Abstract
Objective
To determine that children with arterial ischemic stroke (AIS) due to an identifiable arteriopathy are distinct from those without arteriopathy and that each arteriopathy subtype has unique and recognizable clinical features.
Methods
We report a large, observational, multicenter cohort of children with AIS, age 1 month to 18 years, enrolled in the International Pediatric Stroke Study from 2003 to 2014. Clinical and demographic differences were compared by use of the Fisher exact test, with linear step-up permutation min-p adjustment for multiple comparisons. Exploratory analyses were conducted to evaluate differences between cases of AIS with and without arteriopathy and between arteriopathy subtypes.
Results
Of 2,127 children with AIS, 725 (34%) had arteriopathy (median age 7.45 years). Arteriopathy subtypes included dissection (27%), moyamoya (24.5%), focal cerebral arteriopathy–inflammatory subtype (FCA-i; 15%), diffuse cerebral vasculitis (15%), and nonspecific arteriopathy (18.5%). Children with arteriopathic AIS were more likely to present between 6 and 9 years of age (odds ratio [OR] 1.93, p = 0.029) with headache (OR 1.55, p = 0.023), multiple infarctions (OR 2.05, p < 0.001), sickle cell anemia (OR 2.9, p = 0.007), and head/neck trauma (OR 1.93, p = 0.018). Antithrombotic use and stroke recurrence were higher in children with arteriopathy. Among arteriopathy subtypes, dissection was associated with male sex, older age, headache, and anticoagulant use; FCA-i was associated with hemiparesis and single infarcts; moyamoya was associated with seizures and recurrent strokes; and vasculitis was associated with bilateral infarctions.
Conclusion
Specific clinical profiles are associated with cerebral arteriopathies in children with AIS. These observations may be helpful indicators in guiding early diagnosis and defining subgroups who may benefit most from future therapeutic trials.
Cerebral arteriopathies are reported in 30% to 50% of all children with arterial ischemic stroke (AIS).1,2 Most childhood cerebral arteriopathies are characterized either by dissection or by stenosis and irregularity of the intracranial arteries such as moyamoya and focal cerebral arteriopathy (FCA) of childhood. FCA is characterized by acute, unilateral, segmental stenosis of ≥1 large arteries of the anterior circulation2–5 and is presumed to be inflammatory in origin,2–4,6 although the angiographic appearance can be mimicked by intracranial dissection.7 One-third of cases of FCA may demonstrate initially progressive arterial narrowing, but by definition, arterial narrowing does not progress beyond 3 to 6 months after stroke.8 Most important, childhood cerebral arteriopathies are associated with a 5-fold higher risk of stroke recurrence9 and poor outcome.10 Therefore, their improved understanding is crucial to the development of immediate and preventive treatment strategies.
The difficulty in identifying and classifying childhood cerebral arteriopathies is a major limitation, relating often to the imaging technique, inability to consistently and accurately identify the diagnosis at presentation, and lack of standardized terminology and diagnostic criteria.1,2,8,11,12 Recent cohorts of cerebral arteriopathy have come from the International Pediatric Stroke Study (IPSS),1 in which arteriopathies are classified using gradually developed and refined consensus-based definitions.1,5 Recently, a substudy of the IPSS rereviewed neurovascular imaging of their arteriopathic cases and noted that a substantial proportion (up to 30%) of arteriopathies in their cohort remained challenging to classify.2 Such challenges are a frequent occurrence in clinical practice, suggesting that a clinical fingerprint may be helpful in the diagnosis and classification of childhood cerebral arteriopathies, especially early in the disease course when the arteriopathy has not declared its clinical course (progression, stabilization, improvement). We hypothesized that children with AIS due to an identifiable arteriopathy are distinct from those without arteriopathy and that the arteriopathy subtypes are distinct.
Methods
Study design
The IPSS is a prospective, multicenter, observational registry that enrolls and follows up children with ischemic stroke and related conditions. The IPSS currently comprises >300 investigators (representing 24 countries across 5 continents) and a web-based master database housed at the study coordinating center, The Hospital for Sick Children, Toronto, Ontario, Canada.13 The IPSS design has been previously published.1,10 The present analysis includes children with AIS with and without an underlying cerebral arteriopathy who were enrolled between January 2003 and July 2014 (including previously reported 667 cases enrolled from 2003 to 2007 in the IPSS1 and 355 cases from 2010 to 2014 coenrolled in the IPSS and an IPSS substudy, the Vascular Effects of Infection in Pediatric Stroke [VIPS]2).
Standard protocol approvals, registrations, and patient consents
As per IPSS policy, each site investigator obtained and maintained research ethics approval according to that investigator’s institutional policy to enroll and study eligible cases.
