Abstract
Objective
To quantify the impact of a healthy lifestyle on the risk of Alzheimer dementia.
Methods
Using data from the Chicago Health and Aging Project (CHAP; n = 1,845) and the Rush Memory and Aging Project (MAP; n = 920), we defined a healthy lifestyle score on the basis of nonsmoking, ≥150 min/wk moderate/vigorous-intensity physical activity, light to moderate alcohol consumption, high-quality Mediterranean-DASH Diet Intervention for Neurodegenerative Delay diet (upper 40%), and engagement in late-life cognitive activities (upper 40%), giving an overall score ranging from 0 to 5. Cox proportional hazard models were used for each cohort to estimate the hazard ratio (HR) and 95% confidence interval (CI) of the lifestyle score with Alzheimer dementia, and a random-effect meta-analysis was used to pool the results.
Results
During a median follow-up of 5.8 years in CHAP and 6.0 years in MAP, 379 and 229 participants, respectively, had incident Alzheimer dementia. In multivariable-adjusted models, the pooled HR (95% CI) of Alzheimer dementia across 2 cohorts was 0.73 (95% CI 0.66–0.80) per each additional healthy lifestyle factor. Compared to participants with 0 to 1 healthy lifestyle factor, the risk of Alzheimer dementia was 37% lower (pooled HR 0.63, 95% CI 0.47–0.84) in those with 2 to 3 healthy lifestyle factors and 60% lower (pooled HR 0.40, 95% CI 0.28–0.56) in those with 4 to 5 healthy lifestyle factors.
Conclusion
A healthy lifestyle as a composite score is associated with a substantially lower risk of Alzheimer's dementia.
In 2018, >50 million people worldwide—5.7 million Americans—were living with Alzheimer dementia, posing a significant burden on health and social care.1 As the population ages, it is projected that the prevalence of Alzheimer dementia will triple in the next 30 years, urging the need for prevention and treatment strategies. Up to now, clinical trials investigating various therapies in patients with dementia have failed to modify the course of the disease.2 In contrast, data from epidemiologic studies and clinical trials suggest that primary prevention can delay the onset of the disease.3–5 Therefore, directing the focus toward prevention strategies has become a public health priority.
A number of preventive factors, including lifestyle behaviors and cardiovascular conditions, have been identified that individually contribute to a lower risk of cognitive decline and Alzheimer dementia.4,5 Many of these factors (e.g., diet and exercise) are likely to have synergistic effects on dementia risk, yet few studies have examined the overall effect of multiple modifiable risk factors in combination.6–9 Those that do have focused on cardiovascular risk factors, some of which may not be modifiable solely by the individual.10–14 In the current study of 2 prospective longitudinal studies, we assessed risk of incident Alzheimer dementia according to a composite score of healthy lifestyle behaviors for dementia prevention, including diet,15 exercise,16 abstention from smoking,17 light to moderate alcohol consumption,18 and participation in cognitive activities.19
Methods
Study populations
The Chicago Health and Aging Project (CHAP) is a population-based cohort study that examined risk factors for Alzheimer dementia.20 The study began in 1993 with the enrollment of individuals ≥65 years of age from a geographically defined community of blacks and non-Hispanic whites in the Southside of Chicago. From 1993 to 2012, 10,802 participants (78.7% of eligible residents) had been enrolled in the CHAP study. Of the 10,802 participants, 2,137 individuals without dementia were selected randomly for a detailed clinical assessment of incident Alzheimer dementia over the 18-year follow-up.21 The Rush Memory and Aging Project (MAP) is an ongoing community-based cohort study of aging and risk factors for cognitive decline.22 Since 1997, MAP has enrolled 2,022 adults from retirement facilities, subsidized housings, and individual homes across the Chicago metropolitan area. The data collection and clinical outcomes assessment in the CHAP and MAP cohort studies have followed similar methods, including measures of exposure variables and diagnostic assessment of Alzheimer dementia. A primary distinction between the 2 studies was the conduct of clinical evaluations for incident Alzheimer dementia every 3 years in a stratified random sample in CHAP and annually in MAP.
For the present study, we selected participants whose baseline data were available on diet, lifestyle factors, genetics (i.e., APOE ε4), and clinical assessment for Alzheimer dementia. In CHAP, of 2,137 participants with detailed clinical assessment of incident Alzheimer dementia 1,930 completed the food frequency questionnaire. In MAP, the food frequency questionnaire was introduced in March 2004 when there were 1,306 active participants, of whom 1,068 completed the dietary questionnaires. We excluded participants with no information on lifestyle factors (nCHAP = 22; nMAP = 14), as well as those for whom data were missing on APOE ε4 status (nCHAP = 63; nMAP = 8). In MAP, because the baseline was the date of the initial diet assessment, we excluded 126 participants with dementia at the start of follow-up. After exclusions, 1,845 participants in CHAP and 920 in MAP were included for analysis in this study.
