Abstract
Objective
To develop a disease-specific staging system for CLN3 disease and to test the hypothesis that salient and discrete clinical features of CLN3 disease may be used to define disease stages by analyzed data from an 18-year-long natural history study.
Methods
A proposed staging system, the CLN3 Staging System (CLN3SS), was based on salient and clinically meaningful endpoints. The relationships between stage and age, stage and Unified Batten Disease Rating Scale (UBDRS) physical severity score, and stage and UBDRS capability impairment subscale scores were determined. We used t tests to determine whether the stages were significantly different from each other on the basis of age and scores.
Results
Data were analyzed from 322 evaluations in 108 individuals. There were significant differences among the stages based on age and severity scores. For individuals with longitudinal data, no individual reverted to a less severe stage over time.
Conclusion
The CLN3SS is a disease-specific staging system that can be used to classify individuals into specific strata based on age and disease severity. The CLN3SS has potential applications in clinical trials for cohort stratification.
The neuronal ceroid lipofuscinoses (NCLs; Batten disease) are disorders characterized by neurodegeneration and intracellular autofluorescent lipopigment accumulation.1 The most prevalent form in North America, CLN3 disease (juvenile NCL), has typical onset between 4 and 7 years of age. CLN3 disease evolves in a characteristic and predictable manner with a linear relationship between disease severity and age.2 Vision loss is the most common first symptom, followed by changes in cognition and behavior, onset of seizures, and progressive motor decline with loss of ambulation.3–5 Many individuals develop cardiac arrhythmias and feeding difficulties late in the disease course.3,6 CLN3 disease progresses relatively slowly with ≥20 years between symptom onset and death.4,5 We sought to develop a staging system for CLN3 disease to group patients into discrete categories for potential stratification in clinical trials and to define more homogeneous cohorts. With the recent development of potential disease-modifying therapy7 (e.g., NCT03770572), tools to aid in clinical trial design are increasingly important.
Methods
Individuals with genetically confirmed CLN3 disease were recruited at the University of Rochester Batten Center or the annual Batten Disease Support and Research Association family meeting for an ongoing longitudinal natural history study (NCT01873924).
Demographic and symptom severity data were obtained with the Unified Batten Disease Rating Scale (UBDRS), a global disease severity scale developed to measure disease progression in the NCLs.2,3 The UBDRS consists of 4 quantitative subscales with higher numbers reflecting greater severity: physical assessment (score 0–112), seizure assessment (score 0–54), behavior assessment (score 0–55), and capability assessment (score 0–14). The UBDRS also contains an NCL history section to record approximate age at onset of core clinical features of NCLs from caregiver interview. The UBDRS has been shown to be a valid and reliable tool for quantifying disease severity and progression in CLN3 disease.2,3,8
Data were obtained from 123 individuals with genetically confirmed CLN3 disease in a total of 380 evaluations. Individuals were excluded from analysis if there was onset of seizures or if there were developmental concerns before age 4 years not related to CLN3 disease (2 individuals, 3 evaluations) or clear outlier status based on preservation of independent function up to age 30 years (1 individual, 6 evaluations). Evaluations were excluded if there were incomplete UBDRS data (49 evaluations). For individuals with longitudinal data, data from each evaluation were included.
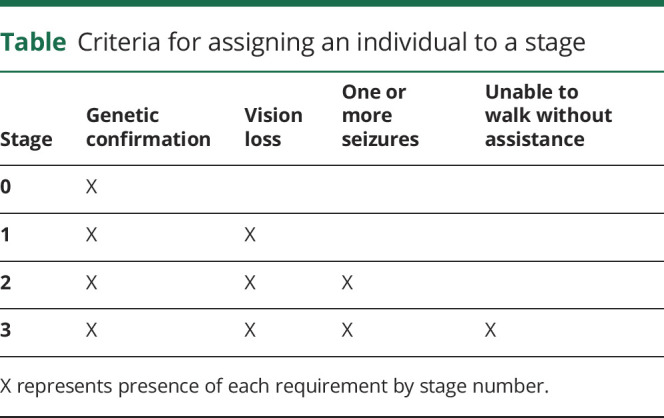
The CLN3 Staging System (CLN3SS) was created using items from the UBDRS that met the following criteria: (1) discrete threshold between presence and absence of a disease characteristic, (2) clinically meaningful transition point, (3) scored objectively, and (4) features unlikely to resolve with treatment. Four stages (0–3) were included in the CLN3SS and were based on genetic confirmation, vision loss, presence of seizures, and loss of independent walking (table).
Table.
Criteria for assigning an individual to a stage
Statistical analyses were done with JMP Pro 14. Because previous work demonstrated a slight but significant sex difference in age at symptom onset,5 a 2-way analysis of variance was performed to determine whether there was a significant sex difference for the relationship between stage and participant age. Because there was no significant difference, data were combined across sexes for all further analyses.