Study Population
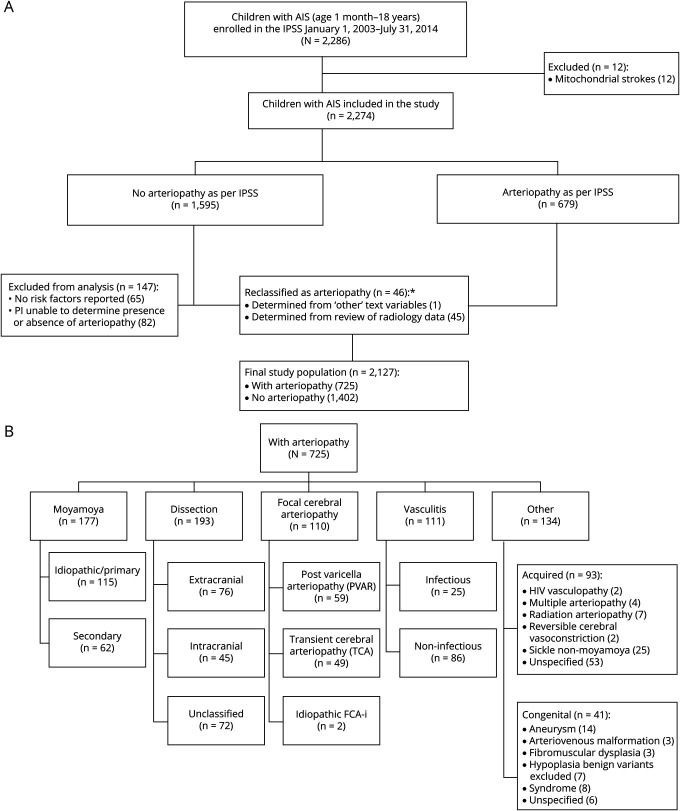
Inclusion criteria were age from 1 month to 18 years and AIS presenting with acute onset of neurologic deficit and a radiologic pattern consistent with arterial territory ischemia. Arteriopathy was defined a priori as any abnormality on vascular imaging (stenosis, irregularity, banding, pseudoaneurysm, dissection flap) except isolated vessel occlusion, which may be due to an embolus rather than a primary blood vessel abnormality.9 Children without arteriopathy had either idiopathic AIS (no obvious risk factor or etiology) or well-described nonarteriopathic risk factor for AIS (e.g., cardioembolic stroke). The presence of arteriopathy and its subtype were determined by the enrolling IPSS site investigators and clinicians involved in the child's care using the IPSS consensus-based definitions.5 Because a centralized review of radiographic films could not be performed due to the lack of an imaging repository in the IPSS dataset, we validated the arteriopathy diagnosis and subtype classification on the basis of the radiographic data available through the free text comment boxes in the IPSS dataset. In cases with unclear descriptions, more data were requested and obtained from the submitting IPSS site investigators (figure 1A). The authors further refined the arteriopathy classification referencing recent literature14 through consensus at 3 teleconferences and 1 face-to-face meeting.
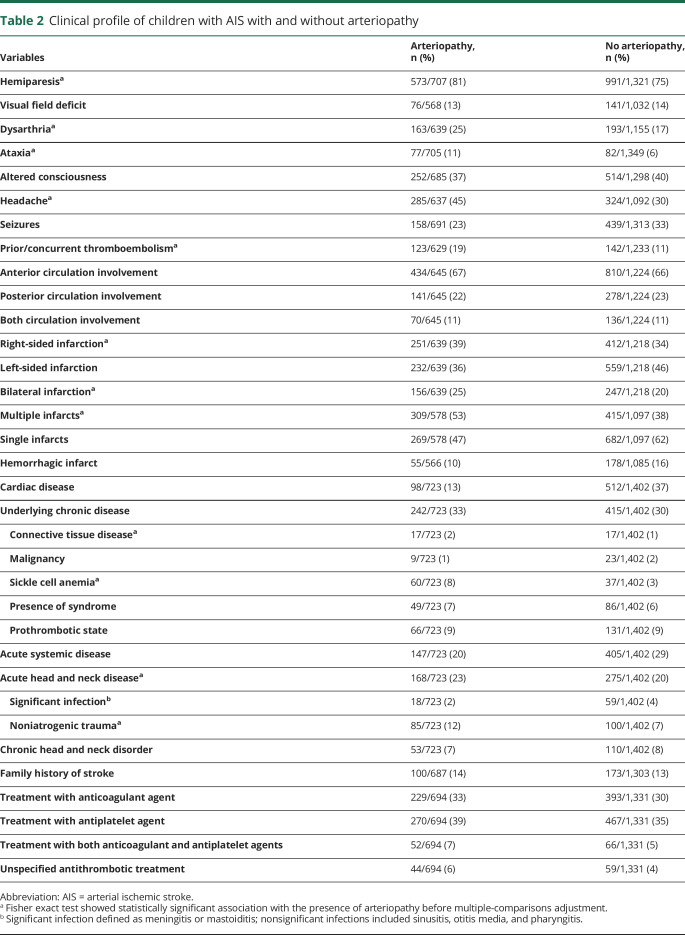
Figure 1. Study population.
(A) International Pediatric Stroke Study (IPSS) cohort of children with arterial ischemic stroke (AIS) and cerebral arteriopathies. (B) IPSS cohort of children with AIS associated with arteriopathy. FCA-i = focal cerebral arteriopathy–inflammatory subtype.
The refined arteriopathy subtypes included 5 mutually exclusive subtypes: (1) FCA, inflammatory type (FCA-i), which included idiopathic or presumed inflammatory FCA, transient cerebral arteriopathy, and postvaricella arteriopathy cases2,3,5,6,15; (2) dissection (extracranial, intracranial); (3) moyamoya (primary moyamoya disease, moyamoya syndrome secondary to other conditions); (4) diffuse cerebral vasculitis (infectious, noninfectious); and (5) nonclassifiable/nonspecific arteriopathies labeled as other arteriopathy (congenital, acquired) (figure 1B). Lack of sufficiently detailed vascular imaging limited our ability to further subcategorize vasculitis. We excluded children with arteriopathy but without stroke.
Data collection and abstraction
Study data were collected at each site by the IPSS site investigators and entered into the web-based IPSS database. The variables studied included demographic data (age at stroke diagnosis, sex, geographic region, race), clinical features (hemiparesis, visual field deficit, speech deficit, ataxia, decreased level of consciousness, headache, seizures, prior/concurrent thromboembolic event), radiographic information (circulation, stroke laterality, number of infarcts, hemorrhage), risk factors for stroke (cardiac disorder, underlying chronic disease, acute systemic disease, acute and chronic head/neck disease, family history of stroke), neuroimaging features (location and number of infarcts), antithrombotic treatments (classified as antiplatelet or anticoagulant agents), and outcomes. Management decisions for each case were guided by the published pediatric stroke management guidelines16 but varied according to the treating physician's preferences and local institutional protocols. Outcomes included neurologic deficits at discharge, stroke recurrence, case fatality, and standardized stroke outcomes at 1 year and last follow-up visit that were collected through a parental Recurrence and Recovery Questionnaire17,18 (mean duration 2.3 years), and Pediatric Stroke Outcome Measure (PSOM)17,18 (mean duration 2.5 years). Poor outcome was defined as a PSOM score >1.