Assessment of lifestyle factors and other covariates
Dietary intake was assessed by the same 144-item food frequency questionnaire in both studies that was validated for use in older Chicago residents.23 Participants were asked how often, on average, they consumed specific foods and beverages with prespecified portion sizes over the past year. To assess the overall diet quality, we calculated the Mediterranean-DASH Diet Intervention for Neurodegenerative Delay (MIND) diet score, which summarizes information on 10 brain healthy food groups (green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, seafood, poultry, olive oil, and wine) and 5 unhealthy food groups (red meats, butter and stick margarine, cheese, pastries and sweets, and fried/fast food).24 Participants reported their average frequency of intake of wine and other alcoholic beverages through the food frequency questionnaires.23 Because we evaluated the alcohol intake separately, we did not include wine in the MIND diet score calculation. Physical activity in both cohorts was assessed by the same validated questionnaire from the 1985 US Health Interview Survey that was adapted for use in older adults.25 Participants reported the time spent in any of 5 moderate or vigorous activities (i.e., walking for exercise, gardening or yard work, calisthenics or general exercise, bicycle riding, and swimming) within the past 2 weeks. Information on smoking status was obtained through the interview at baseline, in which participants specified whether they were current, former, or never smokers.17 Participation in cognitively stimulating activities was assessed with a structured questionnaire of usual time spent in the past year on 7 activities, including reading, writing letters, visiting a library, and playing games such as chess or checkers. Each of these 7 activities was scored on a 5-point scale ranging from 1 for once a year or less to 5 for every day or about every day and then averaged to yield a composite measure of the frequency of participation in cognitively stimulating activities.26 APOE genotype was determined for each participant by the Broad Institute for Population Genetics using the hME Sequenom MassARRAY platform in CHAP and by Polymorphic DNA Technologies in MAP. Participants were classified as APOE ε4 carrier (≥1 ε4 allele) or noncarrier. Race/ethnicity was defined with questions from the 1990 US Census. Education was measured as the number of years of formal schooling completed. Body mass index (BMI; weight in kilograms divided by height in meters squared) was computed from measured weight and height. Information on statins and antihypertensive medication was obtained during the interview when the research assistant had a direct visual inspection of all prescriptions that the participant was receiving. History of heart disease and stroke was determined by self-report questions from the Established Populations for the Epidemiologic Study of the Elderly. Depressive symptoms were assessed with a modified 10-item version of the Center for Epidemiologic Studies Depression (CESD) scale.27
Classification of healthy lifestyle categories
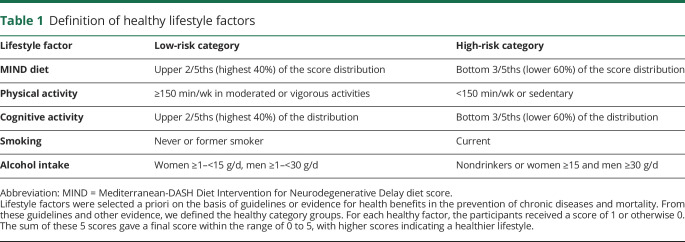
Based on evidence,15–19 guidelines,28,29 and expert knowledge, for health benefits of lifestyle factors in the prevention of dementia, we considered a priori 5 healthy lifestyle behaviors: (1) MIND diet score (without alcohol) in the top 40% of the cohort distribution, (2) cognitive activities in the top 40% of the cohort distribution, (3) not current smoking, (4) moderate or vigorous exercise activities for at least 150 min/wk, and (5) light to moderate alcohol consumption (1–15 g/d in women and 1–30 g/d in men, i.e., up to ≈1 drink a day for women and 2 drinks for men) (table 1).
Table 1.
Definition of healthy lifestyle factors
In the absence of a threshold for a healthy diet and cognitive activities, we imposed an upper 40% cutoff based on the cohort distribution, in line with previous publications.30–32 This cutoff will tend to be achievable for most of the people who plan to modify their lifestyle.
For each healthy lifestyle factor, the participants received a score of 1 if they met the criteria for healthy and 0 if they did not meet the criteria. The sum of these 5 scores yielded a final score within the range of 0 to 5, with higher scores indicating a healthier lifestyle.30
BMI was not included in the score because it is not a behavior and because of both cause and effect relations of obesity in older ages and the risk of dementia.33,34
Clinical diagnosis of Alzheimer dementia
The clinical diagnosis of Alzheimer dementia was determined at each evaluation as previously described.20,35 In short, using data from a structured neurologic examination, medical history, and cognitive performance testing and with the assistance of an algorithmically based rating of cognitive impairment, an experienced clinician determined the diagnosis of Alzheimer dementia on the basis of criteria of the joint working group of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association.36 Data on incident Alzheimer dementia were collected until February 2012 in CHAP and August 2018 in MAP.
Statistical analysis
Baseline characteristics of the study populations are presented as mean and SD, percentages of participants, and medians and quartiles. Participants contributed person-time from the baseline questionnaire until the date of clinical diagnosis for Alzheimer dementia, censored due to mortality/loss to follow-up, or the end of the follow-up period, whichever came first. The maximum follow-up time was 17 years in CHAP and 14 years in MAP. We censored data after 14 years of follow-up in analyses that compared and pooled the studies.
Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) of incident Alzheimer dementia for the categories of healthy lifestyle score. We grouped study participants into 3 categories of lifestyle factor score (0–1, 2–3, and 4–5 factors) and compared the HRs with the lowest category as the referent. We also evaluated the risk of Alzheimer dementia per 1-point increase in a healthy lifestyle factor. All models were adjusted for age (year), sex (male vs female), race (black vs non-Hispanic white), education (years), APOE ε4 carrier (none vs any ε4 allele), and prevalence of cardiovascular disease (including heart disease or stroke, yes vs no) at the baseline. The proportional hazard assumption was assessed through the interaction between time and Schoenfeld residuals.
The analyses were performed separately in each cohort study, and we pooled the HRs to obtain a summarized estimate with the use of an inverse variance–weighted random-effect meta-analysis.37 We used the Cochrane Q statistic and the I2 statistic to examine the heterogeneity of associations among the cohorts.
In the sensitivity analysis, we applied a series of analyses to test the robustness of our findings. First, we evaluated effect modification of the Alzheimer dementia risk factors in each cohort by conducting 6 stratified analyses, including analysis stratified by sex (women and men), by race (black and non-Hispanic white), by APOE ε4 carrier status (noncarrier and carrier), by education (≤12 and 13+ years), by BMI (normal weight and overweight), and by the prevalence of cardiovascular disease (no and yes). Second, to address the potential effect of cardiovascular risk factors on the association between lifestyle and Alzheimer dementia, we also adjusted our multivariable model by BMI, hypertension, dyslipidemia, and diabetes mellitus. Third, to account for the effect of depressive symptoms in our association, we additionally adjusted for the CESD scores. Fourth, to account for possible reverse causation, we conducted an analysis in which we estimated the HRs after excluding Alzheimer dementia events during the first 2.5 years of follow-up (n = 99 in CHAP, n = 60 in MAP). Fifth, to limit the variability of baseline assessment during the follow-up, we limited the follow-up time to 10 years and excluded Alzheimer dementia events after this period. Sixth, to address the concern about the potential adverse effects of light to moderate alcohol intake, we created a healthy lifestyle score that was based on the other 4 healthy factors without alcohol. In this analysis, we adjusted for alcohol intake in the multivariable-adjusted model. Seventh, to address the adverse effect of former smokers, we created a healthy lifestyle score that was based on never smokers as a healthy lifestyle factor. Eighth, given that the role of APOE ε4 allele on Alzheimer dementia risk may be different in blacks and non-Hispanic whites, in CHAP, we conducted another analysis among APOE ε4 carries to evaluate the association of lifestyle score with Alzheimer dementia stratified by race.
All the analyses were performed with R software, CRAN version 3.6.0 (with the survival,38 survey,39 metafor,40 and fmsb packages; R Foundation for Statistical Computing, Vienna, Austria).
Standard protocol approvals, registrations, and patient consents
The study was approved by the institutional review board of Rush University Medical Center. Written informed consent was obtained from all study participants.
Data availability