Between-group comparisons of the ages at each stage were performed with t tests with Bonferroni correction for multiple comparisons. In addition, between-group comparisons of the UBDRS Physical subscale vs stage and UBDRS Capability Assessment subscale vs stage were performed with t tests.
Standard protocol approvals, registrations, and patient consents
Research was performed under a protocol approved by the University of Rochester Research Subjects Review Board (RSRB 20390). Parents or legal guardians provided written permission for their child's participation.
Data availability
The University of Rochester Batten Center maintains all data in a secure database. Deidentified data from this study are available on request to qualified investigators.
Results
Staging was performed on 322 evaluations from 108 individuals. Fifty-one individuals had a single evaluation. The remaining 57 individuals had multiple evaluations ranging from 2 to 15 per individual. The youngest participant was age 4.75 years; the oldest was 29.67 years. Only 1 individual met criteria for stage 0; statistical analyses were limited to comparisons among evaluations at stages 1 (n = 63), 2 (n = 160), and 3 (n = 99).
The mean age at any stage differed significantly from the other stages. The average age at stage 1 was 9.9 ± 2.9 (mean ± SD) years, at stage 2 was 14.2 ± 3.6 years, and at stage 3 was 19.5 ± 4.0 years (figure 1A). Before age 9.5 years, the majority of individuals were in stage 1; between ages 9.5 and 17.75 years, the majority of individuals were in stage 2; and after 17.75 years, the majority of individuals were in stage 3 (figure 1B). Individuals evaluated serially progressed from 1 stage to the next in a unidirectional manner. No individual developed CLN3 disease–related seizures before vision loss, and no individual lost independent walking before the first seizure.
Figure 1. Relationship between age and CLN3 Staging System stage.
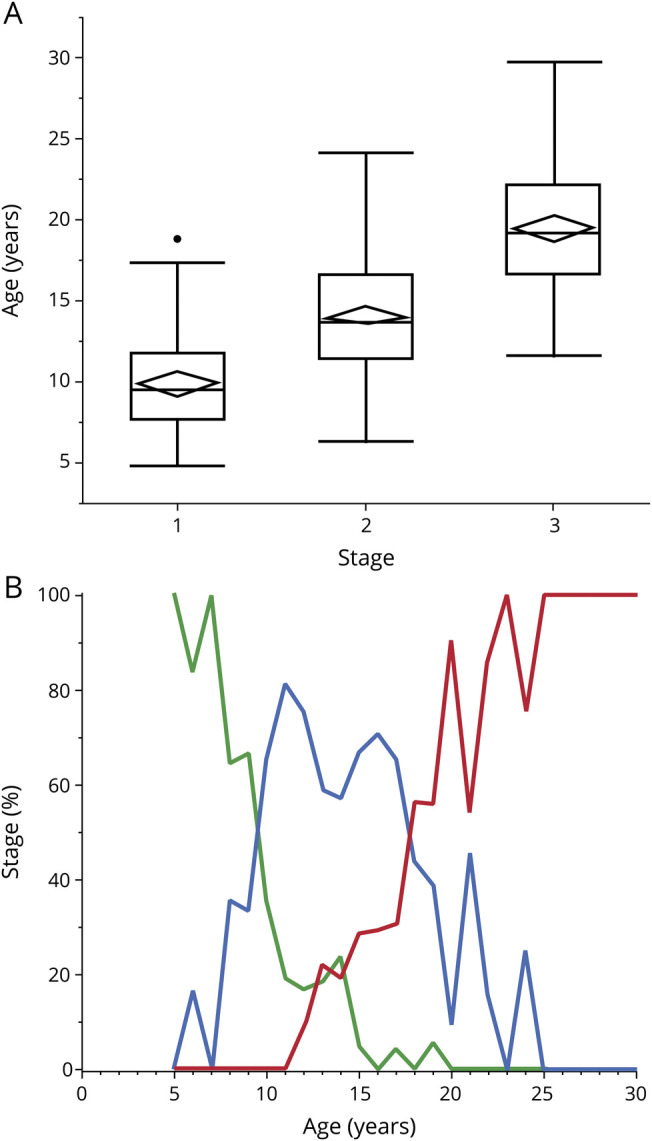
(A) Distributions of participant ages for each of the stages. Mean age and 95% confidence intervals are represented by diamonds; median, quartiles, and range are represented by box-and-whisker plots. Individual points represent outliers, which are >1.5 times the interquartile range above the third quartile or below the first. t Tests showed significant differences between stages (stage 1 vs 2: t = 9.4, p < 0.0001; stage 2 vs 3: t = 20.2, p < 0.0001; stage 1 vs 3: t = 14.6, p < 0.0001). (B) Percent of affected individuals in each stage vs participant age. Green represents stage 1, blue represents stage 2, and red represents stage 3.
