Abstract
Objective
To examine the prognostic ability of the combination of EEG and MRI in identifying patients with good outcome in postanoxic myoclonus (PAM) after cardiac arrest (CA).
Methods
Adults with PAM who had an MRI within 20 days after CA were identified in 4 prospective CA registries. The primary outcome measure was coma recovery to command following by hospital discharge. Clinical examination included brainstem reflexes and motor activity. EEG was assessed for best background continuity, reactivity, presence of epileptiform activity, and burst suppression with identical bursts (BSIB). MRI was examined for presence of diffusion restriction or fluid-attenuated inversion recovery changes consistent with anoxic brain injury. A prediction model was developed using optimal combination of variables.
Results
Among 78 patients, 11 (14.1%) recovered at discharge and 6 (7.7%) had good outcome (Cerebral Performance Category < 3) at 3 months. Patients who followed commands were more likely to have pupillary and corneal reflexes, flexion or better motor response, EEG continuity and reactivity, no BSIB, and no anoxic injury on MRI. The combined EEG/MRI variable of continuous background and no anoxic changes on MRI was associated with coma recovery at hospital discharge with sensitivity 91% (95% confidence interval [CI], 0.59–1.00), specificity 99% (95% CI, 0.92–1.00), positive predictive value 91% (95% CI, 0.59–1.00), and negative predictive value 99% (95% CI, 0.92–1.00).
Conclusions
EEG and MRI are complementary and identify both good and poor outcome in patients with PAM with high accuracy. An MRI should be considered in patients with myoclonus showing continuous or reactive EEGs.
Postanoxic myoclonus (PAM) occurs in approximately 20% of patients surviving after cardiac arrest (CA) and is considered a strong predictor of poor prognosis.1 Recent studies, especially after widespread adoption of targeted temperature management (TTM), revealed that a subset of patients may achieve good outcome.2,3 The combination of clinical and EEG features reliably identifies patients with poor outcome, but has limited positive predictive value of 50%–66% in identifying good outcome.2,3 MRI is a promising tool in CA coma prognostication,4 but its use in PAM has not been formally assessed. We examine the prognostic ability of EEG and MRI to identify patients with good outcome in a multicenter retrospective study.
Methods
Standard protocol approvals, registrations, and patient consents
All local institutional review boards approved this study and granted waiver of informed consent.
Patients
Adult patients with PAM who had an MRI within 20 days after CA were retrospectively identified in 4 prospective CA registries. Details on patient management have been described elsewhere.3,5,6 Collected variables included demographics, administration of TTM, and neurologic examination off sedation at 72 hours. Primary outcome was assessed through coma recovery to command following at discharge; secondary outcome was Cerebral Performance Category7 (CPC; 1–2, good; 3–5, poor) at 3 months. Withdrawal of life-sustaining treatment (WLST) occurred after reaching an interdisciplinary consensus and close involvement with the patient's family.1
EEG analysis
All patients underwent video-EEG recording according to the international 10–20 system, with continuous EEG monitoring (US centers) or repeated routine 20-minute recordings (Lausanne). EEGs were prospectively interpreted by local certified neurophysiologists using the American Clinical Neurophysiology Society terminology.8 EEGs were defined as epileptiform in the presence of periodic or rhythmic spikes, sharp waves, or spike and waves. Burst suppression with identical bursts (BSIB)9 were identified. Best EEG background was categorized as reactive vs unreactive, defined as reproducible change in amplitude or frequency with exclusion of muscle artifacts,10 and as continuous vs ≥50% discontinuous.8 Due to the retrospective character of this study with different EEG assessment times, and to minimize potential effects of sedation, we chose to record best EEG background rather than distinguishing early vs late background.
MRI analysis
MRIs were interpreted by senior neuroradiologists and classified as anoxic in the presence of restricted diffusion-weighted imaging with associated apparent diffusion coefficient (ADC) decrease or T2/fluid-attenuated inversion recovery changes. If anoxic brain injury was present, it was further categorized as cortical, subcortical, or diffuse. Other major abnormalities not associated with anoxic brain injury were noted.
