Abstract
Objective
To determine whether training with a brain–computer interface (BCI) to control an image of a phantom hand, which moves based on cortical currents estimated from magnetoencephalographic signals, reduces phantom limb pain.
Methods
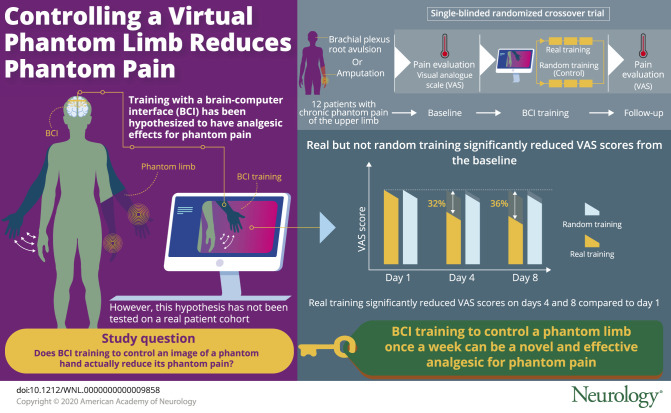
Twelve patients with chronic phantom limb pain of the upper limb due to amputation or brachial plexus root avulsion participated in a randomized single-blinded crossover trial. Patients were trained to move the virtual hand image controlled by the BCI with a real decoder, which was constructed to classify intact hand movements from motor cortical currents, by moving their phantom hands for 3 days (“real training”). Pain was evaluated using a visual analogue scale (VAS) before and after training, and at follow-up for an additional 16 days. As a control, patients engaged in the training with the same hand image controlled by randomly changing values (“random training”). The 2 trainings were randomly assigned to the patients. This trial is registered at UMIN-CTR (UMIN000013608).
Results
VAS at day 4 was significantly reduced from the baseline after real training (mean [SD], 45.3 [24.2]–30.9 [20.6], 1/100 mm; p = 0.009 < 0.025), but not after random training (p = 0.047 > 0.025). Compared to VAS at day 1, VAS at days 4 and 8 was significantly reduced by 32% and 36%, respectively, after real training and was significantly lower than VAS after random training (p < 0.01).
Conclusion
Three-day training to move the hand images controlled by BCI significantly reduced pain for 1 week.
Classification of evidence
This study provides Class III evidence that BCI reduces phantom limb pain.
Phantom limb pain often follows the amputation or deafferentation of a limb and has a large impact on a patient's life.1 Although effective treatment is limited and lacks sufficient evidence,2–5 mirror therapy and related techniques have been hypothesized to reduce pain by strengthening the cortical representation of the phantom hand.2–9 However, some recent studies have suggested that the pain reduction is associated with a weakening of the representation.10,11 In our previous study, we developed a brain–computer interface (BCI) to control a robotic hand12,13 using magnetoencephalography (MEG) signals and tested a hypothesis that training to use the BCI robotic hand, which was controlled based on the cortical representation of the phantom hand by moving the phantom hands, strengthened the cortical representation of the phantom hand to reduce the pain.14 However, contrary to that hypothesis, the pain was significantly increased immediately after the BCI training compared to after a sham training in a crossover trial, although the BCI training succeeded in strengthening the cortical representation of the phantom hand. Therefore, to decrease the pain by attenuating the cortical representation of the phantom hand, the same patients were trained to control the BCI based on the cortical representation of the intact hand by moving the phantom hand. In this case, the pain was significantly decreased immediately after the training compared to the pain changes of the previous sham trainings, which were not randomized with the current training. These results suggested that training to use the BCI based on the cortical representation of the intact hand movements, coupled with the phantom hand, temporarily relieved pain.11 However, the efficacy of the BCI training has not been elucidated by a randomized controlled trial. In this study, we tested the hypothesis that the BCI training based on the cortical representation of the intact hand movements reduces phantom limb pain sustainably.
Methods
Classification of evidence
This interventional study provides Class III evidence that training with BCI reduces phantom limb pain significantly more than sham training. We tested the hypothesis that BCI training reduces phantom limb pain sustainably by a single-blinded randomized sham-controlled crossover trial (figure 1). The primary outcome was reduced pain at day 4, defined as visual analogue scale (VAS) normalized by the VAS scores at day 1. The exclusion/inclusion criteria are defined in the following section. The dropout rate was less than 20%.
Figure 1. Participant flow diagram.
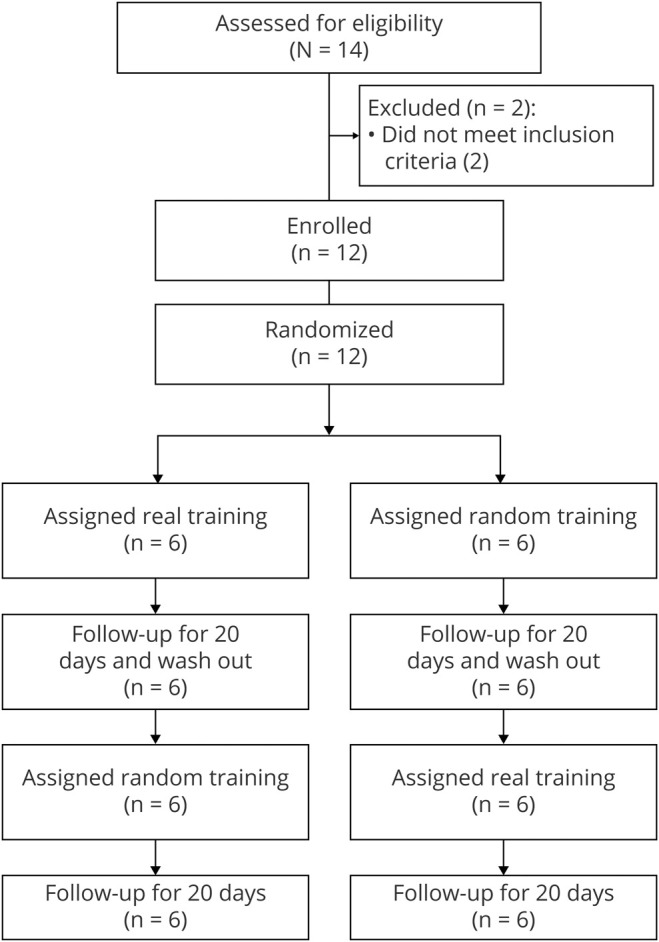
Participant flow diagram with actual number of participants.
