Abstract
Objective
To measure exosomal and plasma levels of candidate blood biomarkers in veterans with history of mild traumatic brain injury (mTBI) and test their relationship with chronic symptoms.
Methods
Exosomal and plasma levels of neurofilament light (NfL) chain, tumor necrosis factor (TNF)–α, interleukin (IL)–6, IL-10, and vascular endothelial growth factor (VEGF) were measured using an ultrasensitive assay in a cohort of 195 veterans, enrolled in the Chronic Effects of Neurotrauma Consortium Longitudinal Study. We examined relationships between candidate biomarkers and symptoms of postconcussive syndrome (PCS), posttraumatic stress disorder (PTSD), and depression. Biomarker levels were compared among those with no traumatic brain injury (TBI) (controls), 1–2 mTBIs, and repetitive (3 or more) mTBIs.
Results
Elevated exosomal and plasma levels of NfL were associated with repetitive mTBIs and with chronic PCS, PTSD, and depression symptoms. Plasma TNF-α levels correlated with PCS and PTSD symptoms. The total number of mTBIs correlated with exosomal and plasma NfL levels and plasma IL-6. Increased number of years since the most recent TBI correlated with higher exosomal NfL and lower plasma IL-6 levels, while increased number of years since first TBI correlated with higher levels of exosomal and plasma NfL, as well as plasma TNF-α and VEGF.
Conclusion
Repetitive mTBIs are associated with elevated exosomal and plasma levels of NfL, even years following these injuries, with the greatest elevations in those with chronic PCS, PTSD, and depression symptoms. Our results suggest a possible neuroinflammatory and axonal disruptive basis for symptoms that persist years after mTBI, especially repetitive.
Sustaining a mild traumatic brain injury (mTBI) can be associated with persistent or worsening neurobehavioral dysfunction years after injury,1–3 and can include a constellation of cognitive, affective, and somatic complaints.1,3 Common traumatic brain injury (TBI) clinical sequelae and comorbidities, such as postconcussive syndrome (PCS), posttraumatic stress disorder (PTSD), and depression, share significant symptom overlap, and might be more severe among individuals who have sustained repetitive TBIs.4–7 However, the biologic mechanisms underlying this association remain largely unknown.
TBI is a complex, heterogeneous injury that pathologically involves neuroaxonal and vascular injury accompanied by robust inflammatory responses. In response to the initial mechanical tissue damage, injured cells release proteins and extracellular vesicles, including exosomes into the extracellular space.8–10 Exosomes have a lipid bilayer membrane, readily cross the blood–brain barrier, and can be isolated from the peripheral circulation, where their contents can be easily accessed and measured. Their cargo may provide easily accessed insights into privileged CNS tissue-specific pathologic processes.10 Candidate protein biomarkers of neuronal damage, inflammation, and vascular injury, such as neurofilament light (NfL) chain,11,12 cytokines,13,14 and vascular endothelial growth factor (VEGF),15,16 respectively, may help inform TBI severity and prognosis, shedding light on underlying pathogenesis of chronic TBI symptom complexes.
In this study, we examined relationships among peripherally circulating (exosomal and plasma) candidate TBI biomarkers (NfL, interleukin [IL]–6, IL-10, tumor necrosis factor [TNF]–α, and VEGF) and persistent neurobehavioral symptoms in a cohort of veterans with a remote history of mTBI.