Data of the MAP cohort study are available via the Rush Alzheimer's Disease Center Research Resource Sharing Hub, which can be found at radc.rush.edu. It has descriptions of the studies and available data. Any qualified investigator can create an account and submit requests for deidentified data.
Results
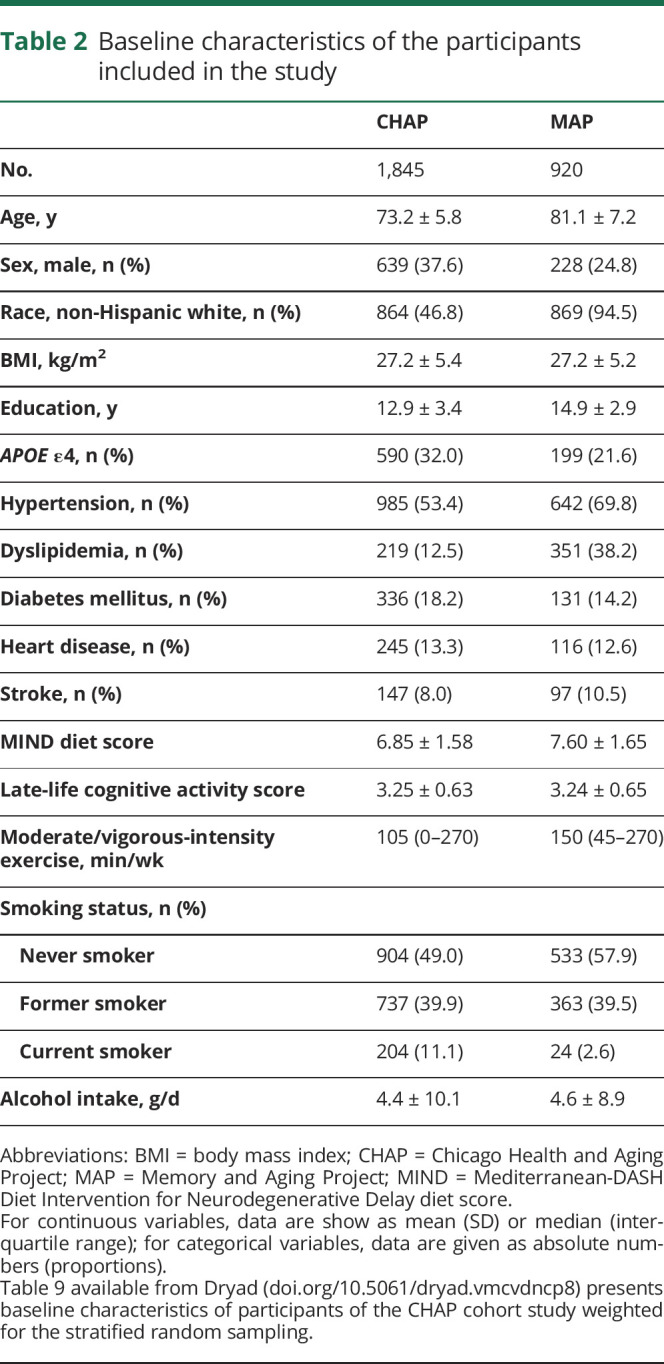
At baseline, the mean age of participants in the CHAP study was 73.2 (SD 5.8) years and in MAP was 81.1 (SD 7.2) years; 62.4% of the CHAP and 75.2% of the MAP participants were women (table 2). A large fraction of the CHAP participants were black (53.2%), whereas the majority of MAP participants were non-Hispanic white (94.5%). Compared to the CHAP, participants of the MAP study reported on average more years of education (12.9 vs 14.9), a higher MIND score (6.85 vs 7.60), and more minutes per week in moderate/vigorous activities (105 vs 150 min/wk). The adherence to healthy lifestyle behaviors was lower in CHAP compared to MAP; 24.4% of CHAP participants and 31.5% of MAP had 4 or 5 healthy lifestyle behaviors. Participants with 2 or 3 healthy behaviors made up the majority of CHAP and MAP population, 58.2% and 55.1%, respectively. Adherence to 0 or 1 healthy behaviors was 17.4% in CHAP and 13.4% in MAP. The contribution of each lifestyle factor on the overall score by cohort is shown in table 1 available from Dryad (doi.org/10.5061/dryad.vmcvdncp8).
Table 2.
Baseline characteristics of the participants included in the study
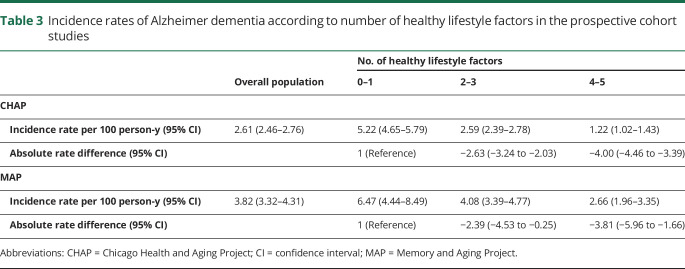
During a median follow-up of 5.8 (interquartile range 3.2–9.7) years in CHAP and 6.0 (interquartile range 3.0–9.0) years in MAP, a total of 379 and 229 participants, respectively, developed Alzheimer dementia. The incidence rates decreased with an increasing number of healthy lifestyle behaviors (table 3). Compared with the incidence rate of Alzheimer dementia in those with 0 or 1 healthy behaviors (CHAP 5.22 [95% CI 4.65–5.79], MAP 6.47 [95% CI 4.44–8.49] per 100 person-years), the absolute rate differences per 100 person-years in those with 2 or 3 healthy behaviors were −2.63 (95% CI −3.24 to −2.03) in CHAP and −2.39 (95% CI −4.53 to −0.25) in MAP, and in those with 4 or 5 healthy behaviors, the absolute rate differences were −4.00 (95% CI −4.46 to −3.39) in CHAP and −3.81 (95% CI −5.96 to −1.66) in MAP.
Table 3.
Incidence rates of Alzheimer dementia according to number of healthy lifestyle factors in the prospective cohort studies
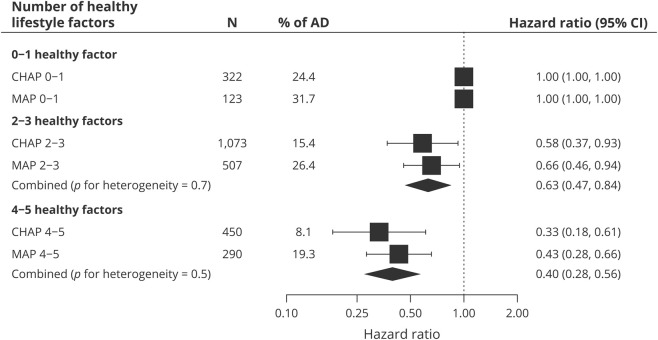
In multivariable models, the HRs for Alzheimer dementia per 1 additional healthy behavior in the score were 0.70 (95% CI 0.59–0.83) in CHAP and 0.74 (95% CI 0.66–0.84) in MAP. Across the 2 cohorts, the risk of incident Alzheimer disease was 27% lower per 1 healthy behavior increase in lifestyle score (pooled HR 0.73, 95% CI 0.66–0.80). Furthermore, compared to participants with 0 or 1 healthy behavior, the HRs of Alzheimer dementia in those with 2 or 3 behaviors were 0.58 (95% CI 0.37–0.93) in CHAP and 0.66 (95% CI 0.46–0.94) in MAP, and in those with 4 or 5 healthy behaviors, the HRs were 0.33 (95% CI 0.18–0.61) in CHAP and 0.43 (95% CI 0.28–0.66) in MAP. Across the 2 cohorts, the risk of incident Alzheimer dementia was 37% lower in those with 2 or 3 healthy behaviors (pooled HR 0.63, 95% CI 0.47–0.84) and 60% lower in those with 4 or 5 healthy behaviors (pooled HR 0.40, 95% CI 0.28–0.56) compared to participants with 0 or 1 healthy behaviors (figure).