Statistical analysis
Data were analyzed using Fisher exact and Student t tests as appropriate. The Benjamini-Hochberg procedure was applied to control the false discovery rate, using a q value of 0.05. Logistic regression was implemented using the Firth method to account for separation of data. Backward stepwise selection with criteria for variable removal set to p = 0.10 and penalized regression (Elastic Net) were evaluated to model predictors of outcome. Model performance was assessed with the area under the receiver operating characteristic curve (AUC) and validated using leave-one-out cross-validation.
Data availability
Data will be shared at the request of other investigators. Supplemental data, including samples of EEG and MRI scans, are available at doi.org/10.5061/dryad.nk98sf7pt.
Results
A total of 78 patients were included, of whom 74 underwent TTM; 189 patients with PAM were excluded because no MRIs were performed. Patients had a cardiac etiology in the majority of our cohort (48/78, 61.5%); 26 (33.3%) presented with shockable rhythm, 44 (56.4%) with PEA, and 8 (10.3%) with asystole. Return of spontaneous circulation was achieved after a mean of 19.2 ± 9.0 minutes (data not available for 11 patients). At discharge, 11 (14.1%) patients recovered to follow commands: 61 (78.2%) died, 3 (3.8%) were in vegetative state (CPC 4), 10 (12.8%) had severe disability (CPC 3), 3 (3.8%) had moderate disability (CPC 2), and 1 (1.3%) had a complete recovery (CPC 1). At 3 months, 61 (78.2%) had CPC 5 (death), 1 (1.3%) CPC 4, 8 (10.3%) CPC 3, 3 (3.8%) CPC 2, 3 (3.8%) CPC 1, and 2 were lost to follow-up.
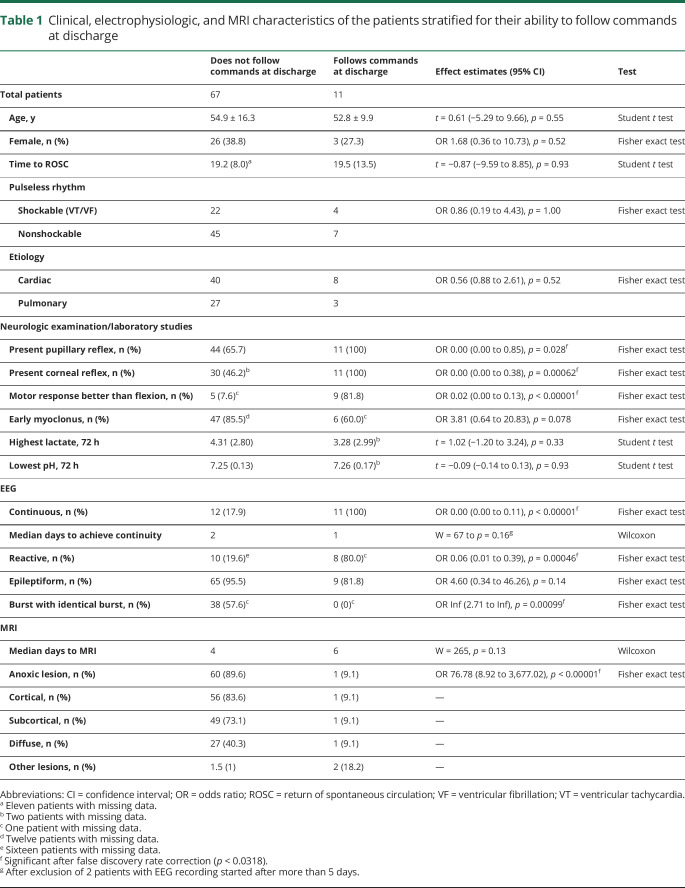
Patient characteristics stratified by coma recovery are presented in table 1. MRI changes consistent with anoxic brain injury were found in 61 (78%), 14 had no MRI lesions, and 3 had other lesions (stroke, subarachnoid hemorrhage). When present, anoxic brain injury affected the cortical (83.6%) or subcortical (73.1%) region in most patients, and diffusely in 40.3%.
Table 1.