Methods
Study design and population
Participants in this study were enrolled in the Chronic Effects of Neurotrauma Consortium (CENC) Multicenter Observational Study, an institutional review board (IRB)–approved, multisite longitudinal study of mTBI late effects among post-9/11 era veterans and service members (SM). The overarching study has been described previously.17 Briefly, the CENC study assesses SM and veterans for every possible concussive event (PCE) throughout the lifetime and assigns an mTBI diagnosis for each event based on the Department of Defense (DoD)–Veterans Affairs (VA) common definition of mTBI. The inclusion criteria were (1) a history of Operation Enduring Freedom (OEF)/Operation Iraqi Freedom (OIF)/Operation New Dawn (OND) deployment confirmed by VA or DoD records (defined as deployed to a region of conflict in support of OEF/OIF/OND while serving in the US military); (2) a history of combat exposure during any deployment, either OEF/OIF/OND or non-OEF/OIF/OND (defined as Deployment Risk and Resiliency Inventory–2 [DRRI-2] score >1 on any item); and (3) adult age >18 years. The exclusion criteria were (1) a history of moderate or severe TBI (defined as initial Glasgow Coma Scale score <13, coma duration >1/2 hour, posttraumatic amnesia duration >24 hours, or traumatic intracranial lesion on head CT); or (2) a history of major neurologic or psychiatric disorder such as stroke, spinal cord injury, or schizophrenia (major defined as resulting in a significant decrement in functional status or loss of independent living capacity). The DRRI-2-D >1 on any item criterion is to ensure that the TBI exposed and unexposed controls have a similar environmental history, that is, all participants will have experienced combat situations during their military career. In this study, the cross-sectional analysis utilized an initial snapshot dataset of baseline evaluations from veterans recruited from the 4 original sites at VA medical centers in Richmond, Virginia; Tampa, Florida; Houston, Texas; and San Antonio, Texas. All participants provided written informed consent. From this dataset, 195 participants were selected based on TBI status and availability of full clinical data and associated plasma in the CENC Biorepository.
Standard protocol approvals, registrations, and patient consents
All participants provided written informed consent to participate, and all sites received approval from and followed the ethical standards of their respective institutional review boards. Participants also provided written consent for blood to be drawn, stored in CENC biorepository, and analyzed.
Determination of TBI
Using a modification of the Ohio State University TBI Identification instrument, participants were screened for PCEs sustained during military deployments and across their entire lifetime, including childhood. Then, each PCE was investigated to determine whether it fulfilled criteria for an mTBI using the Virginia Commonwealth University Retrospective Concussion Diagnostic Interview (VCU rCDI).17 Using the structured interview data and the DoD–VA common definition of mTBI,18 each VCU rCDI provided a preliminary TBI diagnosis of mTBI with loss of consciousness (LOC) and posttraumatic amnesia (PTA), mTBI without LOC/PTA, or not mTBI. The preliminary TBI diagnosis was then reviewed and vetted against the unstructured free text portion of the interview and any available medical documents recorded in proximity to the event (i.e., first responder, emergency department, or in-theater documentation). This process was used by the site principal investigator to confirm or override each preliminary mTBI diagnosis, providing the final diagnosis. The TBI severity of each event was also evaluated and any event more severe than an mTBI was excluded from the study. If any uncertainty regarding the TBI diagnosis remained, the event was adjudicated by a central diagnosis committee consisting of national experts in TBI. Using these TBI determinations, participants were classified into various TBI groupings for these analyses as follows: (1) repetitive (3 or more) mTBI, (2) 1–2 mTBIs, and (3) control (no TBI history). We also compared participants with and without blast exposure, irrespective of TBI designation, as well as mTBI with at least 1 mTBI with LOC/PTA vs mTBI with alteration of consciousness (AOC) only and without LOC/PTA.
Assessment of PCS, PTSD, and depression symptoms
The Neurobehavioral Symptom Inventory (NSI) was used to assess PCS symptom severity. The NSI consists of a 22-item assessment with a 3/4 factor structure (somatic/sensory, affective, and cognitive) with higher total score indicating more severe symptoms.19,20 The PTSD Checklist Military Version (PCL-M) was used to assess the symptoms of PTSD, resulting in a score of 0–80, with higher scores indicating a greater symptom burden. The PCL-M is widely used in military and veteran populations and has high reliability and validity.21,22 Symptoms of depression were measured using the 9-item Patient Health Questionnaire Depression Scale (PHQ-9). The PHQ-9 is a self-administered tool, with higher scores indicating greater symptom severity.23 It consists of 9 criteria upon which the diagnosis of DSM-IV (and DSM-5) depressive disorders is based. The PHQ-9 is half the length of many other depression measures and has comparable sensitivity and specificity.23
Blood sampling and protein assays
Biomarker analysis was carried out under an exempt IRB-approved protocol. Biomarker levels in the plasma and exosomes extracted from plasma were measured.