Figure. HRs of AD according to the combination of healthy lifestyle factors in the prospective cohort studies.
Model adjusted for age, sex, race, education, APOE ε4, and prevalence of cardiovascular disease (including heart disease or stroke). A random-effects meta-analysis was used to combine cohort-specific results. AD = Alzheimer dementia; CHAP = Chicago Health and Aging Project; CI = confidence interval; HR = hazard ratio; MAP = Rush Memory and Aging Project; N = number of participants in each group.
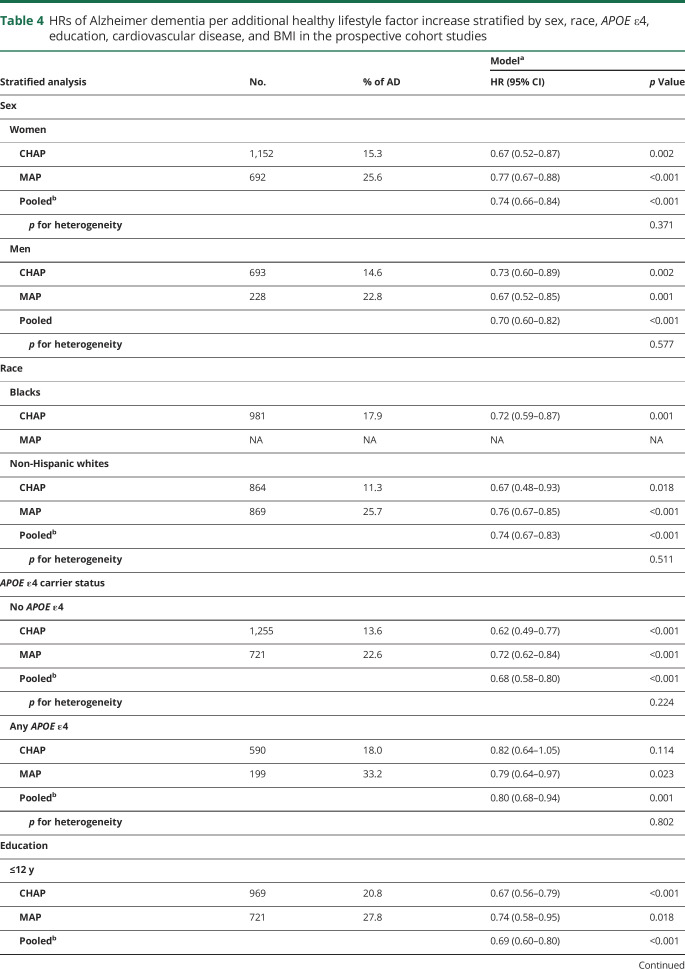
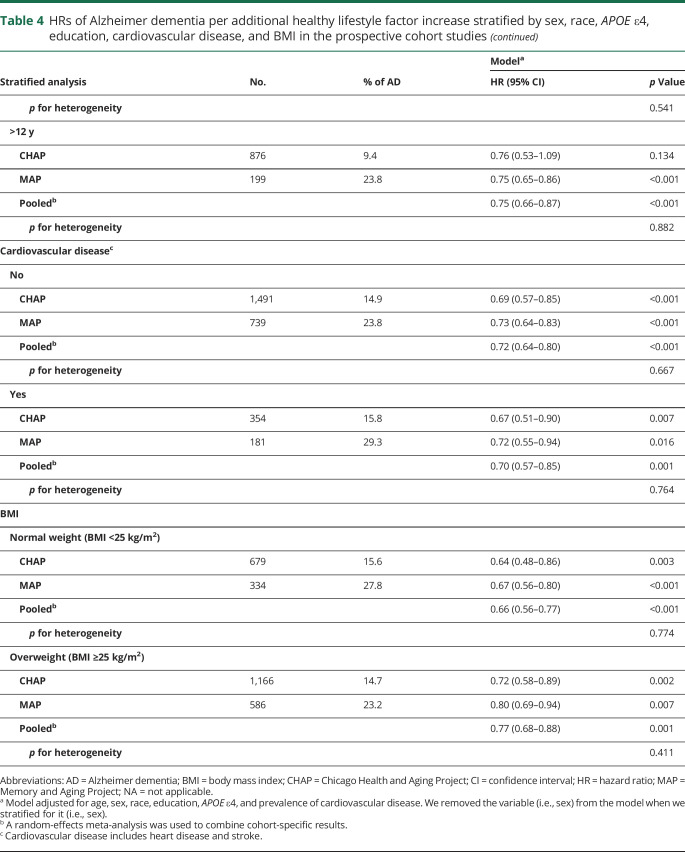
Stratified analysis by sex, race, and APOE ε4, education, BMI, and prevalence of cardiovascular disease yielded results principally similar to those of the overall analysis (table 4). No heterogeneity was observed between cohorts (p for heterogeneity >0.2 for all comparisons). The pooled HRs of Alzheimer dementia per 1 healthy behavior increase in the lifestyle score were 0.74 (95% CI 0.66–0.84) in women and 0.70 (95% CI 0.60–0.82) in men. Among non-Hispanic white participants of CHAP and MAP, the pooled HR was 0.74 (95% CI 0.67–0.83) and in blacks (only CHAP participants included) was 0.72 (95% CI 0.59–0.87). In the stratified analyses by the presence of the APOE ε4 allele, the pooled HR among noncarriers was 0.68 (95% CI 0.58–0.80) and in carriers was 0.80 (95% CI 0.68–0.94). In the CHAP cohort, notably, a strong HR was observed among non–APOE ε4 allele carriers (HR 0.62, 95% CI 0.49–0.77) compared to APOE ε4 allele carriers (HR 0.82, 95% CI 0.64–1.05). In the analysis stratified by education levels, the pooled HRs were 0.69 (95% CI 0.60–0.80) in those with ≤12 years of education and 0.75 (95% CI 0.66–0.87) in those with >12 years. In participants without cardiovascular disease at the baseline, the pooled HRs were 0.72 (95% CI 0.64–0.80); among patients with cardiovascular disease, the HR was 0.70 (95% CI 0.57–0.85). In the stratified analysis by BMI, the pooled HRs were 0.66 (95% CI 0.56–0.77) in normal-weight participants (BMI <25 kg/m2) and 0.77 (95% CI 0.68–0.88) in overweight individuals (table 4).
Table 4.
HRs of Alzheimer dementia per additional healthy lifestyle factor increase stratified by sex, race, APOE ε4, education, cardiovascular disease, and BMI in the prospective cohort studies
The associations between lifestyle and incident Alzheimer dementia were not different when we also adjusted for BMI, hypertension, dyslipidemia, and diabetes mellitus; also adjusted for the CESD scores; excluded Alzheimer dementia events during the first 2.5 years of follow-up to account for reverse causality; or limited the follow-up to 10 years. When we used a healthy score without light to moderate alcohol intake, the HRs of Alzheimer dementia per 1 healthy factor increase in the lifestyle score were 0.71 (95% CI 0.59–0.86) in CHAP and 0.70 (0.61–0.81) in MAP, similar to the primary analysis. Similar results were found when we used never smoking as a healthy lifestyle factor. Focusing the analysis on APOE ε4 allele carriers and stratifying by race in CHAP showed broadly similar associations in blacks (HR 0.81, 95% CI 0.63–1.04) and non-Hispanic whites (HR 0.84, 95% CI 0.49–1.43). Results of the sensitivity analyses are available from Dryad, tables 2–8 (doi.org/10.5061/dryad.vmcvdncp8).
Discussion
