each ARIC visit included detailed questionnaire information and a cardiovascular examination (17). Unless otherwise specified, all information used for study covariates was obtained at visit 4. Age, sex, education, smoking, menopause status, and medication use were self-reported. Sports physical activities were assessed via a modified Baecke questionnaire at visit 3 (18). Waist and hip circumferences were measured in light clothing. Sitting blood pressure was calculated as the average of the first and second measurements. Diabetes was defined by self-report of a physician diagnosis, a fasting blood glucose level ≥126 mg/dL, a nonfasting blood glucose level ≥200 mg/dL, or use of hypoglycemic medications. Total cholesterol and high-density lipoprotein cholesterol (HDL-C) were measured using standardized enzymatic methods. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation based on serum creatinine (19). Prevalent coronary heart disease (CHD) was assessed via annual surveillance telephone follow-up calls, review of hospital discharge diagnoses, and death certificates, and defined as definite or probable myocardial infarction, definite fatal CHD, or cardiac procedure on or before visit 4.
Statistical analysis
All analyses were stratified by sex, given that sex hormone distributions are different by sex and are not overlapping. The study endpoints were the development of incident overall HF, HFpEF, and HFrEF. For all HF, participants were followed from visit 4 until they experienced HF, death, were lost to follow-up, or until December 31, 2017. For HFpEF and HFrEF, the event adjudications started in January 1, 2005, and therefore the analysis was restricted to participants who had not developed HF by January 1, 2005. The time between cohort entry and January 1, 2005, was immortal (participants who developed HF prior to 2015 were censored), and subsequent follow-up time was classified as the analysis follow-up period. Assuming 2-sided type I error probability of .05, with 1 standard deviation (SD) decrease in sex hormones, we have 80% power to detect a regression coefficient (log[HR]) of 0.15 to 0.20 for HFpEF and HFrEF analyses in men and women.
We compared lower levels of sex hormones with higher levels. Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95% confidence intervals (CI) for HF events associated with a 1 SD decrease in loge-transformed sex hormone levels. In addition, to evaluate nonlinear dose–response relationships, we modeled loge-transformed sex hormone levels as restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles of their sex-specific distribution. In the primary analyses, each individual hormone was modeled separately. In secondary analyses, total testosterone, DHEA-S, and SHBG were included in the same model to mutually account for one another. For each outcome, we used 3 models with increasing degrees of adjustment. Model 1 adjusted for demographic factors: age and race/center groups. Model 2 further adjusted for lifestyle variables: education, smoking, waist-to-hip ratio (WHR), and intentional physical activity. In women, model 2 additionally adjusted for use of hormone therapy. Model 3 further adjusted for CVD risk factors: systolic blood pressure, use of antihypertensive medications, total cholesterol, HDL-C, use of lipid-lowering medication, diabetes, eGFR, and prevalent CHD.
For stratified analyses, we examined the associations in prespecified subgroups defined by age and race/ethnicity. For sensitivity analyses, we excluded men and women with CHD at baseline, as well as women who were on hormone therapy. All reported P values were 2-sided and the significance level was set at .05. Statistical analyses were performed using STATA version 16 (StataCorp LP, College Station, TX).
Results
The mean age of participants at baseline was 63.2 (SD 5.7) years for men and 62.8 (5.5) for women. The median levels of total testosterone (nmol/L), DHEA-S (nmol/L), and SHBG (nmol/L) in men were 17.7 (25th percentile to 75th percentile 13.5-22.9), 101.3 (68.0-149.2), 31.6 (23.4-41.6), respectively, and in women they were 0.8 (0.6-1.1), 58.8 (35.8-91.7), 42.3 (26.3-73.5), respectively (Table 1). At baseline, men and women who developed HF were more likely to be older, diabetic, hypertensive, smokers, to have higher WHR, body mass index (BMI), prevalent CHD, and lower levels of education and physical activity. Additionally, HF patients were more likely to have lower levels of total testosterone and DHEA-S among men, and lower levels of DHEA-S and SHBG among women.
Table 1.