Clinical, electrophysiologic, and MRI characteristics of the patients stratified for their ability to follow commands at discharge
An example of a malignant EEG with nonlesional MRI is shown in figure e-1 (doi.org/10.5061/dryad.nk98sf7pt). Anoxic MRI was present in 1 of 11 patients who recovered and in 60 of 67 patients who did not. Best background continuity was assessed after a median of 2 days (range 1–13 days), with 16 of 23 patients achieving continuity on the first day the EEG was applied; 2 patients whose EEGs were started 6 and 13 days after CA were eliminated from further calculations. Initial complete suppression, burst suppression, or myoclonic status evolved into continuous backgrounds in 3 patients. Among the 23 patients with continuous EEG background, all patients who did not have anoxic MRI had coma recovery (n = 11), whereas only 1 of 12 with anoxic MRI recovered. Three of 11 patients who recovered had CPC improvement at 3 months; none of them had anoxic injury on MRI. The single patient who recovered with anoxic MRI was discharged from hospital with CPC 3, and remained unchanged at 3 months.
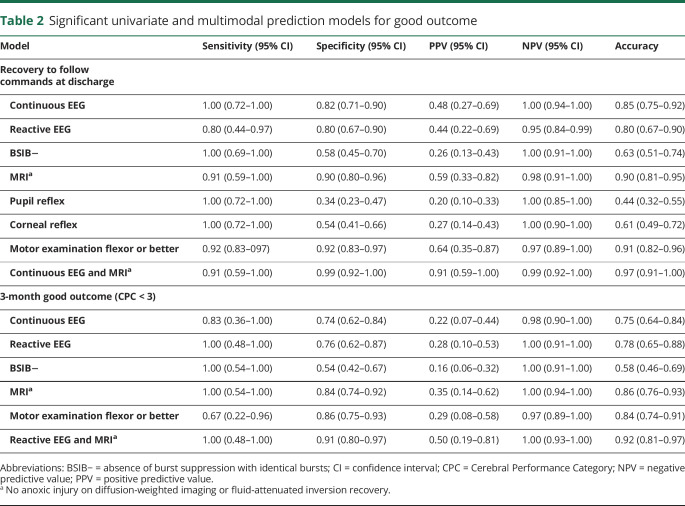
Backwards elimination resulted in a model of combined variables of EEG continuity and absence of anoxic MRI changes that had highest accuracy for coma recovery (0.97; cross-correlation accuracy 0.97, AUC 0.98) with sensitivity of 91% (95% confidence interval [CI], 0.59–1.0), specificity of 99% (95% CI, 0.92–1.0), positive predictive value of 91% (95% CI, 0.59–1.0), and a negative predictive value of 99% (95% CI, 0.92–1.0) in determining coma recovery (table 2 and figure e-2, doi.org/10.5061/dryad.nk98sf7pt). Other models were discarded for overfitting. Similar univariate results were obtained for analysis of 3-month outcome, but the combined variables of EEG reactivity and absence of anoxic MRI change achieved the highest AUC (0.95, table 2 and table e-1, doi.org/10.5061/dryad.nk98sf7pt).
Table 2.
Significant univariate and multimodal prediction models for good outcome
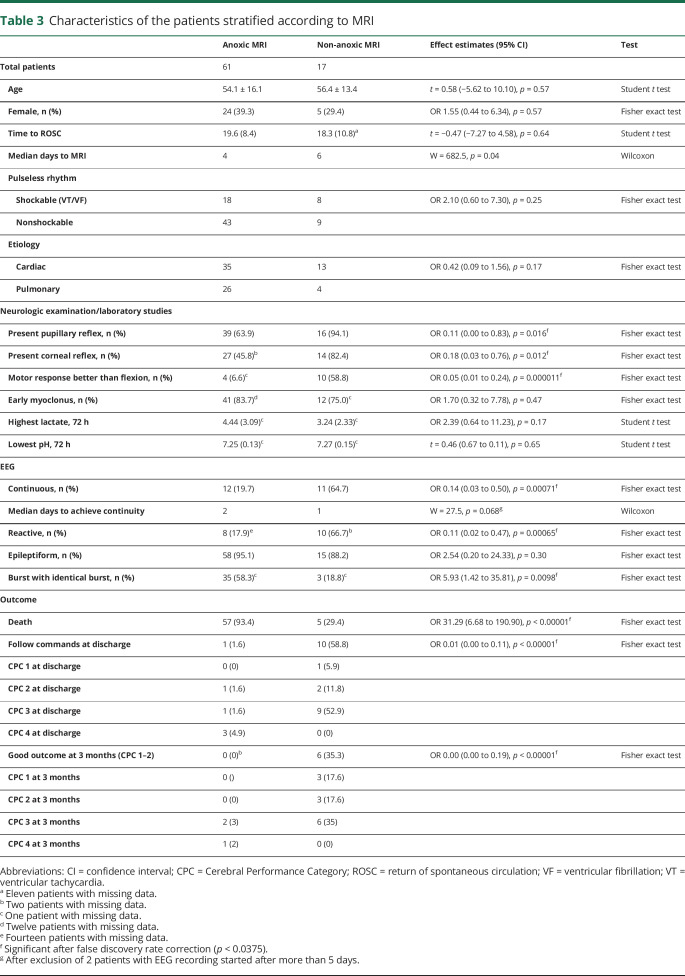
Characteristics of patients with and without anoxic brain injury on MRI were examined further (table 3). MRIs with anoxic injury were obtained sooner than MRIs without (p = 0.045); early MRIs were also more likely to reveal anoxic injury (p = 0.042). Five patients without anoxic brain injury on MRI died. All of them had discontinuous and epileptiform EEG, early myoclonus (within 48 hours from CA), and no motor reaction better than flexion, but all had preserved pupillary reflexes. One patient sustained a second CA in the hospital leading to WLST. One had abnormal MRI with subarachnoid hemorrhage and BSIB. One presented with superrefractory status epilepticus leading to WLST due to failing to wean general anesthetics after 10 days. The 2 remaining patients had absent cortical somatosensory evoked potentials and BSIB.
Table 3.
Characteristics of the patients stratified according to MRI
Discussion