Exosome isolation from human plasma
Exosomes were isolated from 0.5 mL of frozen human plasma containing EDTA by using ExoQuick (System Biosciences Inc., Mountainview, CA). After the plasma was thawed, thrombin was added to each sample and incubated at room temperature for 5–10 minutes. Then samples were centrifuged at 10,000 rpm for 5 minutes and the supernatant was transferred into a clean tube for exosome isolation. ExoQuick solution was added to thrombin-treated plasma samples. Resulting solutions were incubated for 30 minutes at 4°C, then centrifuged at 1,500 g for 30 minutes. The supernatant was aspirated after the centrifugation and the exosome pellet was resuspended in 500 µL phosphate-buffered saline. For the protein quantification, each tube received equal amounts of M-PER mammalian protein extraction reagent to lyse exosomes (Thermo Scientific, Inc., Rockford, IL), containing 3 times the suggested concentrations of protease and phosphatase inhibitors. These suspensions were used to measure biomarker concentrations.
Protein quantification
Exosomal and plasma levels of NfL, IL-6, TNF-α, IL-10, and VEGF were analyzed using a site-specific Simoa HD-1 analyzer (Quanterix, Lexington, MA), a paramagnetic bead-based enzyme-linked immunosorbent assay, according to the manufacturer's protocol. The reported coefficient of variation (intraplate and interplate) values were no higher than 20% for all analytes.
Statistical analysis
Descriptive statistics were calculated for all demographic and clinical variables. Comparisons of demographic and clinical characteristics between groups were conducted using χ2 test or Mann-Whitney U tests. Biomarker comparisons among TBI groups were performed by using logistic regression models, as well as Mann-Whitney test or Kruskal-Wallis test followed by Dunn test correcting for repetitive pairwise comparisons. Spearman correlations were used to examine relationships between biomarkers and PCS, PTSD, and depression symptoms. Generalized linear models (GLMs) including demographics (age and sex) and combinations of clinical TBI characteristics (number of TBIs, time since the last TBI, time since the first TBI, history of TBI caused by blast or associated with PTA or LOC) were constructed to assess the relationships between PCS, PTSD, and depression symptoms and biomarker levels. Model fit was evaluated by looking at the residual plots and distribution plots. All data were analyzed using the R statistical package (version 3.5) and GraphPad Prism version 7.04. GraphPad Prism was also used to produce graphs.