In 2 prospective cohort studies of older adults, an increased number of healthy lifestyle behaviors was associated with a lower risk of Alzheimer dementia. Older adults who adhere simultaneously to 4 or 5 healthy behaviors (i.e., high-quality diet, engagement in cognitive activities, regular physical activity, light to moderate alcohol intake, and not smoking) had 60% lower risk of developing incident Alzheimer dementia than individuals with 0 or 1 healthy behaviors. These associations were independent of other established risk factors of Alzheimer dementia and persisted among white participants who were carriers of the APOE ε4 allele. From these findings and the fact that the lifestyle factors we studied are modifiable and in direct control of the individual, it is imperative to promote them concurrently among older adults as a strategy to delay or prevent Alzheimer dementia.
Previous epidemiologic studies of lifestyle and dementia have evaluated risk factors individually, including diet,15 regular physical exercise,16 and engagement in cognitive activities.19 For example, using the data from the MAP cohort study, we reported that the risk of incident Alzheimer dementia is substantially lower in older adults who adhere to a high-quality MIND diet.15 Similar findings of prevention were reported for engagement in mentally stimulating activities.19 An early meta-analysis of prospective cohort studies summarized that adults who routinely engaged in physical activities have a significantly lower risk of dementia.16 However, given that these lifestyle factors are often interrelated, evaluating them in combination accounts for a cluster effect within individuals.30 Indeed, studies assessing the impact of lifestyle factors on cardiovascular disease30,41 underscored the significance of promoting these lifestyle factors concurrently to optimize preventive effects in the population. Despite the fact that cardiovascular disease and dementia share similar risk factors,42 only a few studies have investigated the effect of lifestyle factors on the risk of dementia,6–9 and most of them have focused on cardiovascular risk factors defined by the American Heart Association43 (i.e., smoking, BMI, physical activity, diet, blood pressure, cholesterol, and glucose) to promote optimal cardiovascular health.10–14 Overall, these studies showed that better cardiovascular health is associated with lower cognitive decline and incident dementia in the Framingham Heart Study,10 Northern Manhattan Study,11 and the Atherosclerosis Risk in Communities Study12 and with a lower risk of dementia in the Three-City study in France13 and Chicago Heart Association Detection Project in Industry study in the United States.14 However, cardiovascular health addressed by these studies includes metabolic factors and conditions (blood cholesterol, blood glucose, blood pressure, and obesity) in addition to health behaviors.43 While these factors are modifiable, they often require medical treatment and are not always under control of the individual. Therefore, from a public health perspective, promoting healthy lifestyle behaviors is a feasible strategy that is likely to have a significant impact on dementia prevention and cardiovascular-related conditions.44
The mechanisms underlining the protective effects of healthy lifestyles in Alzheimer dementia are not entirely understood. A number of studies indicate that healthier diets rich in nutrients and vitamins, physical exercise, and smoking abstinence could initiate a chain of metabolic and molecular alterations that presumably inhibit inflammation and oxidative stress and may reduce amyloid accumulation, neuritic plaques, and neurofibrillary tangles in the brain,45–47 but insights into the specific pathways involved are limited.