Characteristics of study participants at baseline, by sex
| Men | Women | |||||
|---|---|---|---|---|---|---|
| No HF (n = 3234) | Developed HF (n = 873) | P value | No HF (n = 3894) | Developed HF (n = 945) | P value | |
| Age, years | 62.6 (5.6) | 65.1 (5.5) | <.001 | 62.4 (5.5) | 64.7 (5.3) | <.001 |
| Total T, nmol/L | 17.9 (13.8, 23.2) | 17.1 (12.7, 22.2) | <.001 | 0.8 (0.6, 1.1) | 0.8 (0.6, 1.1) | .46 |
| DHEA-S, µg/dL | 102.9 (70.1, 151.4) | 92.6 (60.4, 142.9) | <.001 | 60.1 (36.8, 93.8) | 52.3 (32.1, 80.8) | <.001 |
| SHBG, nmol/L | 31.8 (23.4, 41.6) | 30.9 (23.3, 41.6) | .26 | 43.7 (26.8, 75.6) | 37.5 (24.3, 64.9) | <.001 |
| Race/center, % | .02 | <.001 | ||||
| Minneapolis, MN, Whites | 1063 (32.9) | 256 (29.3) | 1132 (29.1) | 205 (21.7) | ||
| Jackson, MS, Blacks | 844 (26.1) | 251 (28.8) | 952 (24.4) | 256 (27.1) | ||
| Washington, MD, Whites | 839 (25.9) | 205 (23.5) | 942 (24.2) | 197 (20.8) | ||
| Forsyth County, NC, Blacks | 54 (1.7) | 13 (1.5) | 103 (2.6) | 24 (2.5) | ||
| Forsyth County, NC, Whites | 434 (13.4) | 148 (17.0) | 765 (19.6) | 263 (27.8) | ||
| Education, % | <.001 | <.001 | ||||
| <High school | 509 (15.7) | 193 (22.1) | 605 (15.5) | 283 (29.9) | ||
| High school, technical school, or associate degree | 1190 (36.8) | 327 (37.5) | 1876 (48.2) | 396 (41.9) | ||
| College, graduate or professional school | 1535 (47.5) | 353 (40.4) | 1413 (36.3) | 266 (28.1) | ||
| Smoking, % | <.001 | <.001 | ||||
| Never | 981 (30.3) | 186 (21.3) | 2121 (54.5) | 452 (47.8) | ||
| Former | 1807 (55.9) | 538 (61.6) | 1270 (32.6) | 323 (34.2) | ||
| Current | 446 (13.8) | 149 (17.1) | 503 (12.9) | 170 (18.0) | ||
| Physical activity indexa | 2.7 (0.8) | 2.6 (0.8) | <.001 | 2.5 (0.8) | 2.4 (0.7) | <.001 |
| Waist-to-hip ratio | 0.98 (0.1) | 0.99 (0.1) | <.001 | 0.92 (0.1) | 0.94 (0.1) | <.001 |
| BMI, kg/m2 | 28.2 (4.3) | 29.1 (4.8) | <.001 | 28.4 (5.8) | 30.6 (6.9) | <.001 |
| Systolic BP, mmHg | 125.1 (17.0) | 131.1 (19.3) | <.001 | 126.2 (18.7) | 133.8 (20.9) | <.001 |
| Diastolic BP, mmHg | 72.3 (9.6) | 71.7 (11.0) | .12 | 69.9 (10.0) | 69.8 (10.9) | .82 |
| Antihypertension medication, % | 935 (28.9) | 411 (47.1) | <.001 | 1244 (31.9) | 493 (52.2) | <.001 |
| Total cholesterol, mg/dL | 192.6 (33.7) | 190.5 (38.1) | .12 | 208.2 (35.9) | 207.5 (40.0) | .61 |
| HDL-C, mg/dL | 43.3 (13.2) | 41.5 (12.7) | <.001 | 56.3 (16.4) | 53.4 (16.5) | <.001 |
| Triglycerides, mg/dL | 120.0 (87.0, 170.0) | 126.0 (91.0, 180.0) | .008 | 121.0 (88.0, 171.0) | 133.0 (96.0, 194.0) | <.001 |
| Lipid lowering medication | 470 (14.5) | 179 (20.5) | <.001 | 455 (11.7) | 161 (17.0) | <.001 |
| eGFR, mL/min/1.73 m2 | 86.0 (14.0) | 81.9 (18.1) | <.001 | 87.8 (15.2) | 84.1 (19.0) | <.001 |
| Prevalent CHD, % | 293 (9.1) | 216 (24.7) | <.001 | 96 (2.5) | 81 (8.6) | <.001 |
| Diabetes, % | 444 (13.7) | 237 (27.1) | <.001 | 442 (11.4) | 251 (26.6) | <.001 |
| Hormone therapy, % | <.001 | |||||
| Current | 1123 (28.8) | 197 (20.8) | ||||
| Never | 1460 (37.5) | 410 (43.4) | ||||
| Former | 134 (3.4) | 15 (1.6) | ||||
| Unknown | 1177 (30.2) | 323 (34.2) | ||||
Data are mean (SD) or number (percentage) or median (25th percentile to 75th percentile).
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; IR, incidence rates; T, testosterone; DHEA-S, dehydroepiandrosterone sulfate; SHBG, sex hormone binding globulin.
a Data are from visit 3.