Study design and participants
We conducted a randomized single-blinded crossover design trial at Osaka University in Japan. Eligible patients had chronic phantom limb pain of the upper limb, and we selected patients who met all of the following inclusion criteria: (1) phantom hand sensation, (2) chronic pain in the phantom hand, (3) no hand or no actual sensation in the residual hand, (4) no hand or completely plegic hand, and (5) normal comprehension and intellectual capacity according to the Japanese Adult Reading Test (JART-25). Here, “no hand” refers to amputees and “no actual sensation in the residual hand” refers to complete deafferentation. The affected hands of all patients with brachial plexus root avulsion had no sensation and were plegic due to complete avulsion of their roots (as definitively confirmed through MRI or CT with myelogram). We excluded patients with incompletely plegic hands so that the motor function of these patients was the same as the amputees. Participants who were not able to be recorded by MEG were excluded to allow all patients to perform the training using MEG.
Standard protocol approvals, registrations, and patient consents
The study adhered to the Declaration of Helsinki and was performed in accordance with protocols approved by the Ethics Committee of the Osaka University Clinical Trial Center (No. 13381-6, UMIN000013608, study protocol available from Dryad, text 1, doi.org/10.5061/dryad.15dv41nt9). All patients were informed of this study's purpose and possible consequences (i.e., that it sought to induce changes in cortical activity related to pain using the BCI and that the experiments included 2 trainings with different decoders), and written informed consent was obtained. Patients were informed that their pain might increase or decrease after training, although the change would be temporary.
Randomization and masking
The participants were enrolled in the study by 2 medical doctors who had no involvement with the rest of the trial. The experimenter in charge of the BCI system assigned the patients to the trial groups by a block method with a block size of 2. Although this experimenter was aware of the treatment allocation, the participants and the other experimenters who assessed pain were not aware of the allocations. All of the experiment's procedures and settings were exactly the same among trials, except for the selection of the decoder, which was set by the experimenter. Notably, although the assessment of pain was performed blindly, this study was single-blinded because the experimenter in charge of the BCI system was aware of the treatment allocation. Controlling the BCI system blindly was difficult. Therefore, the randomization was also performed by the experimenter in charge of the BCI system. To assess the masking, we asked all participants whether the hand image was controllable. Notably, we did not ask the patients their group allocation directly because we could not do the interview after the last follow-up for all patients.
Procedures
All patients completed 2 sessions with a washout period of more than 3 weeks; each session consisted of experiments on 3 consecutive days using a different training—real or random. At the beginning of the 3-day experiments, each patient's pain was evaluated with a 100-mm VAS. The patients marked a point on a 100-mm horizontal line to indicate their pain intensity; the line represented a range with the worst pain at the right side and no pain at the left side. The patients then performed a movement task for their phantom hand while the MEG signals were recorded. The patients were visually instructed with the Japanese word for “grasp” or “open” to move their phantom hand so that it was either grasping or opening at the presented times, without moving other body parts (figure 2A).15 Next, the patient did the same movement task with their intact hand, while the MEG signals from 84 selected sensors were recorded (figure 2B). From the MEG signals during the intact hand movements, the motor cortical currents were estimated at 126 selected cortical points contralateral to the intact hand (figure 2C). These were converted into z scores using mean and SD estimated from the 50 seconds of resting state data before the movement task. z Scores were averaged in a 400-ms time window from −2,000 to 1,000 ms at 100-ms intervals according to the cue. We constructed a “real decoder” to estimate the likelihood of the intact hand's “opening” movement using the z scores averaged in a 400-ms time window by sparse logistic regression (SLR).16 During this decoder construction, which took about 5 minutes, the patient stayed at rest in the MEG scanner with eyes closed.
Figure 2. Movement task and training to move the virtual hand image controlled by brain–computer interface.