We did not consider the inclusion of BMI in our healthy lifestyle score because of the complex relation of weight as both a risk factor and a cause of dementia. A cohort study in which nearly 2 million people were followed up for 2 decades showed an inverse association between obesity and the risk of dementia, opposing the hypothesis that obesity increases the risk of dementia in old age.33 However, we did adjust for the BMI in the sensitivity analysis, and results were consistent with the primary analysis.
We observed some degree of heterogeneity between the CHAP and MAP cohort studies when we focused our investigation on participants with the APOE ε4 allele. We found a lower risk of Alzheimer dementia with each additional healthy lifestyle behavior in MAP, whereas in CHAP, the presence of the APOE ε4 allele diminished the effect of lifestyle. Consistent with our MAP findings, the 2-year Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial in Finland48 and a large-scale observational study (UK Biobank) in the United Kingdom8 concluded that healthy lifestyle changes may prevent cognitive decline in individuals with the APOE ε4 allele. In contrast, these protective effects of healthy lifestyle among individuals at high genetic risk were not demonstrated in population-based study in the Netherlands.9 The heterogeneity in our findings between cohorts (CHAP vs MAP) could be explained in part by the characteristics of each cohort. A high proportion of the CHAP population is black with lower education and a poor lifestyle profile compared to MAP cohort. The MAP cohort is made up of 94% non-Hispanic whites and is much older. In fact, it was expected that the contribution of APOE ε4 would have been limited in the CHAP cohort given a weaker contribution of genetic risk to dementia among blacks.49 However, in a recent study involving CHAP participants, we showed that the association of the APOE genotypes with cognitive decline was not different between blacks and non-Hispanic whites.50 We also confirmed these findings in our study when we investigated the association of lifestyle factors with Alzheimer dementia within carriers of the APOE ε4 allele stratified by race in CHAP and found similar HRs (nonsignificant) in both blacks and non-Hispanic whites. Although our small sample size in the stratified analysis does not allow us to draw conclusions, we hypothesize that the heterogeneity observed between cohorts among carriers of the APOE ε4 allele could be attributed to the differences in the lifestyle profile and education.
The assessments of diet and physical activity in these studies were self-reported and thus could be prone to measurement error, although these questionnaires were validated.23,25 Lifestyle factors were assessed at baseline, and changes over time were not considered in this study. Although the effect size of each lifestyle factor to Alzheimer dementia is different, we did not weight them in the analysis because of our limited sample size, and our central hypothesis was to evaluate an overall healthy lifestyle as a cluster of factors within an individual. Despite the exclusion of events occurring in the first 2.5 years of follow-up, reverse causality remains a limitation of our study because of the long prodromal phase of Alzheimer dementia. A significant strength of this study is the generalizability of the findings across age and race. Although our 2 cohorts have different mean ages and proportions of blacks, the adherence to a healthy lifestyle had similar effects on incident Alzheimer dementia. Another strength is the accurate diagnosis of Alzheimer dementia through frequent neuropsychological testing and structured clinical neurologic evaluations by clinicians blinded to the lifestyle factors profile.
Our study suggests that a healthy lifestyle as a composite score is associated with a substantially lower risk of Alzheimer dementia.
Acknowledgment
The authors thank all participants of the CHAP and MAP study and the research assistants, psychologists, and physicians for their efforts in the conduct of the fieldwork.