In this multicenter cohort, the combination of EEG and anoxic MRI changes resulted in very high accuracy in predicting neurologic recovery to follow commands in comatose patients with PAM after CA. Whereas identifying favorable clinical and EEG features could predict good outcome in 50% to 67% in previous series of PAM,2,3 addition of MRI increases identification rate to >90%. In agreement with recent data,2,3 our results demonstrate that PAM is not universally associated with poor outcome, with 14% of our patients improving to command following at discharge, and 9% achieving good long-term outcome (CPC 1 or 2 at 3 months).
MRI is one of the latest tools added to the multimodal prediction after CA. However, the absence of anoxic lesions alone forecasts good outcome with only 73% to 75% positive predictive value.1 A recent retrospective analysis not limited to patients with PAM found that a model combining nonmalignant EEG, flexor or better motor response, and MRI (quantitative ADC signal) was associated with good outcome with 100% sensitivity and 91.1% specificity.4
Our data reveal that MRI scans provide substantial prognostic information in addition to EEG and especially to clinical examinations, particularly in patients with intact brainstem reflexes; we utilized best available examination and it is unclear whether serial examinations would have been more informative. MRI studies may be particularly helpful in prognostication in these patients, as clinical examination may be limited due to the medications that may be administered for control of myoclonic status.
This study has several limitations. Our primary outcome of coma recovery (following commands) may indicate only a partial recovery. This was chosen to control for patients with neurologic recovery who may have unfavorable CPC scores for other reasons. Although this is a retrospective analysis, the vast majority of data come from prospective registries. As this was a multicenter study, treatment protocols, EEG recording length, and WLST policies differed; however, this increases the generalizability of results. MRI scans were analyzed qualitatively and may be subject to interrater variability; however, all studies were specifically reanalyzed by trained local neuroradiologists to mitigate this possibility. The best EEG background was recorded, rather than distinguishing early vs late scoring. Due to resource limitations, particularly during nights and weekends, best EEG background may prove more reflective of real-world clinical variability. As clinicians had access to EEG and MRI data, bias due to self-fulfilling prophecy cannot be excluded. Although MRI scans obtained sooner after CA were more likely to reveal anoxia, this observational study was not designed to determine optimal timing to obtain MRIs, but it is possible that clinical indication was more acute in patients with suspected poor prognosis. In this cohort, MRI validity was independent of its timing. Due to the retrospective design of the study with diagnostic studies ordered at the clinician's discretion, only 30% of our PAM population underwent MRI scans. As 95% of patients who did not have an MRI died in-hospital, this examination was likely omitted due to presumed poor prognosis. As such, these results do not reflect the proportion of anoxic brain injury with potentially favorable prognosis in the greater population of PAM. However, even within our selected population, the combination of EEG and MRI showed high correlation with outcome.