Statistical methods
Analyses were performed with SAS version 9.4 statistical software (SAS Institute, Cary, NC). Descriptive analysis, including proportions, means, and frequencies, was used to define participant characteristics. Intracranial dissection, extracranial dissection, FCA-i, moyamoya, and vasculitis were included in the subtype comparison exploratory analyses. Cases with multiple arteriopathies or missing data were excluded from the analysis. Cases with dissection that could not be classified as either intracranial or extracranial dissection were excluded from the arteriopathy subtype analysis. Multiple comparisons (arteriopathy- vs non–arteriopathy-associated AIS, arteriopathy subtypes) were performed with the Fisher exact test with linear step-up permutation min-p adjustment. Values of α ≤0.05 were considered significant. Significant variables were included in a stepwise multiple logistic regression analysis. Poisson regression was used to test the difference of treatment over time, and logistic regression and χ2 tests were used as necessary to describe and measure associations involving multilevel categorical variables.
Data availability
All data used for analysis are presented in the tables and figures in this article. Data will be shared after ethics approval if requested by other investigators for purposes of replicating the results.
Results
A total of 4,294 children were enrolled in the IPSS during the study period. We identified 2,274 (53%) children, 1 month to 18 years of age, with AIS. Of these, 2,127 (93%) children fulfilled the current study inclusion criteria. Of this cohort, 725 (34%) children were identified as having AIS due to arteriopathy, and 1,402 (66%) children had AIS due to nonarteriopathic causes (figure 1A and table 1). In 2,127 children with AIS, nonarteriopathic risk factors (either single or multiple in a study participant) included cardioembolic disorders (29%), chronic diseases (31%), acute systemic illnesses (26%), acute head or neck infections/trauma (21%), chronic head or neck disorders (8%), and family history of stroke (14%) (table 2). In 725 children with arteriopathy, arteriopathy subtypes were categorized as craniocervical dissection in 193 (27%), moyamoya in 177 (24%), FCA-i in 110 (15%), diffuse cerebral vasculitis in 111 (15%), and other arteriopathy in 134 (19%) (figure 1B). Although dedicated vascular imaging was reported in 1,409 of 2,127 (66%) cases (74% noncardiac and 26% cardiac AIS), this number is underreported due to the missing data in the dataset. Of the 718 (34%) cases with no vascular imaging indicated in the IPSS dataset, 164 (23%) were labeled as having an arteriopathy by the site investigators. As stated in the Methods section, arteriopathy cases with insufficient information or unclear diagnosis were rereviewed by the study investigators for any radiology information in the dataset, and those with no radiologic data to support the arteriopathy diagnosis were sent to the reporting site investigator for review of their radiology information to confirm the diagnosis and type of arteriopathy (figure 1A).
Table 1.
Demographic profile of children with AIS with and without arteriopathy
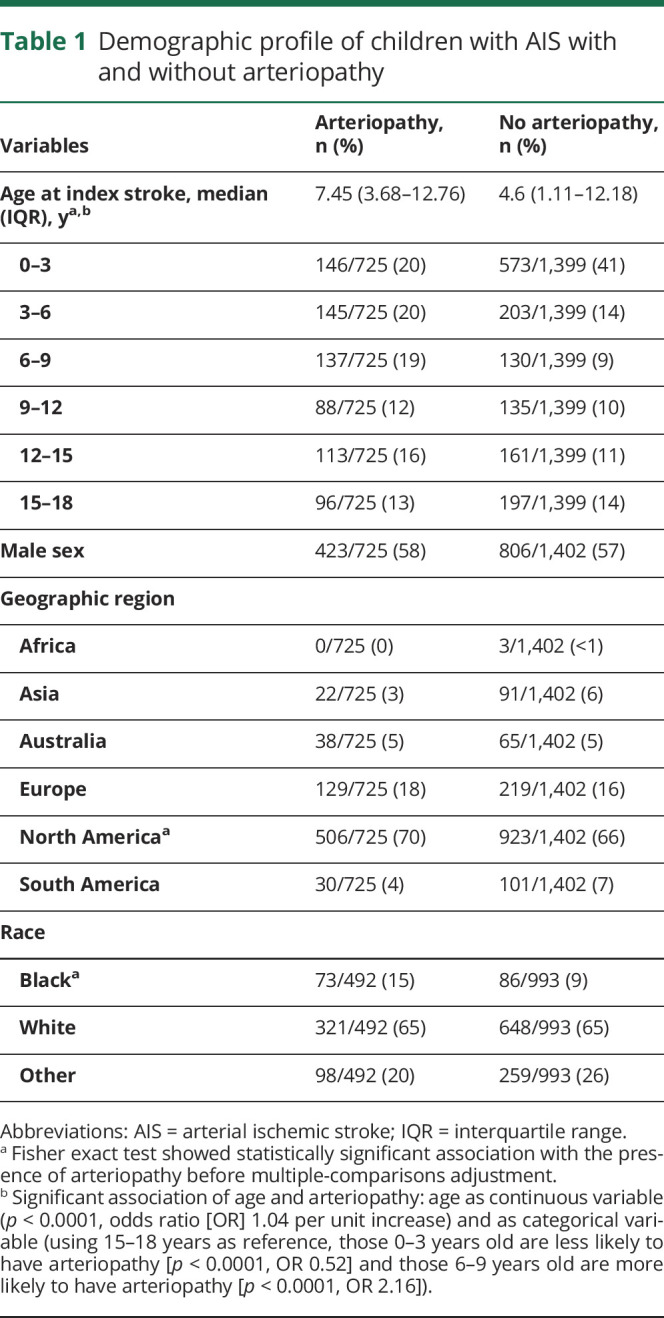
Table 2.