Data availability
The data that support the findings of this study are available on reasonable request to the corresponding author.
Results
Demographic and clinical characteristics
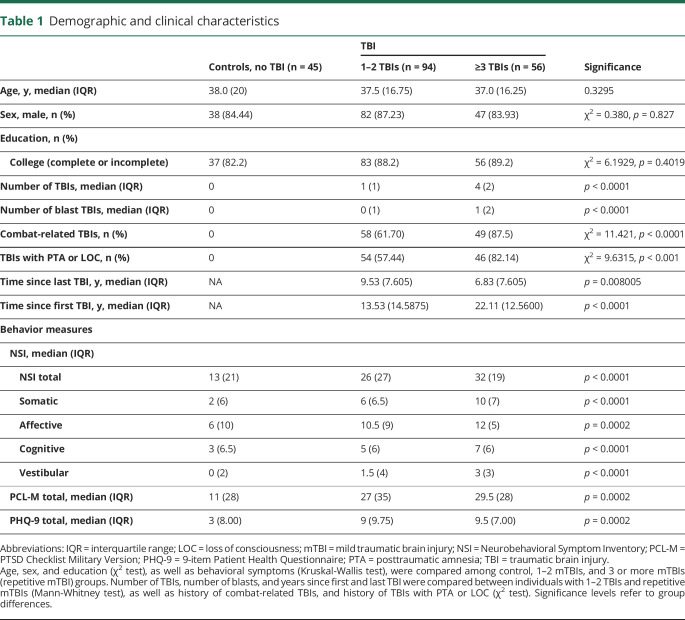
Demographic and clinical characteristics of the 195 participants are described in table 1. The sample was predominately male (85%), with a median age of 38 years (interquartile range [IQR], 20.5) in the control group and 37.5 years (IQR, 18) in the TBI group. No significant differences on the demographic variables of age, sex, or education were observed between the control group (no TBI, n = 45) and TBI-positive groups (1 or 2 TBIs, n = 94; repetitive TBI, n = 56). Education categories were as follows: college, 4 years or more (controls, n = 18; 1 or 2 TBIs, n = 44; repetitive TBI, n = 20); college, 1 year to 3 years (control, n = 19; 1 or 2 TBIs, n = 39; repetitive TBI, n = 30); high school graduate (grade 12 or General Educational Development, controls, n = 7; 1 or 2 TBIs, n = 11; and repetitive TBI, n = 6); and some high school (grades 9 through 11, controls, n = 1; 1 or 2 TBIs, n = 0; and repetitive TBI, n = 0). Individuals with positive TBI history reported significantly more severe PCS (p < 0.0001), PTSD (p = 0.0002), and depression symptoms (p = 0.0002) than the control group. PCS symptoms were more severe in individuals with repetitive TBI than in those with 1 or 2 TBIs (p = 0.0383). The number of TBIs caused by blasts (p < 0.0001) was also significantly higher in the repetitive mTBI group than in those with 1 or 2 mTBIs. Finally, the time in years since the first TBI was longer in the repetitive mTBI group and the time since the most recent TBI (p < 0.0001) was shorter in the 1–2 mTBIs group (p = 0.0080).
Table 1.
Demographic and clinical characteristics
Repetitive mTBI is associated with elevated biomarker levels
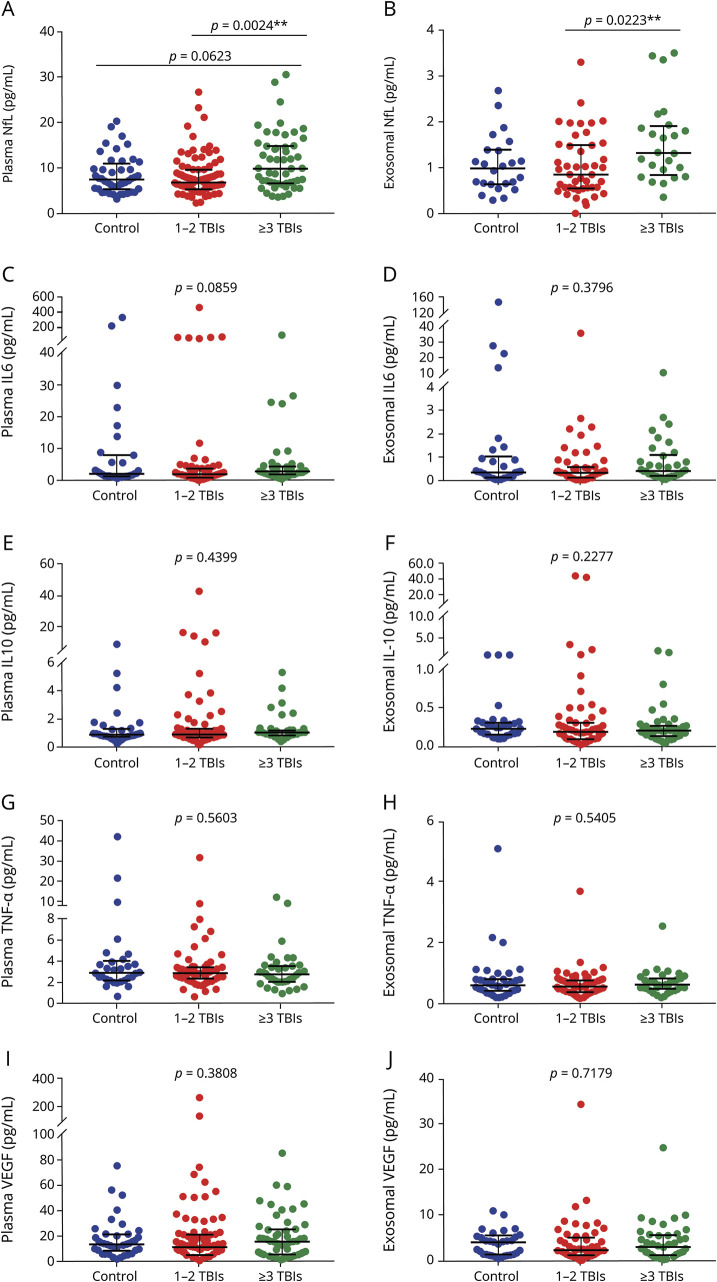