Glossary
- BMI
body mass index
- CESD
Center for Epidemiologic Studies Depression
- CHAP
Chicago Health and Aging Project
- CI
confidence interval
- FINGER
Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability
- HR
hazard ratio
- MAP
Memory and Aging Project
- MIND
Mediterranean-DASH Diet Intervention for Neurodegenerative Delay
Appendix. Authors
Study funding
Study funding by the NIH and National Institute on Aging (R01AG054476, R01AG052583, P30AG10161, R01AG17917, R01AG11101, R01AG051635, RF1AG057532, and R01AG058679).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Alzheimer's Association. 2018 Alzheimer's disease facts and figures. Alzheimers Dement 2018;14:367–429. [Google Scholar]
- 2.Sugino H, Watanabe A, Amada N, et al. Global trends in Alzheimer disease clinical development: increasing the probability of success. Clin Ther 2015;37:1632–1642. [DOI] [PubMed] [Google Scholar]
- 3.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health 1998;88:1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol 2014;13:788–794. [DOI] [PubMed] [Google Scholar]
- 5.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385:2255–2263. [DOI] [PubMed] [Google Scholar]
- 6.Gelber RP, Petrovitch H, Masaki KH, et al. Lifestyle and the risk of dementia in Japanese-American men. J Am Geriatr Soc 2012;60:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabia S, Nabi H, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Health behaviors from early to late midlife as predictors of cognitive function: the Whitehall II study. Am J Epidemiol 2009;170:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA 2019;322:430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Licher S, Ahmad S, Karamujić-Čomić H, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med 2019;25:1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pase MP, Beiser A, Enserro D, et al. Association of ideal cardiovascular health with vascular brain injury and incident dementia. Stroke 2016;47:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardener H, Wright CB, Dong C, et al. Ideal cardiovascular health and cognitive aging in the Northern Manhattan Study. J Am Heart Assoc 2016;5:e002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González HM, Tarraf W, Harrison K, et al. Midlife cardiovascular health and 20-year cognitive decline: Atherosclerosis Risk in Communities study results. Alzheimers Dement 2018;14:579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samieri C, Perier M-C, Gaye B, et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA 2018;320:657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vu THT, Zhao L, Liu L, et al. Favorable cardiovascular health at young and middle ages and dementia in older age-the cha study. J Am Heart Assoc 2019;8:e009730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement 2015;11:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med 2009;39:3–11. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal NT, Bienias JL, Bennett DA, et al. The relation of cigarette smoking to incident Alzheimer's disease in a biracial urban community population. Neuroepidemiology 2006;26:140–146. [DOI] [PubMed] [Google Scholar]
- 18.Sabia S, Fayosse A, Dumurgier J, et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ 2018;362:k2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson RS, Segawa E, Boyle PA, Bennett DA. Influence of late-life cognitive activity on cognitive health. Neurology 2012;78:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein e allele status. Arch Neurol 2003;60:185–189. [DOI] [PubMed] [Google Scholar]
- 21.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dement Dis 2003;5:349–355. [DOI] [PubMed] [Google Scholar]
- 22.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol 2003;158:1213–1217. [DOI] [PubMed] [Google Scholar]
- 24.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement 2015;11:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med 1989;5:65–72. [PubMed] [Google Scholar]
- 26.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology 2007;69:1911–1920. [DOI] [PubMed] [Google Scholar]
- 27.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (center for epidemiological studies depression) depression symptoms index. J Aging Health 1993;5:179–193. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. December 2015. Available at https://health.gov/our-work/food-and-nutrition/2015-2020-dietary-guidelines/. [Google Scholar]
- 29.US Department of Health and Human Services. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. 2018. [Google Scholar]
- 30.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 2000;343:16–22. [DOI] [PubMed] [Google Scholar]
- 31.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ 2008;337:a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhana K, Haines J, Liu G, et al. Association between maternal adherence to healthy lifestyle practices and risk of obesity in offspring: results from two prospective cohort studies of mother-child pairs in the United States. BMJ 2018;362:k2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qizilbash N, Gregson J, Johnson ME, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol 2015;3:431–436. [DOI] [PubMed] [Google Scholar]
- 34.Gustafson D. BMI and dementia: feast or famine for the brain? Lancet Diabetes Endocrinol 2015;3:397–398. [DOI] [PubMed] [Google Scholar]
- 35.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 2006;27:169–176. [DOI] [PubMed] [Google Scholar]
- 36.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Ley SH, Tobias DK, et al. Birth weight and later life adherence to unhealthy lifestyles in predicting type 2 diabetes: prospective cohort study. BMJ 2015;351:h3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 39.Lumley T. Analysis of complex survey samples. J Stat Softw 2004;9:1–19. [Google Scholar]
- 40.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- 41.Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation 2018;138:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorelick PB, Furie KL, Iadecola C, et al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke 2017;48:e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 44.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark TA, Lee HP, Rolston RK, et al. Oxidative stress and its implications for future treatments and management of Alzheimer disease. Int J Biomed Sci 2010;6:225–227. [PMC free article] [PubMed] [Google Scholar]
- 46.Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol 2004;61:668–672. [DOI] [PubMed] [Google Scholar]
- 47.Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL. Nutrients and bioactives in green leafy vegetables and cognitive decline: prospective study. Neurology 2018;90:e214–e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein e genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol 2018;75:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 1998;279:751–755. [DOI] [PubMed] [Google Scholar]
- 50.Rajan KB, Barnes LL, Wilson RS, Weuve J, McAninch EA, Evans DA. Apolipoprotein E genotypes, age, race, and cognitive decline in a population sample. J Am Geriatr Soc 2018;67:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data of the MAP cohort study are available via the Rush Alzheimer's Disease Center Research Resource Sharing Hub, which can be found at radc.rush.edu. It has descriptions of the studies and available data. Any qualified investigator can create an account and submit requests for deidentified data.