Clinical profile of children with AIS with and without arteriopathy
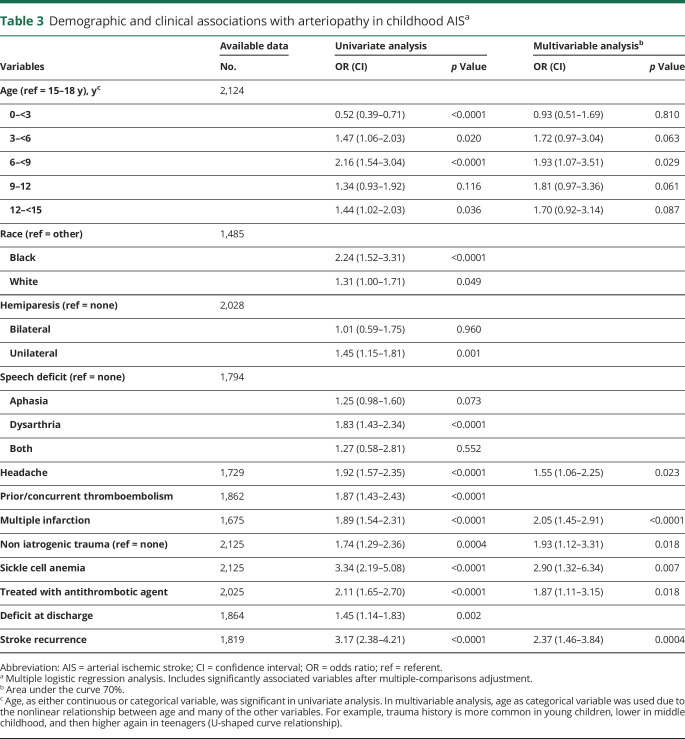
Tables 1 and 2 lists the demographic and clinical features of AIS and specifies all variables that were significantly associated with arteriopathy (p ≤ 0.05) before multiple-comparison adjustment. Results (p values and odds ratios [ORs]) from multivariable analysis are stated in the section below (table 3).
Table 3.
Demographic and clinical associations with arteriopathy in childhood AISa
Arteriopathic vs nonarteriopathic AIS
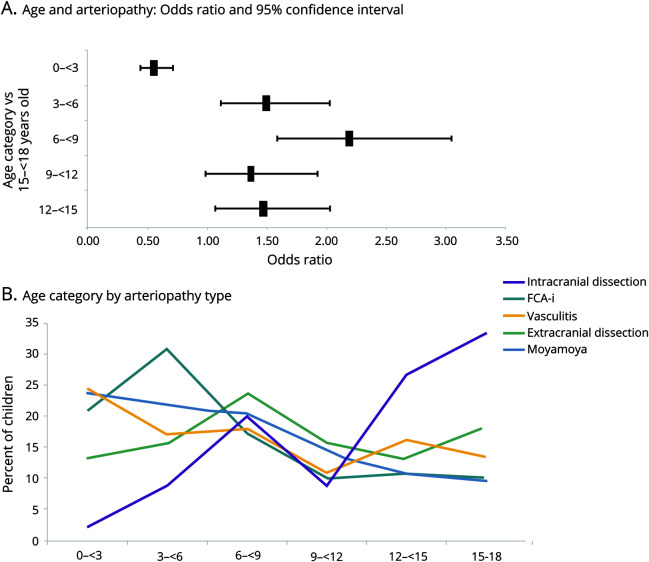
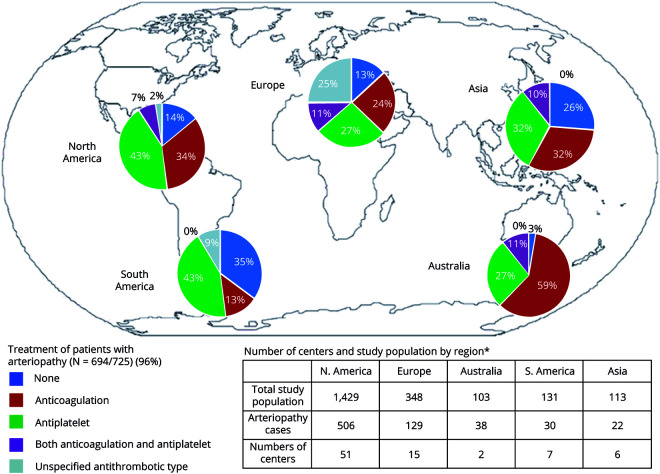
Children with arteriopathy-associated AIS were older compared to children with nonarteriopathic causes (median age 7.45 years [interquartile range 3.68–12.76] vs 4.6 years [interquartile range 1.11–12.18 years], p < 0.0001 [z = 7.05]), with the highest likelihood of AIS due to an arteriopathic cause occurring in 6- to 9-year-old children (p = 0.029, OR 1.93, 95% confidence interval [CI] 1.1–3.5) (figure 2A). There were equal proportions of boys (58% [423 of 725] arteriopathic AIS vs 57% [806 of 1,402] nonarteriopathic AIS) and whites (65% [321 of 725] arteriopathic AIS vs 65% [648 of 993] nonarteriopathic AIS) in each group (table 1). Geographic differences were observed, with Asia (19%) and South America (23%) having smaller proportions of arteriopathic AIS compared to other continents (35%–37%, p < 0.0003 [χ2 = 21, df = 4]) (figure 3). However, these geographic differences were not significant on multivariable analysis.
Figure 2. Association of age.