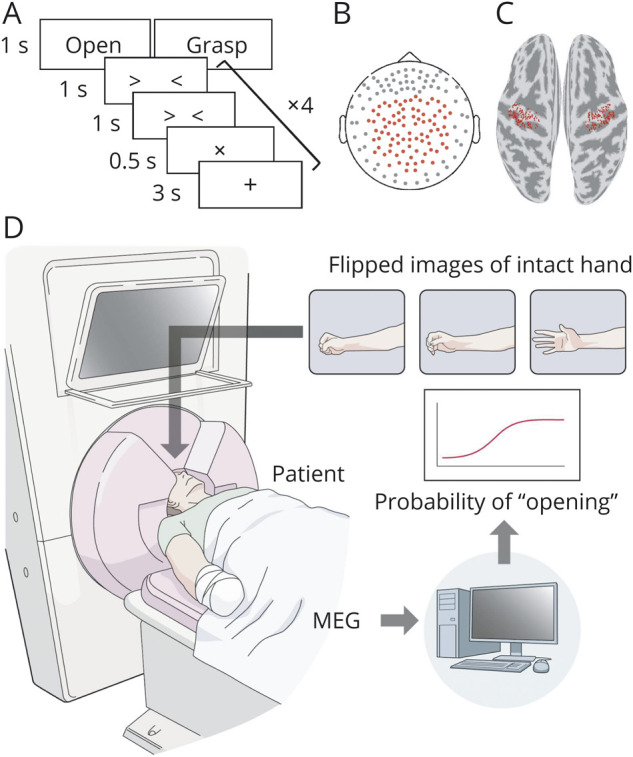
(A) The movement task began with a 3-second visual presentation of a black cross. A Japanese word was shown for 1 second to instruct the participants which movement to perform. After two 1-second timing cues, the execution cue with the cross sign was presented for 0.5 seconds with a sound, and patients performed the indicated movement. Cues with sounds were repeated 4 times for each instruction. Each movement type was assigned in random order 10 times. (B) The locations of the 84 selected sensors are shown as red points. (C) The 126 selected vertices are shown in red on the motor cortex for each hemisphere. (D) Schematic explanation of the training to control the virtual hand image. The hand image was presented to patients during the training and moved according to the output of the decoder every 200 ms. MEG = magnetoencephalography.
After the decoder construction, the patients performed 10 minutes of real or random training 3 times on day 1. During the real training, a virtual image of the patient's phantom hand was presented to the patient and moved according to the likelihood of the hand opening as evaluated by the real decoder using the 400-ms averaged z scores every 200 ms, which was calculated from the MEG signals obtained online (figure 2D and video 1). Notably, we only used the cortical currents estimated from the MEG signals from 84 selected sensors both for the construction of the real decoder and for the real training. During the random training, the same image was controlled by a value that randomly increased or decreased every 200 ms. For both trainings, patients were instructed to control the same images by moving their phantom hand. The difference between the 2 trainings was whether or not the images were controlled based on cortical activities. Trainings were done 6 times on day 2, and 3 times on days 1 and 3. After the training on day 3, the patient performed the movement task for the phantom hand and the intact hand in the same manner as on day 1 to evaluate whether and how the cortical representation of the phantom hand had changed. The amount of training was maximized by keeping the whole MEG recording of each day within 2 hours to avoid patient overload. Finally, immediately after each day's training, we evaluated patient pain by VAS and asked patients to describe whether they could control the hand image during the training. The patients reported their pain with a VAS at a similar time to the training every day from day 4 to day 20. Although the pain was evaluated by short-form McGill Pain Questionnaire 2 (SF-MPQ2) at the beginning of the training for each patient, we used VAS to evaluate pain repeatedly during and after the training.
This video shows an example of the brain–computer interface training during which a patient controlled his hand image by moving his phantom hand. The phantom hand image was shown with the probability of “open hand,” which was estimated from the cortical motor currents online. The phantom hand image was controlled according to the probability. The black line in the left panel shows the estimated probability. The red line shows the time average of the black line for 5 consecutive points.Download Supplementary Video 1 (13.7MB, mp4) via http://dx.doi.org/10.1212/009858_Video_1
Outcomes
The primary outcome was reduction of pain at day 4, defined as the VAS normalized by the VAS scores at day 1 (baseline). The VAS scores were normalized to compare the pain reduction rate among the real and random trainings. As the secondary outcome, the normalized VAS was compared among trainings at the remaining follow-up. Safety and adverse events were evaluated for each trial. If patients failed to report pain, the lost VAS score was treated as missing data.
MEG recordings
Participants were placed in the supine position, with their heads centered in the gantry. Patients were instructed not to move the head during the measurement to avoid motion artifacts. A projection screen presented visual stimuli (Presentation, Neurobehavioral Systems, Albany, CA) from a liquid crystal projector (LVP-HC6800, Mitsubishi Electric, Tokyo, Japan). MEG signals were measured using a 160-channel whole-head MEG system equipped with coaxial-type gradiometers (MEGvision NEO, Yokogawa Electric Corporation, Kanazawa, Japan) housed in a magnetically shielded room.
MEG signals were sampled at 1,000 Hz with an online high-pass filter at 0.1 Hz, a band-rejection filter at 60 Hz, and a low-pass filter at 200 Hz, and acquired online by FPGA DAQ boards (PXI-7854R, National Instruments, Austin, TX) after passing through an optical isolation circuit.
Five head marker coils were attached to each patient's face before the MEG recording to determine the position and orientation of sensors relative to the head. The positions of the coils were measured to evaluate differences in head position before and after each MEG recording, with a maximum acceptable difference of 5 mm.
Cortical current estimation by variational Bayesian multimodal encephalography
A polygon model of the cortical surface was constructed based on structural MRI (T1-weighted; Signa HDxt Excite 3.0T, GE Healthcare UK Ltd., Buckinghamshire, UK) using Freesurfer software (Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown). To align MEG data with individual MRI data, we scanned the 3D facial surface, positions of the marker coils, and 50 points on the scalp of each participant (FastSCAN Cobra, Polhemus, Colchester, VT). 3D facial surface data were superimposed on the anatomical facial surface extracted from the MRI data. Marker coil positions were also measured using the MEG system before each recording and were used to align the MEG system with the MRI through the FastSCAN coordinate.