Overall HF
During median follow-up of 19.2 years, we identified 873 new cases of HF in men and 945 new cases in women. Compared with higher levels, lower plasma levels of total testosterone and DHEA-S were associated with an increased risk of incident HF in men, and lower levels of DHEA-S was associated with an increased risk of incident HF in women. In fully adjusted models, the HRs for HF associated with 1 SD decrease in log-transformed total testosterone and DHEA-S levels in men were 1.10 (95% CI 1.03, 1.17) and 1.07 (1.00, 1.15), respectively (Table 2). In women, the corresponding HRs were 1.05 (0.99, 1.13) and 1.17 (1.09, 1.24), respectively (Table 3). SHBG was not statistically significantly associated with HF in fully adjusted models. The HRs per 1 SD decrease in SHBG were 1.04 (0.96, 1.11) in men and 0.93 (0.85, 1.01) in women.
Table 2.
Hazard ratios (95% CI) for any HF, HFpEF, HFrEF associated with sex hormone levels in men per 1 SD decrease in sex hormone level
| Model 1 a | Model 2 b | Model 3 c | |
| Any HF (N events/person-years = 873/63 483; IR = 13.8 per 1000 person-years) | |||
| Total T (nmoI/L) | 1.18 (1.12, 1.25) | 1.16 (1.09, 1.23) | 1.10 (1.03, 1.17) |
| DHEA-S (ug/dL) | 1.09 (1.01, 1.17) | 1.11 (1.03, 1.19) | 1.07 (1.00, 1.15) |
| SHBG (nmoI/L) | 1.11 (1.04, 1.18) | 1.11 (1.04, 1.19) | 1.04 (0.96, 1.11) |
| HFpEF (N events/person-years = 197/35 857; IR = 5.5 per 1000 person-years) | |||
| Total T (nmoI/L) | 1.20 (1.06, 1.37) | 1.16 (1.02, 1.33) | 1.08 (0.93, 1.25) |
| DHEA-S (µg/dL) | 1.05 (0.89, 1.22) | 1.06 (0.91, 1.24) | 1.04 (0.89, 1.21) |
| SHBG (nmol/L) | 1.15 (1.00, 1.32) | 1.13 (0.98, 1.31) | 1.06 (0.91, 1.24) |
| HFrEF (N events/person-years = 298/35 857; IR = 8.3 per 1000 person-years) | |||
| Total T (nmoI/L) | 1.06 (0.94, 1.20) | 1.04 (0.92, 1.17) | 0.92 (0.81, 1.06) |
| DHEA-S (µg/dL) | 1.02 (0.90, 1.15) | 1.04 (0.92, 1.18) | 1.01 (0.89, 1.15) |
| SHBG (nmoI/L) | 1.09 (0.98, 1.22) | 1.10 (0.98, 1.23) | 1.01 (0.89, 1.15) |
All sex hormones are log-transformed and standardized. Hazard Ratios are associated with 1 SD decrease in log sex hormones (total T: 0.51 nmoI/L; DHEA-S: 0.61 µg/dL; SHBG: 0.47 nmoI/L). Bolded results are statistically significant.
Abbreviations: HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IR, incidence rates; T, testosterone; DHEA-S, dehydroepiandrosterone sulfate; SHBG, sex hormone binding globulin.
a Model 1 (demographics): adjusts for age and race center groups.
b Model 2 (+lifestyle factors): model 1 + education, waist-to-hip ratio, physical activity, and smoking.
c Model 3 (+CVD risk factors): model 2 + systolic blood pressure, use of antihypertensive medications, total cholesterol, HDL-C, use of lipid lowering medications, diabetes, eGFR, and prevalent CHD.
Table 3.
Hazard ratios (95% CI) for any HF, HFpEF, HFrEF associated with sex hormone levels in women per 1 SD decrease in sex hormone level
| Model 1 a | Model 2 b | Model 3 c | |
| Any HF (N events/person-years = 945/80 659; IR = 11.7 per 1000 person-years) | |||
| Total T (nmoI/L) | 1.00 (0.94, 1.07) | 1.02 (0.95, 1.09) | 1.05 (0.99, 1.13) |
| DHEA-S (µg/dL) | 1.16 (1.08, 1.23) | 1.19 (1.11, 1.27) | 1.17 (1.09, 1.24) |
| SHBG (nmoI/L) | 1.08 (1.01, 1.16) | 1.01 (0.93, 1.09) | 0.93 (0.85, 1.01) |
| HFpEF (N events/person-years = 336/47 493; IR = 7.1 per 1000 person-years) | |||
| Total T (nmoI/L) | 1.01 (0.90, 1.13) | 1.02 (0.91, 1.15) | 1.05 (0.94, 1.18) |
| DHEA-S (µg/dL) | 1.11 (1.00, 1.24) | 1.15 (1.03, 1.28) | 1.12 (1.00, 1.25) |
| SHBG (nmoI/L) | 1.17 (1.04, 1.30) | 1.11 (0.97, 1.26) | 1.02 (0.88, 1.18) |
| HFrEF (N events/person-years = 203/47 493; IR = 4.3 per 1000 person-years) | |||
| Total T (nmoI/L) | 1.02 (0.88, 1.18) | 1.02 (0.88, 1.18) | 1.06 (0.91, 1.22) |
| DHEA-S (µg/dL) | 1.13 (0.98, 1.30) | 1.14 (0.99, 1.31) | 1.10 (0.96, 1.27) |
| SHBG (nmoI/L) | 1.00 (0.86, 1.16) | 0.96 (0.81, 1.14) | 0.88 (0.74, 1.06) |
All sex-hormones are log-transformed and standardized. Hazard ratios are associated with 1 SD decrease in log sex hormones (total T: 0.48 nmoI/L; DHEA-S: 0.72 µg/dL; SHBG: 0.74 nmoI/L). Bolded results are statistically significant.