We compared biomarker levels among individuals with no mTBI history (controls), 1–2 mTBIs, and 3 or more mTBIs (repetitive mTBI) (figure 1). We observed significant group differences for exosomal (p = 0.0226) and plasma NfL (p = 0.0030). Pairwise comparisons showed differences between 1–2 mTBIs and repetitive mTBI groups for exosomal (p = 0.0223) and plasma NfL (p = 0.0024). For plasma NfL, a marginally significant difference was observed between control and repetitive mTBI groups (p = 0.0623). No other significant differences among control, 1–2 mTBIs, and repetitive mTBIs groups were observed. When participants were divided into control and TBI (1–2 and 3 or more mTBIs) groups, there were no significant differences in the plasma or exosomal concentrations of any biomarker: NfL (exosomal, p = 0.4852; plasma, p = 0.5458), IL-6 (exosomal, p = 0.9365; plasma, p = 0.83135), IL-10 (exosomal, p = 0.1087; plasma, p = 0.4339), TNF-α (exosomal, p = 0.6682; plasma, p = 0.4674), or VEGF (exosomal, p = 0.5162; plasma, p = 0.4857). We compared controls to individuals with mTBI with LOC or PTA and those with mTBI with AOC only, but no significant differences were observed in NfL (exosomal, p = 0.7579; plasma, p = 0.8251), IL-6 (exosomal = 0.881; plasma, p = 0.755), IL-10 (exosomal, p = 0.1893; plasma, p = 0.693), TNF-α (exosomal, p = 0.1893; plasma, p = 0.5417), or VEGF (exosomal, p = 0.6848; plasma, p = 0.3257).
Figure 1. Exosomal and plasma levels of biomarkers.
(A–J) Participants were divided into 3 groups: control, 1–2 mild traumatic brain injuries (TBIs), and 3 or more TBIs (repetitive mild TBI). Significant group differences were observed for plasma and exosomal neurofilament light (NfL). Biomarker concentrations represented as median + interquartile range. IL = interleukin; TNF-α = tumor necrosis factor–α; VEGF = vascular endothelial growth factor.
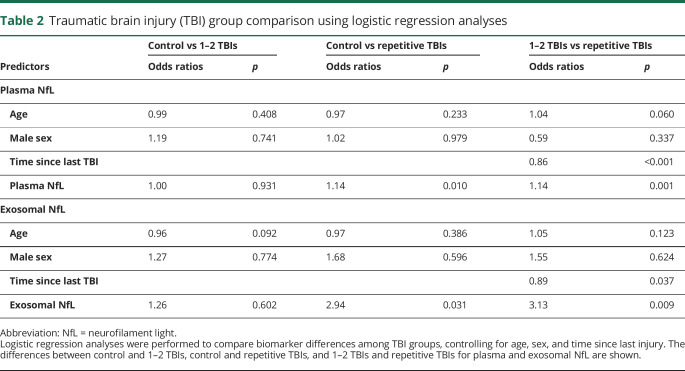
We also compared groups using logistic regression models. Controlling for age and sex, we assessed biomarker differences comparing controls vs TBI, control vs 1–2 mTBIs, and control vs repetitive mTBI. Controlling for age, sex, and time in years since last TBI, we compared 1–2 mTBI and repetitive mTBI groups (table 2). We observed that levels of exosomal and plasma NfL were elevated in both the 1–2 mTBIs and repetitive mTBI groups when compared to controls.
Table 2.