(A) Association of age and arteriopathy in children with arterial ischemic stroke (AIS). (B) Age distribution in children with AIS by arteriopathy type. FCA-i = focal cerebral arteriopathy–inflammatory subtype.
Figure 3. Distribution of arteriopathy and first reported treatment of arteriopathy by geographic region.
Children with arteriopathy associated AIS were more likely to present with headache (p = 0.023, OR 1.55, 95% CI 1.0–2.2) and multiple infarctions (p < 0.0001, OR 2.05, 95% CI 1.4–2.9) than those with nonarteriopathic AIS. Sickle cell anemia (p = 0.007, OR 2.9, 95% CI 1.3–6.3) and head or neck trauma (p = 0.018, OR 1.93, 95% CI 1.1–3.3) were risk factors for AIS associated with arteriopathy (table 3).
Children with arteriopathy-associated AIS were more likely to be treated with antithrombotic treatment (86% [595 of 694] arteriopathic vs 74% [985 of 1,331] nonarteriopathic AIS, p = 0.018, OR 1.87). Although not statistically significant, an increase in the use of antithrombotic treatment over time was noted (73% in 2003–2006, 78% in 2007–2010, and 83% in 2011–2014, p = 0.082) (table 3). We observed significant differences in treatment patterns between geographic regions/continents in arteriopathic AIS (p = 0.006 [χ2 = 14, df = 4]). The proportion of cases of AIS with arteriopathy receiving antithrombotic treatment was highest in Australia (97%), Europe (87%), and North America (86%) compared to Asia (74%) and South America (65%) (figure 3).
Discharge outcome was available in 88% and longitudinal outcomes were available in 37% of cases (mean duration 2.4 years, range 3 months–12.1 years). Children with arteriopathy-associated AIS had a higher likelihood of deficits at discharge (82% [538 of 659] arteriopathic vs 75% [909 of 1,205] nonarteriopathic AIS, p = 0.002). Longitudinal outcomes, measured by the PSOM, were not different between the 2 groups both at 1 year (52% [63 of 121] arteriopathic vs 51% [109 of 214] nonarteriopathic AIS) and at last measured PSOM (57% [159 of 279] vs 52% [266 of 510]). Overall ischemic stroke recurrence risk was 12% (226 of 1,819), with significantly higher risk in arteriopathic compared to nonarteriopathic AIS (21% [131 of 614] arteriopathic vs 8% [95 of 1,205] nonarteriopathic AIS, p = 0.0004, OR 2.37, 95% CI 1.5–3.8) (table 3).
AIS characteristics by arteriopathy subtype
Each arteriopathy subtype demonstrated interesting differences compared to other arteriopathies (figure 2B and tables e-1 and e-2, doi.org/10.5061/dryad.7d7wm37r3). Cases with intracranial dissection were typically >15 years of age (27%, p = 0.035, OR 2.3, 95% CI 1.1–5.1), while extracranial dissection was associated with male sex (80%, p = 0.001, OR 3.17, 95% CI 1.6–6.4). With age as a continuous variable, a per-unit increase in age was associated with an increased risk for intracranial dissection (p < 0.0001, OR 1.18), FCA-i (p = 0.015, OR 0.95), or moyamoya (p = 0.026, OR 0.96). Black race was common with moyamoya (24% vs 3%–11%, p = 0.002, OR 3.81, 95% CI 1.6–8.8) and white race with FCA-i (81% vs 49%–77%, p = 0.009, OR 2.79, 95% CI, 1.3–6.0).
In univariate analysis, statistically significant variations in geographic distribution of arteriopathy subtypes were observed. Post hoc analysis looking at proportion of subtypes within each region was conducted to account for the variability in the number of sites enrolling in IPSS per region (of 86 sites: South America 9 [10%], North America 54 [63%], Europe 15 [17%], Australia 2 [2%], and Asia 6 [7%]). Compared to other subtypes, FCA-i diagnosis was highest in Europe (37 of 82 [45%], p < 0.0001 [χ2 = 47, df = 4]) and Australia (16 of 35 [45%], p = 0.001 [χ2 = 18, df = 4]), and moyamoya diagnosis was highest in North America (138 of 364 [38%], p < 0.0001 [χ2 = 45, df = 4]). No significant differences in the proportion of subtypes were observed in South America and Asia (table e-1, doi.org/10.5061/dryad.7d7wm37r3).
In a comparison of arteriopathy subtypes (table e-2, doi.org/10.5061/dryad.7d7wm37r3), clinical presentations that were more prevalent with a specific arteriopathy subtype included head or neck trauma with both intracranial (38%, p < 0.0003, OR 4.19, 95% CI 1.9–9.0) and extracranial (37%, p < 0.0001, OR 5.02, 95% CI 2.5–10.3) dissection; headache (78%, p = 0.0004, OR 4.20, 95% CI 1.9–9.2) with intracranial dissection; posterior circulation involvement (52%, p < 0.0001, OR 3.65, 95% CI 1.9–6.7) with extracranial dissection; seizures (31%, p = 0.004, OR 2.62, 95% CI 1.3–5.1), bilateral infarction (37%, p < 0.0004, OR 3.97, 95% CI 1.9–8.4), and anterior circulation involvement (80%, p < 0.0007, OR 3.5, 95% CI 1.7–7.2) with moyamoya; unilateral hemiparesis (88%, p = 0.02, OR 2.95, 95% CI 1.1–7.5) and single infarcts (76%, p < 0.0001, OR 5.31, 95% CI 2.6–10.8) with FCA-i; and bilateral infarction (36%, p = 0.03, OR 1.71, 95% CI 1.0–2.8) and acute systemic findings (37%, p < 0.0001, OR 3.58, 95% CI 2.2–5.9) with vasculitis.