Abbreviations: HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IR, incidence rates; T, testosterone; DHEA-S, dehydroepiandrosterone sulfate; SHBG, sex hormone binding globulin.
a Model 1 (demographics): adjusts for age and race-center groups.
b Model 2 (+lifestyle factors): model 1 + education, waist to hip ratio, physical activity, smoking, and hormone therapy use.
c Model 3 (+CVD risk factors): model 2 + systolic blood pressure, use of antihypertensive medications, total cholesterol, HDL-C, use of lipid lowering medications, diabetes, eGFR, and prevalent CHD.
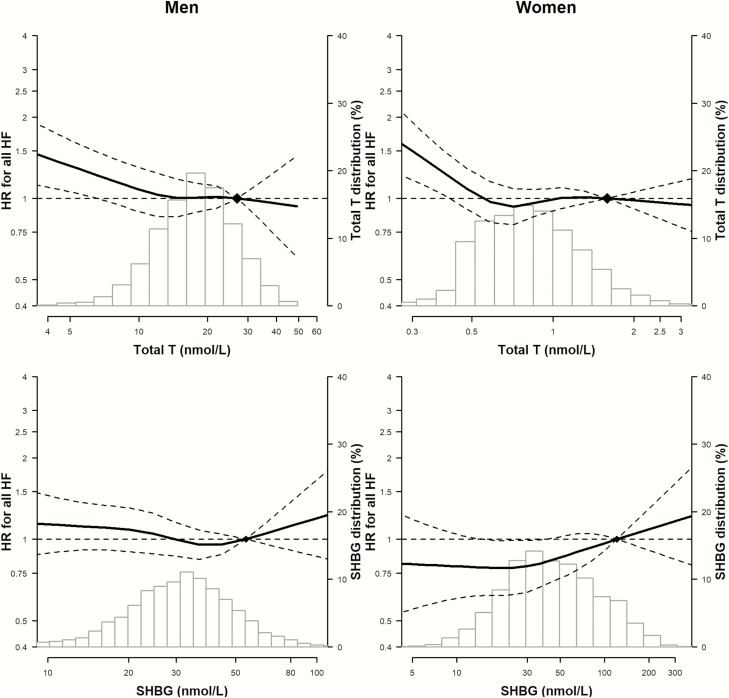
In spline regression analyses, with increasing levels of total testosterone, the risk of incident HF decreased progressively in men, while it initially decreased and then plateaued in women (Fig. 1). The P values for the nonlinear spline components of total testosterone, DHEA-S, and SHBG for HF in men were .34, .16, .39, respectively, and in women they were .007, .21, .48, respectively, indicating that the associations of these sex hormones with HF were approximately linear, except for total testosterone in women, which leveled off at the upper extreme.
Figure 1.
Hazard ratios for HF and HF subtypes associated with total testosterone, DHEA-S, and SHBG, by sex. The histograms represent the distribution of sex hormones. The curves represent the adjusted HR of any HF by log sex hormone levels from fully adjusted models. The dose–response association was estimated by using a linear and a cubic spline term for sex hormones in the multivariable cox regression. Curves represent adjusted HR (solid line) and 95% confidence interval (dashed lines) based on restricted cubic splines for each sex hormones with knots at the 5th, 35th, 65th, and 95th percentiles of their sample distributions. The reference values (diamonds) were set at the 90th percentile. The model was adjusted for variables in model 3.