Traumatic brain injury (TBI) group comparison using logistic regression analyses
TBI characteristics and biomarker levels
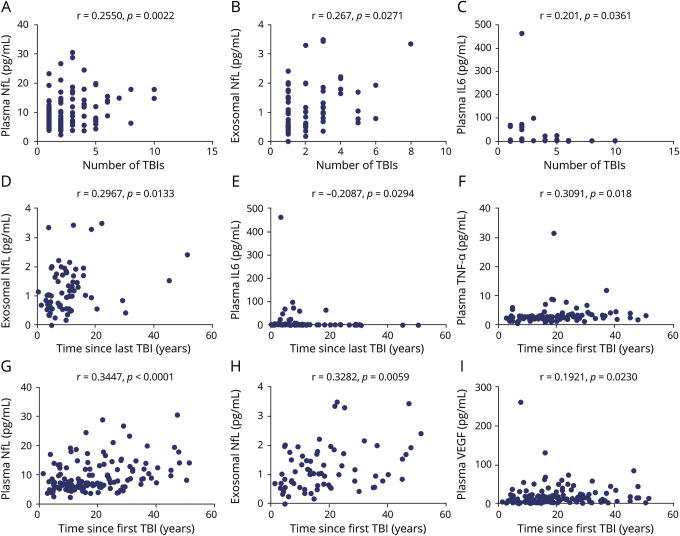
The results of the correlation analyses between TBI characteristics and biomarker levels are displayed graphically in figure 2. The total number of lifetime mTBIs positively correlated with higher exosomal (r = 0.2661, p = 0.0271) and plasma NfL (r = 0.255, p = 0.0022), as well as plasma IL-6 (r = 0.201, p = 0.0361). We also observed that the number of years since first TBI positively correlated with higher levels of exosomal (r = 0.3282, p = 0.0059) and plasma NfL (r = 0.3447, p < 0.0001), plasma TNF-α (r = 0.3091, p = 0.018), and plasma VEGF (r = 0.1921, p = 0.0230). Similarly, the number of years since last TBI positively correlated with the levels of exosomal NfL (r = 0.2967, p = 0.0133). However, levels of IL-6 decreased with time as the number of years since last TBI negatively correlated with levels of IL-6 (r = −0.2087, p = 0.0294). Correlations between years since last TBI and both plasma NfL (r = 0.1608, p = 0.0559) and plasma VEGF (r = 0.1524, p = 0.0722) were marginally significant. No significant correlations were observed for number of blasts and any candidate plasma or exosomal biomarker.
Figure 2. Correlations between traumatic brain injury (TBI) characteristics and biomarker levels.
(A–I) Only significant correlations are shown. IL = interleukin; NfL = neurofilament light; TNF-α = tumor necrosis factor–α; VEGF = vascular endothelial growth factor.
Biomarker levels are linked to the severity of PCS, PTSD, and depression symptoms
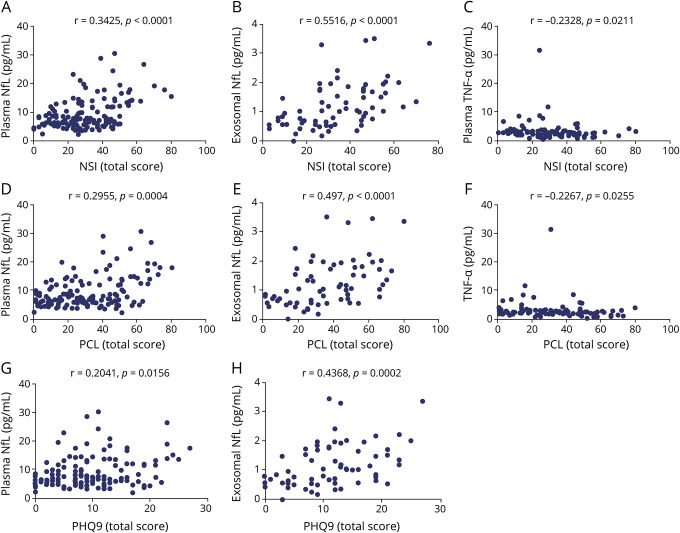
The results of correlations between TBI-type symptoms and biomarker levels are shown graphically in figure 3. NSI total score correlated moderately with exosomal NfL (r = 0.5516, p < 0.0001) and mildly with both plasma NfL (r = 0.3425, p < 0.0001) and plasma TNF-α (r = −0.2328, p = 0.0211). We also evaluated possible correlations between each of the NSI subscales (i.e., somatic, affective, cognitive, and vestibular) and biomarker levels. Overall, exosomal NfL also correlated moderately with most NSI subscales (somatic, r = 0.5268, p < 0.0001; cognitive, r = 0.399, p = 0.0008; vestibular, r = 0.4604, p < 0.0001; affective, r = 0.4542, p = 0.0001, respectively). Similarly, the levels of plasma NfL positively correlated with higher scores in the NSI somatic (r = 0.3779, p < 0.0001), cognitive (r = 0.2621, p = 0.0017), vestibular (r = 0.3529, p < 0.0001), and affective subscales (r = 0.2679, p = 0.0013). Higher scores in the somatic (r = −0.2349, p = 0.0199) and cognitive subscales (r = −0.2671, p = 0.0075) weakly correlated with decreased plasma concentrations of TNF-α.