A higher proportion of cases with dissection were treated with an anticoagulation (62% extracranial [p = 0.0008, OR 2.79, 95% CI 1.5–5.1] and 53% intracranial dissection [p = 0.02 significant only in univariate analysis] vs 15%–39% other arteriopathy subtypes), whereas a higher proportion of cases with moyamoya received antiplatelet treatment (55% moyamoya [p = 0.001, OR 2.80, 95% CI 1.1–4.1] vs 29%–37% other arteriopathy subtypes).
Both discharge and long-term outcomes were not statistically different among the arteriopathy subtypes, except that moyamoya arteriopathy was associated with the highest odds of having recurrent ischemic strokes (33%, p = 0.01, OR 2.17, 95% CI 1.1–4.1). In cases with moyamoya, stroke recurrence may have been affected by the timing of revascularization surgery. However, dates of surgery were inconsistently available, and this information could not be presented.
Discussion
We present the largest series of childhood AIS patients with and without arteriopathy, comparing their presentation, risk factors, treatments, and outcome. The proportion of children with arteriopathic AIS in our sample, both total and across geographic regions, is largely similar to what has previously been reported.1,19 Lower frequencies of arteriopathic AIS in Asia and South America may have been due to underdiagnosis because MRI and other vascular imaging techniques are less available in these regions, although this finding might be an artifact of limited sites participating in the IPSS and needs further evaluation. Higher prevalences of young children with arteriopathy and male sex in childhood AIS have been previously reported1,20 and are confirmed by this study. Recently, VIPS investigators reanalyzed their 355 cases with AIS and found 127 (36%) children with a radiologically confirmed arteriopathy. In their cohort, moyamoya disease more frequently affected children <8 years of age, FCA-i affected children between 8 and 15 years of age, and dissection affected all ages.19 In the current IPSS cohort of children (including VIPS cases), cases with FCA-i were more likely to present between 3 and 6 years of age and cases with dissection were more common at >12 years of age, differing somewhat from the smaller VIPS dataset. In total, the bimodal distribution seen in all arteriopathies may be secondary to an increased prevalence of FCA-i and moyamoya among younger children and intracranial dissection among older children. These findings may be particularly important in future outcome studies because recovery from stroke is likely modified by age due to the location-specific vulnerability and plasticity in the developing brain.21,22
Many previously described clinical features and observations are confirmed by the current IPSS dataset, including the frequent occurrence of deficits at discharge, stroke recurrence, and multiple ischemic infarctions in children with arteriopathy.9,10 The last is likely accounted for by arteriopathic involvement of multiple arteries or arterial segments and recurrent artery-to-artery thromboembolism. In addition, in children with sickle cell anemia, the sickle-associated arteriopathy combines with an increased baseline recurrent stroke risk from sickle cell anemia. Other study findings included frequent involvement of anterior circulation with moyamoya arteriopathy23 and posterior circulation with extracranial dissection.24,25 Furthermore, the finding of a high proportion of cases with seizures and bilateral infarction in children with moyamoya is also not entirely surprising because moyamoya arteriopathy involves bilateral arterial territories and causes varying ages of ischemic strokes as the disease slowly progresses.23,26 On the same note, the presence of single infarcts with resultant hemiparesis in cases with FCA-i and bilateral infarction and acute systemic findings in cases with vasculitis can be explained by their etiopathologic involvement (typical unilateral focal arterial involvement in cases with FCA-i and bilateral diffuse arterial involvement in association with infectious or inflammatory conditions in cases with vasculitis).
Considering the large multinational IPSS dataset, the findings of the current study are generalizable to and highly representative of the childhood AIS population. While male sex and black race have previously been reported as risk factors for dissection and childhood AIS in general,20,27 to the best of our knowledge, the very high percentage of male patients with extracranial dissection (80%) in our study is the highest reported to date. This finding may be secondary to increased head trauma in male patients,27 although biological sex differences may also have contributed. A study comparing cases of pediatric ischemic stroke to controls has demonstrated a 1.3-fold increase in the risk for cerebral thromboembolism with each 1-nmol/L increase in testosterone in boys.28 Furthermore, recent descriptions of V3 vertebral dissections in an exclusively male cohort29 also suggest increased susceptibility to extracranial dissections among male patients. The proportion of cases of FCA in our cohort is smaller than reported previously (15% vs up to 25%)2 and may be due to a lack of vascular imaging in all our patients. Geographic differences noted in this study are also interesting. Moyamoya diagnosis had the highest proportion in North America, whereas FCA-i diagnosis had the highest proportion in Europe and Australia. These findings may be related to the association between moyamoya and sickle cell anemia (p < 0.0001) and between sickle cell anemia and black race (p < 0.0001) in conjunction with the higher number of black children in North America (of 371 with AIS with race and region data, 45 of 48 [94%] of black children were in North America). The association of moyamoya and black race does not hold while controlling for the presence of sickle cell anemia. Of 25 patients with sickle cell anemia AIS with race data, 5 of 5 (100%) with vasculitis and 18 of 20 (90%) with moyamoya were black children (Fisher exact test p = 0.99).
The association of headache with arteriopathic childhood AIS is a clinically relevant finding in the current cohort.30 While headache in childhood stroke has been reported, particularly with craniocervical arterial dissection,24,30 this study demonstrates that the association is broad (across arteriopathic stroke types) and robust, with very high rates of headache in both extracranial (63%) and intracranial (78%) dissections. On the basis of these data, it is important for clinicians to consider and exclude dissection early in children with AIS who present with significant headache.30
The high rate of antithrombotic use acutely in children with arteriopathic AIS noted in the current cohort is similar to the previously reported increased use of antithrombotic treatment over time.10,31 This finding is largely reflective of current clinical practice and is likely driven by the increased awareness of high recurrence risk in cases with arteriopathy. The high rate of anticoagulation use acutely in children with extracranial dissection is reflective of the pediatric stroke management guidelines in use during the study period.16,30 The high rate of anticoagulation use in intracranial dissection (53%) is surprising, however, and may be based on a few studies that suggested that anticoagulation is safe in cases of childhood arteriopathy.32 Of note, despite published management guidelines for childhood stroke, ≈20% of cases of AIS received no acute antithrombotic treatment. It is unclear from these data whether this finding is due to a lack of adherence to published guidelines or, more likely, the withholding of antithrombotic therapy in circumstances when therapy may not be clearly indicated such as brain tumor, several intracranial infections, or sickle cell disease–associated strokes. This indicates an area in which further research, education, and outreach are required.