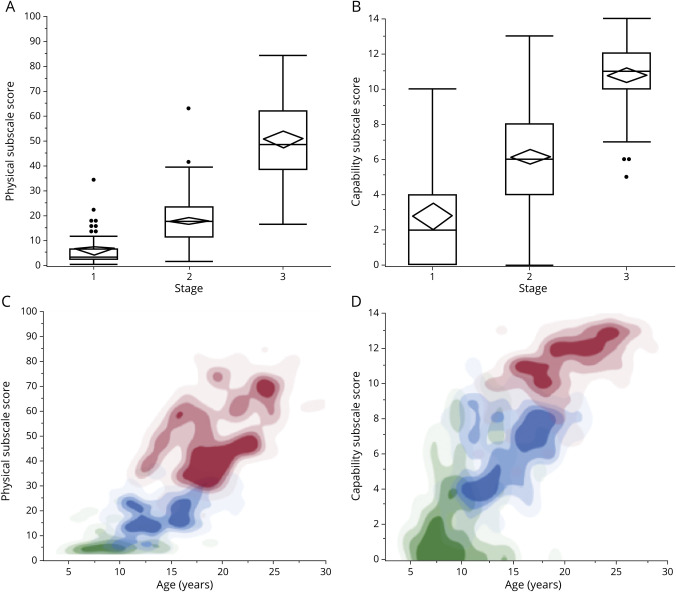
Stage predicted disease severity as measured by the UBDRS Physical subscale and the UBDRS Capability subscale. The Physical subscale score was 5.3 ± 5.8 (mean ± SD) at stage 1, 17.5 ± 9.6 at stage 2, and 50.5 ± 15.9 at stage 3 (figure 2A). The Capability subscale score was 2.7 ± 2.9 (mean ± SD) at stage 1, 6.1 ± 2.4 at stage 2, and 10.6 ± 2.1 at stage 3 (figure 2B). For both subscales, age-related severity differed among stages (figure 2, C and D).
Figure 2. Relationships among UBDRS subscale severity scores, CLN3 Staging System stages, and participant age.
Distributions of Unified Batten Disease Rating Scale (UBDRS) (A) Physical and (B) capability subscale scores for each of the stages. Mean severity scores and 95% confidence intervals are represented by diamonds; median, quartiles, and range are represented by box-and-whisker plots. Individual points represent outliers, which are >1.5 times the interquartile range above the third quartile or below the first. (A) t Tests showed significant differences between stages (stage 1 vs 2: t = 7.1, p < 0.0001; stage 2 vs 3: t = 24.4, p < 0.0001; stage 1 vs 3: t = 22.7, p < 0.0001). (B) t Tests showed significant differences between stages (stage 1 vs 2: t = 9.4, p < 0.0001; stage 2 vs 3: t = 20.2, p < 0.0001; stage 1 vs 3: t = 14.6, p < 0.0001). Data density contour plots, by stage, of relationship between age and severity for UBDRS (C) Physical and (D) Capability subscale scores. Stage 1 data are represented in green; stage 2, in blue; and stage 3, in red. Data density is represented by color density.
Discussion
We developed the CLN3SS, a disease-specific staging system for CLN3 disease, based on clinically meaningful features. The CLN3SS separates affected individuals into 4 distinct stages. These stages are based on core features of CLN3 disease that occur serially over the course of disease progression. The core features of vision loss, seizure onset, and loss of independent ambulation are recognized by parents as central causes of disability in affected individuals (Augustine et al., personal communication, 2019). Assignment to a CLN3SS stage can be done easily with low burden to the examiner and the affected individual. This staging requires minimal training on the part of the rater and for screening purposes can be done without physical examination.
In addition to the significant relationship between stage and age, we found significant relationships between stage and physical symptom severity and between stage and impaired functional capability. These results suggest that the stages can be used to stratify patients into major subgroups. According to our relatively large dataset, all affected individuals progressed from a lower to higher stage in a unidirectional manner. Thus, small fluctuations in symptom severity or response to currently available medical therapies do not affect the staging.
Disease staging has been used to stratify patients for treatment or to predict outcomes in other diseases.9,10 We hypothesize that the CLN3SS can be used similarly in CLN3 disease. Furthermore, it may be useful to stratify participants in future clinical trials to stage-specific outcomes or interventions. Although the CLN3SS has not been evaluated in a clinical trial setting, it can be applied retrospectively and thus could be used post hoc to evaluate response to treatment in ongoing clinical trials.
A limitation of the CLN3SS is low sensitivity to change over short time periods. Thus, it is not likely to be a good endpoint for short-term clinical trials but could still be useful to stratify cohorts for treatment. We had insufficient data to properly evaluate stage 0, even though it has excellent face validity.