Figure 3. Correlations between symptoms and biomarker levels within the traumatic brain injury (TBI) group.
(A–H) Only significant correlations are shown. IL = interleukin; NfL = neurofilament light; NSI = Neurobehavioral Symptom Inventory; PCL-M = PTSD Checklist Military Version; PHQ-9 = 9-item Patient Health Questionnaire; TNF-α = tumor necrosis factor–α; VEGF = vascular endothelial growth factor.
Moreover, PCL-M scores correlated with exosomal (r = 0.497, p < 0.0001) and plasma NfL (r = 0.2955, p = 0.0004), and plasma TNF-α (r = −0.2267, p = 0.0255), and again moderately for exosomal NfL measures and only weakly for plasma NfL and TNF-α measures. A marginally significant correlation between PCL-M scores and exosomal IL-6 (r = 0.1893, p = 0.0865) was also observed. PHQ-9 scores correlated with exosomal (r = 0.4368, p = 0.0002) and plasma NfL (r = 0.2041, p = 0.0156).
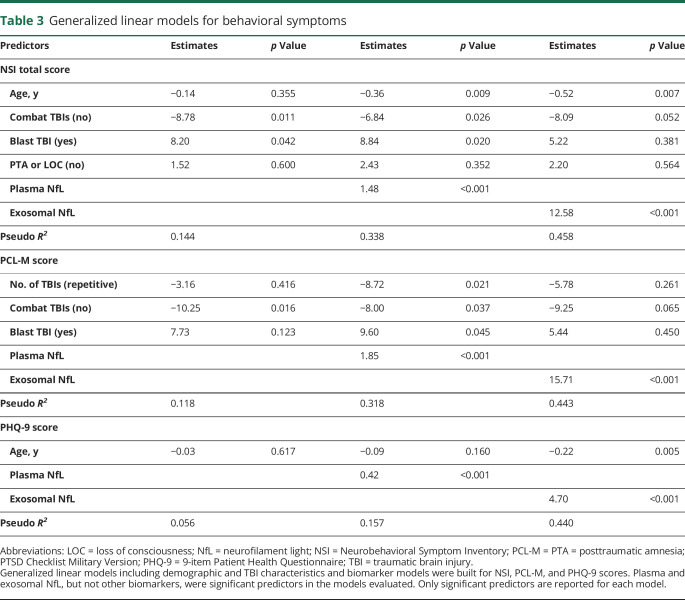
Results of the GLM models corrected for demographic and clinical characteristics to evaluate associations between biomarker levels and NSI, PCL-M, and PHQ-9 scores are shown in table 3. For each behavioral measure, models included age, sex, time since last TBI, number of mTBIs, combat TBIs (yes or no), blast TBI (yes or no), PTA/LOC status (TBI with PTA or LOC, or TBI without PTA or LOC), as well as exosomal and plasma levels of biomarkers. We observed an association between higher NSI, PCL-M, and PHQ-9 scores and increased levels of both exosomal and plasma NfL.
Table 3.