Despite the significant association between arteriopathy and risk for recurrent strokes, we found no statistically significant difference in short- and long-term outcomes between arteriopathic and nonarteriopathic AIS. The large amount of missing outcome data and the lack of longer follow-up duration to see the impact of stroke recurrence likely contributed to these nonsignificant results.
Limitations of this study include those typically associated with large cohort studies. Data may have been overreported or underreported. This finding is particularly germane to our follow-up results in that patients with a higher degree of medical impairment are more likely to seek medical care and may be overrepresented in follow-up data. These study participants were enrolled at academic centers, likely leading to referral bias; for example, AIS due to cardiac disease may be overrepresented due to the concentration of children with congenital heart disease at those centers.
In this study, few variables had significant amounts of missing data; namely, race was missing in 63% and PSOM data were missing in 37%. However, for most variables as listed in tables 1 and 2, the percentage of missing data in the current dataset is within the acceptable ranges for a large dataset (median 4.8%, mean 7.9%, range 0%–24.8%). While the large patient volume is strength of our cohort, all radiographic data are based on available radiology reports and the site investigator's interpretation, without centralized confirmation. Because the interrater reliability is only modest among trained raters,11 imprecise classification of the AIS etiology and arteriopathy subtypes may have confounded our results. Furthermore, although the numbers are small, dedicated vascular imaging was not performed in all noncardiac cases of AIS. Other problems with validity and reliability may exist in these data.
Children with arteriopathic stroke have differentiating features compared to those without arteriopathic stroke and between each arteriopathy subtype. These observations may be helpful in guiding clinicians in managing arteriopathic cases of AIS by choosing appropriate investigations (e.g., head and neck vascular imaging) and treatment strategies. The evaluation of arteriopathy subtypes in our analysis may be particularly useful because initial radiographic diagnosis of arteriopathy does not always accurately determine the final classification; indeed, almost one-third of arteriopathies are challenging to classify. Among the 355 VIPS cases with AIS, 127 (35.7%) had definite arteriopathy and 34 (9.5%) had possible arteriopathy. Of those with definite arteriopathy, only 109 (30.7%) cases could be classified with high certainty into an arteriopathy subtype.2 Another example, intracranial dissection, may be diagnosed on conventional angiogram performed outside of the acute phase of stroke, and 6% of patients with initial diagnosis of FCA progress to the diagnosis of moyamoya.8 Therefore, these observations may assist clinicians in the earlier diagnoses of specific arteriopathy subtypes and appropriate treatment strategies. The results will also serve to define patient subgroups who are at higher risk for adverse outcomes and are likely to benefit most from future therapeutic trials. Specifically, these findings may assist in identifying pediatric patients for inclusion in steroid trials in the FCA-i population, studies evaluating the efficacy of antithrombotic management in extracranial dissection (in particular, utility of anticoagulant therapy because pediatric patients reportedly have higher rates for recurrent stroke compared to adult patients),25 and trials examining the role of long-term transfusion in sickle cell disease.
Acknowledgment
The authors are extremely thankful to all the children with AIS and their families who participated in the International Pediatric Stroke Study and feel privileged to be able to provide medical care to them.
Glossary
- AIS
arterial ischemic stroke
- CI
confidence interval
- FCA
focal cerebral arteriopathy
- FCA-i
focal cerebral arteriopathy, inflammatory type
- IPSS
International Pediatric Stroke Study
- OR
odds ratio
- PSOM
Pediatric Stroke Outcome Measure
- VIPS
Vascular Effects of Infection in Pediatric Stroke
Appendix 1. Authors
Appendix 2. coinvestigators

Study funding
Mubeen F. Rafay is supported by a research operating grant from the Children Hospital Research Institute of Manitoba. Dr. Timothy J. Bernard is supported by the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services (HHS) under grant 2H30MC24049, Mountain States Hemophilia Network. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by, HRSA, HHS, or the US government. Dr. Gabrielle deVeber and Dr. Heather J. Fullerton are supported by NIH R01 NS062820. Dr. Bernhard Weschke has received funding from the European Community's Seventh Framework Program (FP7/2007-2013) under grant 602391 (epistop.eu). The IPSS is graciously supported by The Auxilium Foundation.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Amlie-Lefond C, Bernard TJ, Sebire G, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation 2009;119:1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wintermark M, Hills NK, deVeber GA, et al. Arteriopathy diagnosis in childhood arterial ischemic stroke: results of the Vascular Effects of Infection in Pediatric Stroke Study. Stroke 2014;45:3597–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chabrier S, Rodesch G, Lasjaunias P, Tardieu M, Landrieu P, Sebire G. Transient cerebral arteriopathy: a disorder recognized by serial angiograms in children with stroke. J Child Neurol 1998;13:27–32. [DOI] [PubMed] [Google Scholar]