Cortical currents were estimated from MEG data using variational Bayesian multimodal encephalography (ATR Neural Information Analysis Laboratories, Kyoto, Japan).17 The program estimated 4,004 single-current dipoles equidistantly distributed and perpendicular to the cortical surface. An inverse filter was calculated to estimate the cortical current of each dipole using MEG signals from all trials from 0 to 1 second during the movement task, with the baseline of current variance estimated from the signals from −1.5 to −0.5 seconds. We used a uniform prior. The hyperparameters m0 and γ0 were set to 100 and 10, respectively. The lead field was computed using the boundary elementary method with coregistered sensor positions and the polygon model using the data obtained immediately before each 10-minute training. We only used the cortical currents of the sensorimotor cortex ipsilateral to the phantom hand from the whole cortical currents estimated from the MEG signals of 84 selected sensors.
Preparation of the decoder for BCI
To construct a decoder, the experimenter determined the timing from the cue that had the most information for the intact hand movements. A support vector machine with a radial basis function kernel was used to classify the movement types of the intact hand by 10-fold cross-validation for the time-averaged z score at each timing from −2,000 to 1,000 ms with several hyperparameters that were selected by the experimenter.13 Then, the experimenter selected one timing of data, which has high classification accuracy evaluated by the support vector machine.
The z scores of the selected timing of data were used for decoder construction to infer the intact hand movements of grasping and opening by SLR.16 We used SLR to estimate the likelihood of one movement represented as a logistic function. During the training, the constructed decoder estimated the likelihood of the “opening” movement using the z scores estimated from the MEG signals online every 200 ms. MATLAB R2013a (MathWorks, Natick, MA) was used for the calculations.
Training to move a virtual phantom hand controlled by BCI
We took 8–10 stepped pictures of the patient's intact hand from grasping to opening. Each picture was flipped right to left to make a virtual image of the patient's phantom hand; this image was presented to the patient during the training and moved according to the likelihood of opening the hand (figure 2D and video 1). The likelihood was evaluated as a number from 0 to 1. The numbers were then divided by 8–10 steps and assigned to the flipped pictures.
During the random training, the same image was controlled by randomly generated values, starting from 0 at the beginning to which was added −0.1 or 0.1, as selected randomly using the MATLAB “randi” function, every 200 ms. If the absolute value exceeded 1, the value was not changed. This process generated a gradual increase and decrease of the value from −1 to 1.
Statistical analysis
Sample size calculation was based on the pain reduction rate measured using VAS scores in our previous study11 in which VAS scores were reduced by 0.9 (SD 0.22) (VAS after training/VAS before training) on average after training. Pain reduction in 3 consecutive 10-minute trainings was therefore expected to be 0.729 (0.93). Assuming a difference in the score of 0.27 between the training with the real and the random decoder, approximately 12 patients were necessary to achieve 80% power (simplified calculation as 2-sided t test for paired samples; α = 5%).
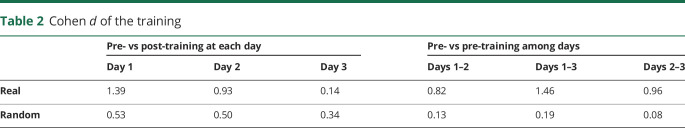
Statistical analyses were performed using MATLAB R2013a. Data sets were initially assessed for normality using the Kolmogorov-Smirnov test. VAS scores were evaluated before and after each day's trainings (pretraining and posttraining) for the 2 conditions (real training and random training) on each of the 3 days (day 1, day 2, and day 3) by 3-way analysis of variance (ANOVA). Statistical significance was considered at p < 0.05. For each real and random training, VAS scores were compared before and after training among the 3 days by 2-way ANOVA with Bonferroni correction. VAS score differences were evaluated by paired Student t test with Bonferroni correction. The effect size of the pain reduction was evaluated by Cohen d.
Pre- and post-training VAS scores for each training day were averaged and reported as the means with SDs, and daily pretraining scores were normalized by the day 1 pretraining score. The normalized VAS scores from days 1–10 and the normalized VAS averaged from day 11–20 were compared between the real and random trainings among days by 2-way ANOVA with p < 0.05 (real vs random and days). Normalized VAS scores between real and random trainings were compared by paired Student t test for each day with p < 0.05. We used multiple imputations with sequential regression for the missing VAS score during the follow-up to create and analyze 20 severally imputed datasets. Calculations were performed using IBM SPSS. The estimates and the SDs were combined using Rubin rules.
Data availability
The data that support the findings of this study are available on request from the corresponding author and from Dryad, tables e-3 through e-6 (doi.org/10.5061/dryad.15dv41nt9).