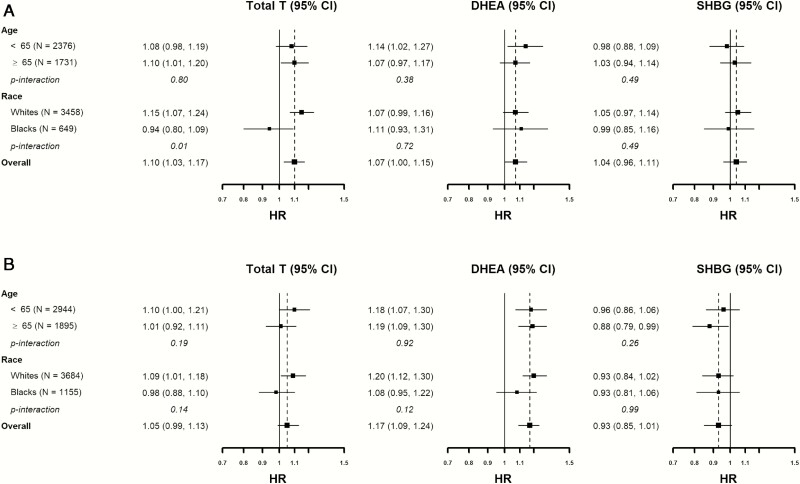
The associations between sex hormones and incident HF were generally consistent across age and race/ethnicity subgroups (Fig. 2), except that the association between total testosterone with HF was null in Black men (P interaction = .01). When restricting analyses to women not on hormone therapy, those free of CHD at baseline, or including all sex hormones in the same model, the associations were consistent with the primary analyses (13).
Figure 2.
Associations between sex hormone levels with incident HF by age and race/ethnic subgroups. (A) Hazard ratio for men. (B) Hazard ratio for women.
HF subtypes
Since January 1, 2005, and during a median follow-up of 13.0 years, we identified 533 new cases of HFpEF (197 cases in men and 336 in women) and 501 new cases of HFrEF (298 cases in men and 203 in women). The associations between lower levels of sex hormones with subtypes of HF were of similar magnitude to those with overall HF, but with wider confidence intervals because of the smaller number of events, and they became statistically insignificant except for the associations of lower total testosterone with increased HFpEF risk in men (Table 2, Model 2), and lower DHEA-S with HFpEF in women (Table 3, Model 3).
Discussion
In this large community-based prospective cohort, low levels of total testosterone and DHEA-S in men, and low levels of DHEA-S in women, were independently associated with an increased risk of incident HF. The estimated associations were of a similar direction and magnitude for HFpEF and HFrEF, but they were not statistically significant due to the smaller number of events and the wider confidence intervals. While the associations were similar in all age and race/ethnicity subgroups, the associations of total testosterone with incident HF were null in Black men. Our findings suggest that sex hormones may play a role in the development of HF, but may not contribute to differences in HF development between men and women.
While a number of studies have investigated the associations of endogenous sex hormones and CVD, few prospective studies have evaluated the association of sex hormones and the development of HF in particular, and their findings are inconsistent. Among men, a prior analysis of a subset of 1558 participants in ARIC (140 incident HF events over 12.8 years of follow-up) did not find a statistically significant association between testosterone levels and incident HF (20). A mendelian randomization study in the UK Biobank found that genetically predicted testosterone was positively associated with the risk of incident HF (21). Among women in the Multi-Ethnic Study of Atherosclerosis (MESA), total testosterone, DHEA, and SHBG were not statistically significantly associated with incident HF, although total testosterone was positively associated with incident CVD and CHD, and DHEA inversely associated with HFrEF (8). Additionally, genetically predicted testosterone was not associated with the risk of incident HF among women in the UK Biobank (21).
A possible explanation for the contradictory findings is the complexity of sex hormone effects and the potential for multiple interactions, ranging from steroid production to hormone metabolism, binding, transportation by receptors, and final effects in target tissues (22). For example, some effects of testosterone are mediated through its aromatization to estradiol, thereby altering its biological activity in peripheral and target tissues (23). Reflecting this biological complexity, there are opposing theories suggesting protective and detrimental effects of androgens on HF. Testosterone and DHEA may prevent vascular atherogenesis and thrombosis through reducing inflammatory cytokines, endothelial dysfunction, plaque instability, and carotid intimal thickness (24-26). Additionally, androgens in men were inversely associated with blood pressure (27), obesity (28), diabetes (29), and LV volume (27, 30), which may contribute to reduced risk for HF.
On the contrary, androgens also have proinflammatory and vasoconstrictive properties, inducing increased blood pressure and other vascular impairment (31). Androgens may have a growth-promoting effect on muscle cells, leading to enlarged cardiomyocytes and cardiac hypertrophy, a myocardial structural abnormality commonly associated with HFpEF (32, 33). The positive associations between androgen levels and HF risk factors were more likely to be observed in women. Indeed, multiple studies showed that high levels of testosterone were associated with increased risk of metabolic syndrome, endothelial dysfunction, atherosclerosis, and chronic heart disease in postmenopausal women, but not men (8, 29, 34-36). However, in this study we did not find a statistically significant association of total testosterone with new HF risk in women, and DHEA was inversely associated with HF risk. While the complex biological effect of androgens could explain heterogeneous findings across studies, it remains unclear how to predict the cardiovascular effects of androgen in a given population.