- 4.Fullerton HJ, Stence N, Hills NK, et al. Focal cerebral arteriopathy of childhood. Stroke 2018;49:2590–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sebire G, Fullerton H, Riou E, DeVeber G. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr 2004;16:617–622. [DOI] [PubMed] [Google Scholar]
- 6.Sebire G, Meyer L, Chabrier S. Varicella as a risk factor for cerebral infarction in childhood: a case-control study. Ann Neurol 1999;45:679–680. [DOI] [PubMed] [Google Scholar]
- 7.Dlamini N, Freeman JL, Mackay MT, et al. Intracranial dissection mimicking transient cerebral arteriopathy in childhood arterial ischemic stroke. J Child Neurol 2011;26:1203–1206. [DOI] [PubMed] [Google Scholar]
- 8.Braun KP, Bulder MM, Chabrier S, et al. The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain 2009;132:544–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fullerton HJ, Wintermark M, Hills NK, et al. Risk of recurrent arterial ischemic stroke in childhood: a prospective international study. Stroke 2016;47:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldenberg NA, Bernard TJ, Fullerton HJ, Gordon A, DeVeber G. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol 2009;8:1120–1127. [DOI] [PubMed] [Google Scholar]
- 11.Bernard TJ, Beslow LA, Manco-Johnson MJ, et al. Inter-rater reliability of the CASCADE criteria: challenges in classifying arteriopathies. Stroke 2016;47:2443–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun KP, Rafay MF, Uiterwaal CS, Pontigon AM, DeVeber G. Mode of onset predicts etiological diagnosis of arterial ischemic stroke in children. Stroke 2007;38:298–302. [DOI] [PubMed] [Google Scholar]
- 13.Database for Stroke in Infants and Children. 18 A.D. Available at: clinicaltrials.gov/ct2/show/NCT00084292?cond=pediatric+stroke&rank=5. Accessed May 16, 2020. [Google Scholar]
- 14.Bernard TJ, Manco-Johnson MJ, Lo W, et al. Towards a consensus-based classification of childhood arterial ischemic stroke. Stroke 2012;43:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebire G. Transient cerebral arteriopathy in childhood. Lancet 2006;368:8–10. [DOI] [PubMed] [Google Scholar]
- 16.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke 2008;39:2644–2691. [DOI] [PubMed] [Google Scholar]
- 17.Kitchen L, Westmacott R, Friefeld S, et al. The Pediatric Stroke Outcome Measure: a validation and reliability study. Stroke 2012;43:1602–1608. [DOI] [PubMed] [Google Scholar]
- 18.Lo WD, Ichord RN, Dowling MM, et al. The Pediatric Stroke Recurrence and Recovery questionnaire: validation in a prospective cohort. Neurology 2012;79:864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wintermark M, Hills NK, deVeber GA, et al. Clinical and imaging characteristics of arteriopathy subtypes in children with arterial ischemic stroke: results of the VIPS study. AJNR Am J Neuroradiol 2017;38:2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golomb MR, Fullerton HJ, Nowak-Gottl U, DeVeber G. Male predominance in childhood ischemic stroke: findings from the International Pediatric Stroke Study. Stroke 2009;40:52–57. [DOI] [PubMed] [Google Scholar]
- 21.Allman C, Scott RB. Neuropsychological sequelae following pediatric stroke: a nonlinear model of age at lesion effects. Child Neuropsychol 2013;19:97–107. [DOI] [PubMed] [Google Scholar]
- 22.Westmacott R, Askalan R, MacGregor D, Anderson P, DeVeber G. Cognitive outcome following unilateral arterial ischaemic stroke in childhood: effects of age at stroke and lesion location. Dev Med Child Neurol 2010;52:386–393. [DOI] [PubMed] [Google Scholar]
- 23.Rafay MF, Armstrong D, Dirks P, MacGregor DL, DeVeber G. Patterns of cerebral ischemia in children with moyamoya. Pediatr Neurol 2015;52:65–72. [DOI] [PubMed] [Google Scholar]
- 24.Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology 2001;57:1155–1160. [DOI] [PubMed] [Google Scholar]
- 25.Rafay MF, Armstrong D, DeVeber G, Domi T, Chan A, MacGregor DL. Craniocervical arterial dissection in children: clinical and radiographic presentation and outcome. J Child Neurol 2006;21:8–16. [DOI] [PubMed] [Google Scholar]
- 26.Smith ER, Scott RM. Moyamoya: epidemiology, presentation, and diagnosis. Neurosurg Clin N Am 2010;21:543–551. [DOI] [PubMed] [Google Scholar]
- 27.Hills NK, Johnston SC, Sidney S, Zielinski BA, Fullerton HJ. Recent trauma and acute infection as risk factors for childhood arterial ischemic stroke. Ann Neurol 2012;72:850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Normann S, de Veber G, Fobker M, et al. Role of endogenous testosterone concentration in pediatric stroke. Ann Neurol 2009;66:754–758. [DOI] [PubMed] [Google Scholar]
- 29.Rollins N, Braga B, Hogge A, Beavers S, Dowling M. Dynamic arterial compression in pediatric vertebral arterial dissection. Stroke 2017;48:1070–1073. [DOI] [PubMed] [Google Scholar]
- 30.Billinghurst L, Hills N, Jastrzab L, et al. Headache presentation in childhood arterial ischemic stroke differs by arteriopathy subtype. Stroke 2017;48:A174. [Google Scholar]
- 31.DeVeber G, Kirton A, Booth FA, et al. Epidemiology and outcomes of arterial ischemic stroke in children: the Canadian Pediatric Ischemic Stroke Registry. Ann Neurol 2017;69:58–70. [DOI] [PubMed] [Google Scholar]
- 32.Bernard TJ, Goldenberg NA, Tripputi M, Manco-Johnson MJ, Niederstadt T, Nowak-Gottl U. Anticoagulation in childhood-onset arterial ischemic stroke with non-moyamoya arteriopathy: findings from the Colorado and German (COAG) Collaboration. Stroke 2009;40:2869–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used for analysis are presented in the tables and figures in this article. Data will be shared after ethics approval if requested by other investigators for purposes of replicating the results.