Results
Patients with phantom limb pain
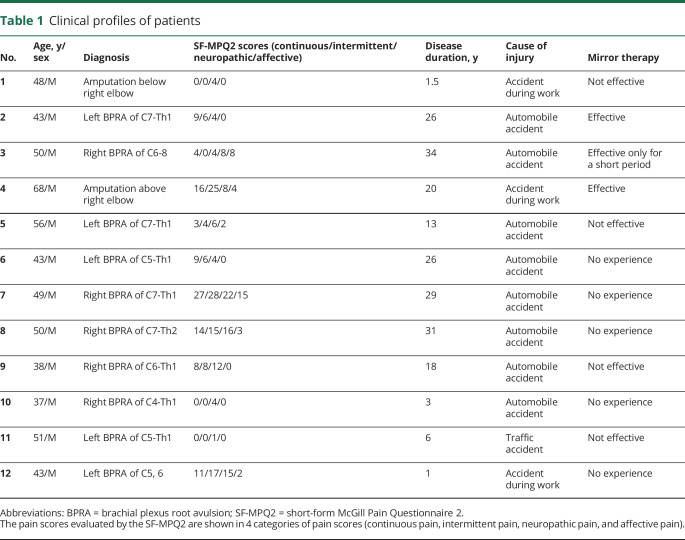
Between August 1, 2015, and June 30, 2018, 14 patients with chronic phantom limb pain were recruited at Osaka University Hospital in Japan (figure 1).18 Two patients were excluded because they could move their affected hands slightly and were found to have incomplete avulsion of their roots during detailed examination. The follow-up with the participants ended on July 3, 2018, at which point the number of participants was 12. Twelve patients with phantom limbs due to brachial plexus root avulsion or amputation of the forearm participated in this study (table 1 and table e-1, doi.org/10.5061/dryad.15dv41nt9). Pain was characterized by the Japanese version of the SF-MPQ219 before the experiment.
Table 1.
Clinical profiles of patients
The patients were randomly allocated into 2 groups (figure 1). On day 1, the total scores of SF-MPQ2 before the training were not significantly different between the 2 groups (mean [SD]; real first, 35.3 [25.0]; random first, 32.8 [31.5]; p = 0.88). However, the VAS scores were significantly different between 2 groups (real first, 60.8 [25.1], 1/100 mm; random first, 22.8 [6.9]; p = 0.005), because we did not allocate the participants based on the pain scales. During the follow-up (day 5–10), a total of 11 out of 144 scores (7.6%) were missing due to the patient's carelessness or misunderstanding (tables e-3 through e-6, doi.org/10.5061/dryad.15dv41nt9).
BCI training reduced pain during the 3-day trainings
Among the 3 consecutive days of training, the training significantly changed VAS scores, although the differences of VAS scores were not significant between real and random trainings (pre- vs post-training, p = 0.0004 < 0.05; real vs random, p = 0.71; days, p = 0.42, interaction p > 0.05). As a post hoc test, VAS scores were significantly reduced during real training (pre- vs post-training, p = 0.0030 < 0.025; days, p = 0.36; interaction p = 0.13; figure 3A), but not during random training (pre- vs post-training, p = 0.047 > 0.025; days, p = 0.90; interaction p = 0.96, figure 3B). Notably, pain was reduced with a large effect size on day 1 and day 2 of the real trainings (table 2 and figure 3A). However, for random training days, VAS scores were not significantly decreased, with smaller effect sizes (table 2 and figure 3B). Moreover, pretraining pain was reduced during the 3 days of real training, and these VAS scores significantly decreased from day 1 to day 3 and from day 2 to day 3 but not from day 1 to day 2 (table 2 and figure 3A). Pretraining VAS scores for the random training were not significantly changed across the 3 days (table 2 and figure 3B). Real training significantly reduced pain immediately after training as well as at the beginning of training on day 3, which demonstrated a cumulative pain reduction effect, although the VAS scores were not significantly different among real and random trainings during the 3 days of trainings and the VAS changes were not specific to the real training.
Figure 3. Real training decreased pain.
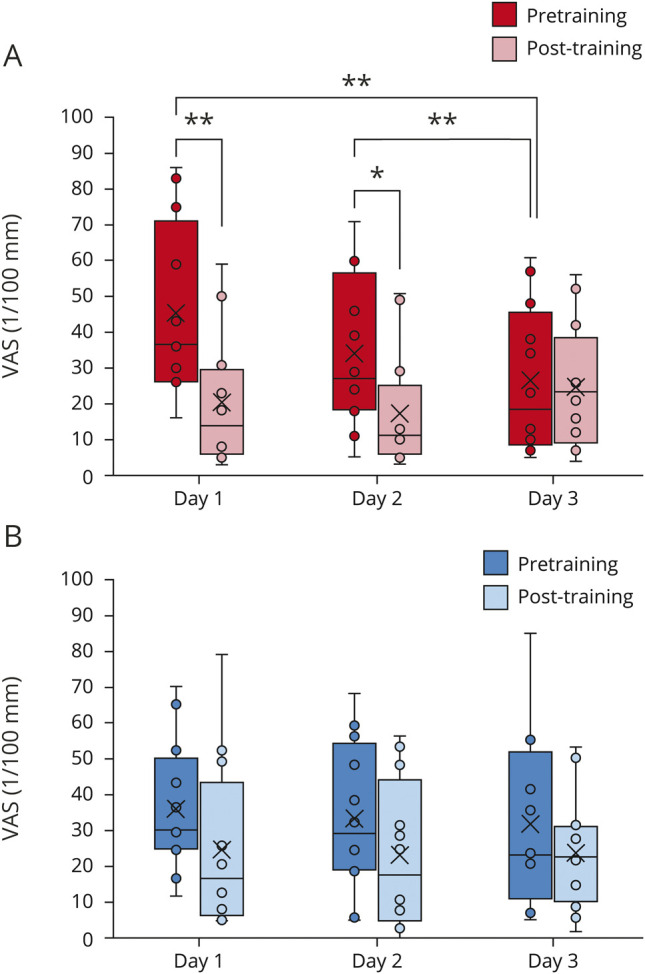
The visual analogue scale (VAS) scores are shown as box and whisker plots between pretraining (filled) and posttraining (shaded) on the 3 days of real training (red; A) and random training (blue; B). The cross represents the mean. The dot shows the VAS scores. *p < 0.05, **p < 0.01, paired Student t test, Bonferroni corrected.