We could not identify any study of SHBG levels and incident HF in men. In postmenopausal women participating in the MESA study, SHBG was not associated with incident HF (8), but it was associated with less concentric LV remodeling (33). The primary role of SHBG is to bind steroid sex hormones with high affinity, and it is hypothesized that this reduces their free concentrations and biological activity (37). In women from our study, SHBG was inversely associated with HF in demographic-adjusted model, but the association became positive in the fully adjusted model. In men, the inverse trend between SHBG and HF was attenuated and became statistically insignificant in the fully adjusted model, indicating that the regulatory effect of SHBG on testosterone may be different for men and women.
In our study, the associations between total testosterone, DHEA-S, and incident HF followed similar patterns for men and women. On the other hand, DHEA-S has distinct sex-specific properties. In women, it is the main adrenal precursor and contributes substantially to the production of other androgens (38), while in men, DHEA-S is only a weak form of androgen and its contribution to other androgens is small (39). Despite the biological differences, the inverse associations between DHEA-S and incident HF in both men and women suggested that DHEA-S may affect the development of HF through common pathways that are not sex specific. Additionally, the lack of significant associations for HFpEF and HFrEF indicates that sex hormones may not be the principal contributors of the sex related differences in HF phenotypes, although our analysis of HF subtypes was limited by the smaller numbers of events in each subtype. More research is needed to better understand sex differences in hormone mechanisms involved in development and progression of HF.
In addition to observational studies, clinical trials have evaluated the effect of testosterone therapy on HF and CVD outcomes, with controversial findings (40). In randomized controlled trials conducted among men with HF, administration of exogenous testosterone improved exercise capacity, but did not modify cardiovascular risk (41-43). Similarly, among women with chronic HF, low-dose testosterone supplementation improved functional capacity, insulin resistance, muscle strength, but did not improve LV function (44). These clinical trials, however, were limited by small numbers of participants, short duration of follow-up, different testosterone doses and formulations, and did not directly assess the effect of testosterone therapy on the development of HF or its subtypes. While the TRAVERSE trial, an ongoing clinical trial of a transdermal testosterone gel in 6000 men who have low serum testosterone, will be powered to evaluate the effect of testosterone therapy on incident CVD outcomes (NCT03518034), incident HF will not be evaluated as the primary or secondary outcomes. Our study thus supports TRAVERSE to additionally adjudicate HF hospitalizations, and clinical trials to examine whether testosterone supplementation reduces incident HF events.
The major strengths of our study include the large sample size of men and women, the prospective design with 20 years of follow-up, the adjudication of HFpEF and HFrEF events, and the ability to account for multiple potential confounders and use of hormone therapy in women. However, several limitations need to be considered. First, we could only capture HF cases through in-patient hospitalizations or death certificates, which might miss milder outpatient HF cases who were not hospitalized due to less severe symptoms. However, the percentage of HF patients in community-based outpatient settings who are hospitalized for HF is relatively high, with 74% hospitalized within 1.7 years (45). Second, adjudication and categorization of HF subtypes only started after 2005. As a result, the shorter follow-up and smaller number of HFpEF and HFrEF events limited our power to detect associations between sex hormones and HF subtypes.
Third, we used sex hormone levels measured at visit 4 for this analysis, as this was the visit that had sex hormones measured in the largest number of participants (~9000). Of note, sex hormones were remeasured 14 years later at visit 5 (2011-2013) among approximately 3700 individuals, but given the long time gap between visit 4 and visit 5, the significant attrition of participants between visit 4 and visit 5, and the short follow-up time after visit 5, we did not evaluate longitudinal changes in sex hormone levels with HF outcomes. Additionally, we measured the concentrations of sex hormones in plasma, but their concentrations can be different in cardiac tissues. Fourth, we did not have information on estradiol levels and could not evaluate the association of estradiol or of the ratio of testosterone versus estradiol with HF outcomes. Fifth, we measured testosterone using immunoassays, which could be unreliable when the testosterone levels are low (46). In a validation analysis among 1558 men attending ARIC visit 4, the Pearson correlation coefficient of testosterone measured by immunoassay compared with mass spectrometry (gold standard) was 0.86, and the data were not available to perform this same correlation analysis in women. Finally, we used an observational design, and we cannot discard the possibility of residual confounding or selection bias.
In conclusion, in this large community-based study, low levels of endogenous testosterone and DHEA-S in men and low levels of DHEA-S in postmenopausal women were associated with the development of HF. These associations were similar in HFpEF and HFrEF, suggesting that sex hormones may not determine sex differences in HF phenotypes, but our analysis of HF subtypes was limited by the small number of events. Although low androgen levels could contribute to the risk of HF later in life, the potential benefit of testosterone therapy in HF prevention or progression is still unproven.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the ARIC study for their important contributions. A full list of participating ARIC investigators and institutions can be found at https://www2.cscc.unc.edu/aric/.