Disease staging has high potential utility for experimental therapeutics in rare diseases such as CLN3 disease that progress slowly over >2 decades of life. Future work is needed to confirm the validity and performance of the CLN3SS in a clinical trial setting. In addition, other stages may be justified in the future if data warrant.
Acknowledgment
The authors are grateful to the patients and their families for their participation in the research.
Glossary
- CLN3SS
CLN3 Staging System
- NCL
neuronal ceroid lipofuscinoses
- UBDRS
Unified Batten Disease Rating Scale
Appendix. Authors
Study funding
Funding was provided by NIH grants U54NS065768, R01NS060022, and U01NS101946 and funding from Our Promise to Nicholas Foundation, Batten Disease Support and Research Association, Noah's Hope-Hope 4 Bridget Foundation, Wilber Smith Pediatric Neurology Fund at the University of Rochester, and the University of Rochester Office for Medical Education.
Disclosure
M. Masten and J. Williams report no disclosures relevant to the manuscript. J. Vermilion receives research funding from an American Academy of Neurology Clinical Research Scholarship. H. Adams has served on scientific advisory boards for Taylor's Tale, Neurogene Inc, and Amicus Therapeutics and has served as a consultant to Neurogene Inc and the Beyond Batten Disease Foundation. She currently receives funding from research contracts with Abeona Therapeutics, Neurogene, Inc and from NIH grants U01NS101946, R01NS104010, R01HL098332, UM1HL098147, and U01DK066143. A. Vierhile receives funding from research contracts with Abeona Therapeutics and NIH grant U01NS101946. A. Collins and F. Marshall report no disclosures relevant to the manuscript. E. Augustine has served on scientific advisory panels for Taylor's Tale and BioMarin Pharmaceutical and has served as a consultant to Neurogene Inc, the Beyond Batten Disease Foundation, Regenxbio, and Signant Health. She currently receives funding from research contracts with Abeona Therapeutics, Our Promise to Nicholas Foundation, and the Batten Disease Support and Research Association and from NIH grants U01NS101946, U24TR002260, UL1TR002001, K12NS098482, U24NS107165, R01NS094292, and P50NS108676. J. Mink serves as associate editor of Neurology; has served on scientific advisory boards for Neurogene Inc and Amicus Therapeutics; has served on a data monitoring committee for Censa, Inc; and has served as a consultant for Abide Therapeutics, Censa, Inc, Amicus Therapeutics, Neurogene Inc, and the Beyond Batten Disease Foundation. He is funded by research contracts from Abeona, Inc, Our Promise to Nicholas Foundation, Noah's Hope-Hope 4 Bridget, and The Batten Disease Support and Research Association and by NIH grants U54NS065768, R01NS06002, R01EY030183, U24NS107165, and U01NS101946. Go to Neurology.org/N for full disclosures.
References
- 1.Schulz A, Kohlschütter A, Mink J, Simonati A, Williams R. NCL disease: clinical perspectives. Biochim Biophys Acta 2013;1832:1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon JM, Adams H, Rothberg PG, et al. Quantifying physical decline in juvenile neuronal ceroid lipofuscinosis (Batten disease). Neurology 2011;77:1801–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall FJ, de Blieck EA, Mink JW, et al. A clinical rating scale for Batten disease: reliable and relevant for clinical trials. Neurology 2005;65:275–279. [DOI] [PubMed] [Google Scholar]
- 4.Mink JW, Augustine EF, Adams HR, Marshall FJ, Kwon JM. Classification and natural history of the neuronal ceroid lipofuscinoses. J Child Neurol 2013;28:1101–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cialone J, Adams H, Augustine EF, et al. Females experience a more severe disease course in Batten disease. J Inherit Metab Dis 2012;35:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostergaard JR, Rasmussen TB, Molgaard H. Cardiac involvement in juvenile neuronal ceroid lipofuscinosis (Batten disease). Neurology 2011;76:1245–1251. [DOI] [PubMed] [Google Scholar]
- 7.Augustine EF, Beck CA, Adams HR, et al. Short-term administration of mycophenolate is well-tolerated in CLN3 disease (juvenile neuronal ceroid lipofuscinosis). JIMD Rep 2019;43:117–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Augustine EF, Adams HR, Beck CA, et al. Standardized assessment of seizures in patients with juvenile neuronal ceroid lipofuscinosis. Dev Med Child Neurol 2015;57:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Disease staging clinical and coded criteria, 2009. Available at: hcup-us.ahrq.gov/db/nation/nis/DiseaseStagingV5_26ClinicalandCodedCriteria.pdf. Accessed August 2, 2019.
- 10.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The University of Rochester Batten Center maintains all data in a secure database. Deidentified data from this study are available on request to qualified investigators.