The combination of EEG and MRI achieved identification of both good and poor outcome in patients with PAM with high accuracy. Although most existing measures excel in identifying patients with poor outcome, absence of anoxic lesion on MRI, in conjunction with other clinical and EEG prognostic predictors, identifies patients with good prognosis and may decrease premature WLST.1 Our study suggests that an MRI should be considered in all patients with PAM who have continuous or reactive EEGs.
Glossary
- ADC
apparent diffusion coefficient
- AUC
area under the receiver operating characteristic curve
- BSIB
burst suppression with identical bursts
- CA
cardiac arrest
- CI
confidence interval
- CPC
Cerebral Performance Category
- PAM
postanoxic myoclonus
- TTM
targeted temperature management
- WLST
withdrawal of life-sustaining treatment
Appendix. Authors
Footnotes
Editorial, page 149
Study funding
No targeted funding reported.
Disclosure
I. Beuchat received support from the Swiss National Science Foundation (early post-doctoral mobility fellowship grant). A. Sivaraju received support from the Swebilius Foundation. E. Amorim received support from the Neurocritical Care Society (NCS research training fellowship), American Heart Association (postdoctoral fellowship), and MIT and Philips (MIT–Philips Clinician Award). E.J. Gilmore received funding from the NIH Loan Repayment Program. V. Dunet and A.O. Rossetti report no disclosures. M.B. Westover received support from the NIH (1K23NS090900, 1R01NS102190, 1R01NS102574, 1R01NS107291) and the Glenn Foundation. L. Hsu reports no disclosures. B. Scirica reports the following financial relationships over the past 24 months: institutional research grant to Brigham and Women's Hospital from AstraZeneca, Eisai, Novartis, and Merck; consulting fees from AbbVie, Allergan, AstraZeneca, Boehringer Ingelheim, Covance, Eisai, Elsevier Practice Update Cardiology, GlaxoSmithKline, Lexicon, Medtronic, Merck, NovoNordisk, and Sanofi; and equity in Health [at] Scale. B. Scirica is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women's Hospital from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, and Zora Biosciences. D. Silva and K. Tang report no disclosures. J.W. Lee reports contract work for DigiTrace and Advance Medical and research funding from NIH/National Institute of Neurological Disorders and Stroke. Go to Neurology.org/N for full disclosures.
References
- 1.Rossetti AO, Rabinstein AA, Oddo M. Neurological prognostication of outcome in patients in coma after cardiac arrest. Lancet Neurol 2016;15:597–609. [DOI] [PubMed] [Google Scholar]
- 2.Elmer J, Rittenberger JC, Faro J, et al. Clinically distinct electroencephalographic phenotypes of early myoclonus after cardiac arrest. Ann Neurol 2016;80:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhakar MB, Sivaraju A, Maciel CB, et al. Electro-clinical characteristics and prognostic significance of post anoxic myoclonus. Resuscitation 2018;131:114–120. [DOI] [PubMed] [Google Scholar]
- 4.Bevers MB, Scirica BM, Avery KR, Henderson GV, Lin AP, Lee JW. Combination of clinical exam, MRI and EEG to predict outcome following cardiac arrest and targeted temperature management. Neurocrit Care 2018;29:396–403. [DOI] [PubMed] [Google Scholar]
- 5.Szumita PM, Baroletti S, Avery KR, et al. Implementation of a hospital-wide protocol for induced hypothermia following successfully resuscitated cardiac arrest. Crit Pathw Cardiol 2010;9:216–220. [DOI] [PubMed] [Google Scholar]
- 6.Oddo M, Rossetti AO. Early multimodal outcome prediction after cardiac arrest in patients treated with hypothermia. Crit Care Med 2014;42:1340–1347. [DOI] [PubMed] [Google Scholar]
- 7.Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA 2004;291:870–879. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society's Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 9.Hofmeijer J, Tjepkema-Cloostermans MC, van Putten MJ. Burst-suppression with identical bursts: a distinct EEG pattern with poor outcome in postanoxic coma. Clin Neurophysiol 2014;125:947–954. [DOI] [PubMed] [Google Scholar]
- 10.Tsetsou S, Novy J, Oddo M, Rossetti AO. EEG reactivity to pain in comatose patients: importance of the stimulus type. Resuscitation 2015;97:34–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared at the request of other investigators. Supplemental data, including samples of EEG and MRI scans, are available at doi.org/10.5061/dryad.nk98sf7pt.