Financial Suport: This work was funded by the American Heart Association Go Red for Women Strategically Focused Research Network grant 16SFRN27870000. The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Dr. Zhao is also funded by the Blumenthal Scholars Preventive Cardiology Fund and Dr. Michos is funded by the Amato Fund for Women’s Cardiovascular Health Research, both at Johns Hopkins University.
Glossary
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- DHEA-S
dehydroepiandrosterone sulfate
- eGFR
Estimated glomerular filtration rate
- HDL-C
high-density lipoprotein cholesterol
- HF
heart failure
- HR
hazard ration
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- pEF
preserved ejection fraction
- rEF
reduced ejection fraction
- SD
standard deviation
- SHBG
sex hormone-binding globulin
- WHR
waist-to-hip ratio
Additional Information
Disclosure Summary: Dr. Hoogeveen has received grant support and consulting fees from Denka Seiken outside the submitted work. Dr. Ballantyne: grants/research support (paid to institution, not individual) and consultant: Abbott Diagnostic, Denka Seiken, Roche Diagnostic. Drs. Hoogeveen and Ballantyne are coinventors on a provisional patent (patent #61721475) entitled Biomarkers to Improve Prediction of Heart Failure Risk filed by Roche and Baylor College of Medicine on their behalf.
Data Availability
The ARIC cohort participates in the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository (BioLINCC). The ARIC data are available upon request through the following website: https://biolincc.nhlbi.nih.gov/studies/aric/ to prospective researchers, and our work can be easily replicable from the methods described in the paper.
References
- 1. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591-602. [DOI] [PubMed] [Google Scholar]
- 2. Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138(2):198-205. [DOI] [PubMed] [Google Scholar]
- 3. Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391(10120):572-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beale AL, Nanayakkara S, Segan L, et al. Sex differences in heart failure with preserved ejection fraction pathophysiology: a detailed invasive hemodynamic and echocardiographic analysis. JACC Heart Fail. 2019;7(3):239-249. [DOI] [PubMed] [Google Scholar]
- 5. Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116(23):2694-2701. [DOI] [PubMed] [Google Scholar]
- 6. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(10):3007-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruige JB, Mahmoud AM, De Bacquer D, Kaufman JM. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;97(11):870-875. [DOI] [PubMed] [Google Scholar]
- 8. Zhao D, Guallar E, Ouyang P, et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol. 2018;71(22):2555-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benn M, Voss SS, Holmegard HN, Jensen GB, Tybjærg-Hansen A, Nordestgaard BG. Extreme concentrations of endogenous sex hormones, ischemic heart disease, and death in women. Arterioscler Thromb Vasc Biol. 2015;35(2):471-477. [DOI] [PubMed] [Google Scholar]
- 10. Laughlin GA, Goodell V, Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab. 2010;95(2):740-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barrett-Connor E, Goodman-Gruen D. Prospective study of endogenous sex hormones and fatal cardiovascular disease in postmenopausal women. BMJ. 1995;311(7014):1193-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129(4):687-702. [PubMed] [Google Scholar]
- 13. Zhao D, Guallar E, Ballantyne CM, et al. Data associated with the publication: Atherosclerosis Risk in Communities study. Johns Hopkins University Data Archive, V1 2020. 10.7281/T1/NH6EGH. Deposited July 6 2020. https://archive.data.jhu.edu/privateurl.xhtml?token=1b099177-071f-4182-8075-6f079e8f77a4 [DOI] [Google Scholar]
- 14. Roetker NS, MacLehose RF, Hoogeveen RC, et al. Prospective study of endogenous hormones and incidence of venous thromboembolism: the atherosclerosis risk in communities study. Thromb Haemost. 2018;118(11):1940-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5(2):152-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang PP, Wruck LM, Shahar E, et al. Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005–2014): ARIC Study Community Surveillance. Circulation 2018;138(1):12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223-233. [DOI] [PubMed] [Google Scholar]
- 18. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936-942. [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Srinath R, Hill Golden S, Carson KA, Dobs A. Endogenous testosterone and its relationship to preclinical and clinical measures of cardiovascular disease in the atherosclerosis risk in communities study. J Clin Endocrinol Metab. 2015;100(4):1602-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo S, Au Yeung SL, Zhao JV, Burgess S, Schooling CM. Association of genetically predicted testosterone with thromboembolism, heart failure, and myocardial infarction: mendelian randomisation study in UK Biobank. BMJ. 2019;364:l476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller VM, Mankad R. Sex steroids and incident cardiovascular disease in post-menopausal women: new perspective on an old controversy. J Am Coll Cardiol. 2018;71(22):2567-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Biegon A, Alexoff DL, Kim SW, et al. Aromatase imaging with [N-methyl-11C]vorozole PET in healthy men and women. J Nucl Med. 2015;56(4):580-585. [DOI] [PubMed] [Google Scholar]