Table 2.
Cohen d of the training
The only adverse event was increased pain during the training compared to that at the beginning for 2 patients (1.7%) in the real training and 7 patients (58.3%) in the random training. The 2 patients who experienced pain during real training had brachial plexus root avulsion and also experienced pain during random training.
The VAS scores of the day 1 pretraining were not significantly different between the first and second arms of the crossover trial (first, 41.8 [15.0]; second, 40.1 [9.3]; p = 0.68). However, the VAS scores of the day 1 pretraining were significantly different between the real and random training due to the differences of the baseline VAS scores (real, 45.3 [24.3]; random, 36.6 [18.5]; p = 0.019). According to patient interviews after each training, no patient realized that the random training was unconnected to their movements (table e-2, doi.org/10.5061/dryad.15dv41nt9).
Real training reduced pain for 5 days after the 3-day trainings
As a primary outcome of this study, the analgesic effects of the training were evaluated after the 3-day trainings. From day 1 to day 4, VAS scores were significantly reduced after the real training (45.3 [24.2] at day 1 to 30.9 [20.6] at day 4; p = 0.009 < 0.025) but not after the random training (36.6 [18.5] to 36.7 [25.0]; p = 0.98). The VAS scores of the real training were significantly smaller than those of random training at day 4 (p = 0.048 < 0.05). Moreover, the VAS scores normalized by day 1 pretraining scores were significantly smaller for real training than random training at day 4 (real, 0.68 [0.30]; random, 0.94 [0.32]; Cohen d = 1.31, p = 0.00038 < 0.05). The 3-day real training significantly reduced pain for patients with phantom limb pain.
The normalized VAS scores were significantly different for real and random training in the follow-up (real vs random, p < 0.0001; among days, p = 0.001; interaction, p = 0.70). Real training normalized scores were significantly smaller than random training scores even 5 days after the 3-day training except for those at days 6 and 7 (figure 4). This result suggests that the 3-day real training induced significant pain reduction that was sustained for 5 days after training.
Figure 4. Normalized visual analogue scale (VAS) scores after the training.
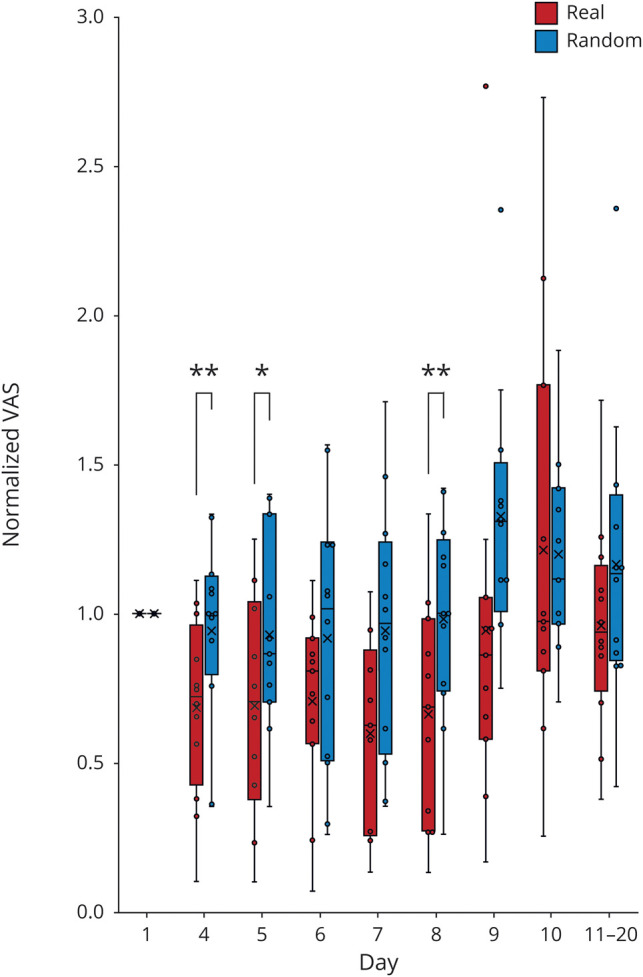
Normalized VAS scores are shown as box and whisker plots for each day of real training (red) and random training (blue). The cross represents the mean. The box represents the upper and lower quartiles. The whisker represents the maximum and minimum. The dot shows the VAS scores. *p < 0.05, paired Student t test.
It should be noted that the pain reduction rate between day 1 and day 4 ([day 4 − day 1]/day 1) was not significantly correlated with the patient's demographics such as age (correlation coefficient 0.12), disease duration (0.05), and the VAS at day 1 (−0.013).
Discussion
This study demonstrates that training to move a virtual phantom hand controlled by BCI significantly reduces phantom limb pain, with the reduction sustained for 5 days after a 3-day training. VAS scores were significantly reduced by 32% at day 4 after real training but not after random training. Pain was also significantly reduced by 36% at day 8 after real training. This finding suggests that training to use a BCI once a week would be a novel treatment to control phantom limb pain.