- 24. Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87(8):3632-3639. [DOI] [PubMed] [Google Scholar]
- 25. Herrington DM, Gordon GB, Achuff SC, et al. Plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate in patients undergoing diagnostic coronary angiography. J Am Coll Cardiol. 1990;16(6):862-870. [DOI] [PubMed] [Google Scholar]
- 26. Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS. Testosterone as a protective factor against atherosclerosis–immunomodulation and influence upon plaque development and stability. J Endocrinol. 2003;178(3):373-380. [DOI] [PubMed] [Google Scholar]
- 27. Svartberg J, von Mühlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromsø Study. Eur J Endocrinol. 2004;150(1):65-71. [DOI] [PubMed] [Google Scholar]
- 28. Haffner SM, Karhapää P, Mykkänen L, Laakso M. Insulin resistance, body fat distribution, and sex hormones in men. Diabetes. 1994;43(2):212-219. [DOI] [PubMed] [Google Scholar]
- 29. Oh JY, Barrett-Connor E, Wedick NM, Wingard DL; Rancho Bernardo Study . Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25(1):55-60. [DOI] [PubMed] [Google Scholar]
- 30. Ventetuolo CE, Ouyang P, Bluemke DA, et al. Sex hormones are associated with right ventricular structure and function: The MESA-right ventricle study. Am J Respir Crit Care Med. 2011;183(5):659-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oskui PM, French WJ, Herring MJ, Mayeda GS, Burstein S, Kloner RA. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. J Am Heart Assoc. 2013;2(6):e000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pirompol P, Teekabut V, Weerachatyanukul W, Bupha-Intr T, Wattanapermpool J. Supra-physiological dose of testosterone induces pathological cardiac hypertrophy. J Endocrinol. 2016;229(1):13-23. [DOI] [PubMed] [Google Scholar]
- 33. Subramanya V, Zhao D, Ouyang P, et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: the Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas. 2018;108:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel SM, Ratcliffe SJ, Reilly MP, et al. Higher serum testosterone concentration in older women is associated with insulin resistance, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2009;94(12):4776-4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mathews L, Subramanya V, Zhao D, et al. Endogenous sex hormones and endothelial function in postmenopausal women and men: the Multi-Ethnic Study of Atherosclerosis. J Womens Health. 2019;28(7):900-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Subramanya V, Zhao D, Ouyang P, et al. Association of endogenous sex hormone levels with coronary artery calcium progression among post-menopausal women in the Multi-Ethnic Study of Atherosclerosis (MESA). J Cardiovasc Comput Tomogr. 2019;13(1):41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev. 2017;38(4):302-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spark RF. Dehydroepiandrosterone: a springboard hormone for female sexuality. Fertil Steril 2002; 77(Suppl 4):S19-S25. [DOI] [PubMed] [Google Scholar]
- 39. Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59(3):551-555. [DOI] [PubMed] [Google Scholar]
- 40. Kloner RA, Carson C 3rd, Dobs A, Kopecky S, Mohler ER 3rd. Testosterone and cardiovascular disease. J Am Coll Cardiol. 2016;67(5):545-557. [DOI] [PubMed] [Google Scholar]
- 41. Toma M, McAlister FA, Coglianese EE, et al. Testosterone supplementation in heart failure: a meta-analysis. Circ Heart Fail. 2012;5(3):315-321. [DOI] [PubMed] [Google Scholar]
- 42. Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Testosterone and cardiovascular risk: meta-analysis of interventional studies. J Sex Med. 2018;15(6):820-838. [DOI] [PubMed] [Google Scholar]
- 43. Gagliano-Jucá T, Basaria S. Testosterone replacement therapy and cardiovascular risk. Nat Rev Cardiol. 2019;16(9):555-574. [DOI] [PubMed] [Google Scholar]
- 44. Iellamo F, Volterrani M, Caminiti G, et al. Testosterone therapy in women with chronic heart failure: a pilot double-blind, randomized, placebo-controlled study. J Am Coll Cardiol. 2010;56(16):1310-1316. [DOI] [PubMed] [Google Scholar]
- 45. Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344-350. [DOI] [PubMed] [Google Scholar]
- 46. Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89(2):534-543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The ARIC cohort participates in the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository (BioLINCC). The ARIC data are available upon request through the following website: https://biolincc.nhlbi.nih.gov/studies/aric/ to prospective researchers, and our work can be easily replicable from the methods described in the paper.