The pain reductions in this study were comparable to those of other visual feedback treatments. Mirror therapy reportedly reduces phantom limb pain by 37.6% according to a VAS for 4 weeks of training.20 Similarly, visual feedback training using augmented reality decreases phantom limb pain by 32% according to a numeric rating scale.7 Notably, these previous studies took longer trainings than our current study and demonstrated that longer training periods decreased the pain more. In our 3-day trainings, the real training significantly reduced pain at day 1 with 3 trainings per day and at day 2 with 6 trainings per day, but not at day 3 with 3 trainings per day. In the short term, 2 days with 3 to 6 BCI trainings might be enough to reduce pain by an amount comparable to other visual feedback treatments. Applying 2 days of this BCI training every week might improve pain reduction in the long term.
Compared with other feedback trainings, our training controlling the BCI virtual hand has some similarities and differences with advantages and disadvantages. Theoretically, the training to move the virtual hand controlled by the BCI with a real decoder is similar to mirror therapy, during which patients move their phantom hand while in fact moving the intact hand, which activates the cortical representation of the intact hand while moving the phantom hand. In our training, patients learned to induce cortical activity for the intact hand while moving the phantom hand. However, because it is difficult to activate the intact hand representation without thinking about intact hand movements, it is difficult to achieve this goal with mirror therapy. The training to control the BCI affords patients the opportunity to activate this representation without moving the intact hand, possibly making it easier to achieve the ultimate goal. Moreover, by monitoring cortical activities, the BCI training should be better at inducing targeted cortical activities compared to the other trainings that do not record the cortical activities. Therefore, if the cortical activity of the phantom hand is the therapeutic target, the BCI training will improve the efficacy to modulate the cortical activity to reduce the pain. The mirror therapy was effective in only 3 out of 7 patients in this study as their prior experiences. But the BCI training reduced the pain for 5 out of the 7 patients and for 9 out of all 12 patients. This suggests that BCI training may be effective for more patients than mirror therapy.4 It should be noted, however, that BCI training costs much more than mirror therapy. We propose that methods based on the same mechanism should be developed to make implementation of BCI training in clinic more cost-effective.
In our previous study, we demonstrated that BCI training with the phantom hand decoder temporarily increased pain in association with the improved accuracy to classify the MEG signals of grasping and opening the phantom hand.11 On the other hand, the BCI training with the intact hand decoder decreased pain with a reduction in classification accuracy.11 Here, we evaluated the classification accuracy as the measure of the cortical representation.21 Similarly, in the current study, the classification accuracy of phantom hand movements was significantly decreased after the real training (data available from Dryad, text 2, doi.org/10.5061/dryad.15dv41nt9) in association with the pain reduction. Patients with a larger reduction of pain tended to reduce the classification accuracy after the training. It was suggested that the cortical representation should be changed to reduce the classification accuracy to reduce the pain.
It should be noted that this study has some limitations. Although the number of participants was comparable to previous studies,7–9 it was nonetheless a small number, which limited the study. Also, we recruited patients with phantom limb pain due to both amputation and brachial plexus root avulsion. Although the pain might be different between the 2 patient groups, the effect of the training was similar among the patients and showed consistent changes in pain and cortical representation. Our results demonstrated that training to control BCI reduces pain similarly among these patients. In addition, the length of the training (3 days) is short to evaluate its long-term clinical effect. This highlights the need for further studies to evaluate the long-term clinical effects of the BCI trainings for a longer period of time. Moreover, in the current study, we evaluated the baseline pain only once before each 3-day training; however, even in the same patient, pain varies over time and can change spontaneously. It will be better to evaluate the baseline pain for a longer period in the future study.
Our 3-day training demonstrated a significant analgesic effect that lasted 1 week compared to the random trainings. Although the study had several limitations, the observed pain reduction evaluated in the randomized crossover trial strongly suggests that the BCI training is effective for reducing phantom limb pain. Moreover, the effect size suggests that the BCI training will be a promising method to reduce phantom limb pain compared to other treatments. Our results strongly support the application of the training to control BCI for longer periods for the clinical relief of phantom limb pain.
Glossary
- ANOVA
analysis of variance
- BCI
brain–computer interface
- MEG
magnetoencephalography
- SF-MPQ2
short-form McGill Pain Questionnaire 2
- SLR
sparse logistic regression
- VAS
visual analogue scale
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study funding
This research was conducted under the Strategic Research Program for Brain Sciences (SRPBS) (JP19dm0307008) from the Japan Agency for Medical Research and Development (AMED). This research was also supported in part by the Japan Science and Technology Agency (JST) Precursory Research for Embryonic Science and Technology (JPMJPR1506), Core Research for Evolutional Science and Technology (JPMJCR18A5), and Exploratory Research for Advanced Technology (JPMJER1801); Grants-in-Aid for Scientific Research from KAKENHI (JP17H06032, JP15H05710, JP18H05522, and JP18H04085); Brain/MINDS (19dm0207070h0001) and Brain/MINDS Beyond (19dm0307103h0001) from AMED; TERUMO Foundation for Life Sciences and Arts; SONPO Foundation; Daiichi Sankyo Foundation of Life Science; and Versus Arthritis (21537) IITP grant funded by MSIT (2019-0-01371).
Disclosure
T. Yanagisawa had grants from AMED (JP19dm0307008), JST (JPMJPR1506, JPMJCR18A5, JPMJER1801), KAKENHI (JP17H06032, JP15H05710), TERUMO Foundation, Daiichi Sankyo Foundation, and SONPO Foundation during the conduct of the study. R. Fukuma reports no disclosures. B. Seymour had grants from Versus Arthritis (21537) and IITP funded by MSIT (2019-0-01371). M. Tanaka reports no disclosures. K. Hosomi had grants from Brain/MINDS (19dm0207070h0001) and Brain/MINDS Beyond (19dm0307103h0001) from AMED and KAKENHI (JP18H05522 and JP18H04085) during the conduct of the study. Dr. Hosomi belongs to a joint research department established with sponsorship by Teijin Pharma Limited. O. Yamashita reports no disclosures. H. Kishima had grants from Brain/MINDS (19dm0207070h0001) and Brain/MINDS Beyond (19dm0307103h0001) from AMED and KAKENHI (JP18H05522 and JP18H04085) during the conduct of the study. Y. Kamitani reports no disclosures. Y. Saitoh had grants from AMED (JP19dm0307008) during the conduct of the study and belongs to a joint research department established with sponsorship by Teijin Pharma Limited. Go to Neurology.org/N for full disclosures.
References
- 1.Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci 2006;7:873–881. [DOI] [PubMed] [Google Scholar]
- 2.Richardson C, Kulkarni J. A review of the management of phantom limb pain: challenges and solutions. J Pain Res 2017;10:1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortiz-Catalan M. The stochastic entanglement and phantom motor execution hypotheses: a theoretical framework for the origin and treatment of phantom limb pain. Front Neurol 2018;9:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbin J, Seetha V, Casillas JM, Paysant J, Perennou D. The effects of mirror therapy on pain and motor control of phantom limb in amputees: a systematic review. Ann Phys Rehabil Med 2016;59:270–275. [DOI] [PubMed] [Google Scholar]
- 5.Thieme H, Morkisch N, Rietz C, Dohle C, Borgetto B. The efficacy of movement representation techniques for treatment of limb pain: a systematic review and meta-analysis. J Pain 2016;17:167–180. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran VS, Rogers-Ramachandran D, Cobb S. Touching the phantom limb. Nature 1995;377:489–490. [DOI] [PubMed] [Google Scholar]
- 7.Ortiz-Catalan M, Guethmundsdottir RA, Kristoffersen MB, et al. Phantom motor execution facilitated by machine learning and augmented reality as treatment for phantom limb pain: a single group, clinical trial in patients with chronic intractable phantom limb pain. Lancet 2016;388:2885–2894. [DOI] [PubMed] [Google Scholar]
- 8.Osumi M, Ichinose A, Sumitani M, et al. Restoring movement representation and alleviating phantom limb pain through short-term neurorehabilitation with a virtual reality system. Eur J Pain 2017;21:140–147. [DOI] [PubMed] [Google Scholar]
- 9.Chan BL, Witt R, Charrow AP, et al. Mirror therapy for phantom limb pain. N Engl J Med 2007;357:2206–2207. [DOI] [PubMed] [Google Scholar]
- 10.Kikkert S, Mezue M, O'Shea J, et al. Neural basis of induced phantom limb pain relief. Ann Neurol 2019;85:59–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanagisawa T, Fukuma R, Seymour B, et al. Induced sensorimotor brain plasticity controls pain in phantom limb patients. Nat Commun 2016;7:13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuma R, Yanagisawa T, Saitoh Y, et al. Real-time control of a neuroprosthetic hand by magnetoencephalographic signals from paralysed patients. Sci Rep 2016;6:21781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuma R, Yanagisawa T, Yorifuji S, et al. Closed-loop control of a neuroprosthetic hand by magnetoencephalographic signals. PLoS One 2015;10:e0131547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuma R, Yanagisawa T, Yokoi H, et al. Training in use of brain-machine interface-controlled robotic hand improves accuracy decoding two types of hand movements. Front Neurosci 2018;12:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanagisawa T, Hirata M, Saitoh Y, et al. Electrocorticographic control of a prosthetic arm in paralyzed patients. Ann Neurol 2012;71:353–361. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita O, Sato MA, Yoshioka T, Tong F, Kamitani Y. Sparse estimation automatically selects voxels relevant for the decoding of fMRI activity patterns. Neuroimage 2008;42:1414–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshioka T, Toyama K, Kawato M, et al. Evaluation of hierarchical Bayesian method through retinotopic brain activities reconstruction from fMRI and MEG signals. Neuroimage 2008;42:1397–1413. [DOI] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:726–732. [DOI] [PubMed] [Google Scholar]
- 19.Maruo T, Nakae A, Maeda L, et al. Validity, reliability, and assessment sensitivity of the Japanese version of the short-form McGill pain questionnaire 2 in Japanese patients with neuropathic and non-neuropathic pain. Pain Med 2014;15:1930–1937. [DOI] [PubMed] [Google Scholar]
- 20.Finn SB, Perry BN, Clasing JE, et al. A randomized, controlled trial of mirror therapy for upper extremity phantom limb pain in Male amputees. Front Neurol 2017;8:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quian Quiroga R, Panzeri S. Extracting information from neuronal populations: information theory and decoding approaches. Nat Rev Neurosci 2009;10:173–185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video shows an example of the brain–computer interface training during which a patient controlled his hand image by moving his phantom hand. The phantom hand image was shown with the probability of “open hand,” which was estimated from the cortical motor currents online. The phantom hand image was controlled according to the probability. The black line in the left panel shows the estimated probability. The red line shows the time average of the black line for 5 consecutive points.Download Supplementary Video 1 (13.7MB, mp4) via http://dx.doi.org/10.1212/009858_Video_1
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author and from Dryad, tables e-3 through e-6 (doi.org/10.5061/dryad.15dv41nt9).