Generalized linear models for behavioral symptoms
Discussion
Findings from this study demonstrate that veterans who sustained remote mTBIs, and especially those with repetitive TBIs, have alterations in candidate biomarker activity, which in turn are associated with chronic neurologic and behavioral symptoms. In this study, we evaluated peripherally circulating exosomal as well as soluble plasma levels of candidate protein biomarkers in veterans years after both their most recent and their initial mTBI. Specifically, we observed higher exosomal and plasma levels of NfL, a neuronal marker of axonal injury, in those with history of repetitive mTBIs and that these increased exosomal and plasma levels of NfL correlate with the severity of PCS, PTSD, and depressive symptoms. We also found an association between concentrations of TNF-α, an inflammatory marker, and PCS and PTSD symptoms. These findings contribute to the ongoing efforts to establish prognostic biomarkers for persistent neurobehavioral symptoms in chronic mTBI and shed light on potential mechanisms underlying these symptom complexes common after mTBI, especially after repetitive TBIs among active duty SM and veterans.
NfL is a major component of the neuronal cytoskeleton, particularly of large myelinated neuronal axons, and has very recently gained attention as a promising diagnostic and prognostic biomarker in a host of neuroinflammatory and neurodegenerative disorders, as well as sports-related concussion.11,24–26 Elevated levels of NfL are believed to reflect axonal damage due to injury or degeneration and have been reported in athletes with history of recurrent head trauma and associated with chronic neurologic symptoms.2,12 Less is known regarding the role of NfL in the chronic period following TBIs, and especially the role of repetitive mTBIs in veterans with chronic symptoms. Cytokines, including interleukin IL-6, IL-10, and TNF-α, are key components of inflammatory responses in the CNS,14 while VEGF15,16,27 has been implicated in the modulation of inflammatory responses after vascular injury. Cytokine levels typically peak within hours after a TBI, declining days after the injury.13,28
Pathologic processes underlying mTBIs remain poorly understood. In individuals who sustain 1 or more mTBIs, there are often transient alterations in neuronal function and temporary neurologic symptoms.29 However, a subset of patients with mTBI develop lasting symptoms,30 with evidence linking repetitive mTBIs with poorer outcomes,3,6,7 as well as repetitive subconcussive exposures to postmortem diagnosis of chronic traumatic encephalopathy in contact sports athletes and military SM and veterans.31 Our finding of persistently elevated neuronal and neuroinflammatory markers, including a dose-dependent relationship between mTBI totals and increasing symptom burden, may provide insight into the underlying pathogenesis of persistent symptom development after repetitive mTBI.
Elevated NfL levels have been linked to axonal injury or degeneration in TBI and several neurodegenerative disorders, suggesting that our findings reflect chronic, persistent axonal degeneration or dysregulation remotely after repetitive mTBIs.11,24,25 Further, the correlation between increased levels of exosomal and plasma NfL and number of years since first injury suggests an ongoing and progressive axonal dysregulation that may be critical in the persistent symptom complexes seen chronically after repetitive mTBI. In athletes, NfL has been linked to both repetitive concussive and subconcussive head impacts.12,32,33 Elevated NfL in the CSF among hockey players with a history of repetitive mTBIs has been associated with the presence of PCS for over 1 year.12 Our findings provide further evidence that increased NfL levels are linked to repetitive head traumas and may play a role in chronic symptom persistence following repetitive TBIs.
We also observed that levels of TNF-α are associated with the severity of NSI and PTSD symptom scores. These results confirm previous findings from our group that described increased blood TNF-α levels and chronic TBI symptoms in military personnel.34 PTSD has previously been linked to low-grade systemic inflammation, characterized by elevated levels of inflammatory markers.35–38 Our group and others have shown that chronic PTSD is associated with peripheral elevations of proinflammatory cytokines in civilian and military populations.34,39,40 Other lines of investigation have also associated PTSD with chronic inflammation and immune system activation.35,41,42 PTSD has been linked to diseases with an inflammatory component such as diabetes, atherosclerosis, and coronary disease.35,41 Our results suggest a link between chronic, especially repetitive mTBIs and PTSD symptoms through inflammatory mediators including TNF-α.
The measurement of blood-based biomarkers is a safe, accessible, and inexpensive method to monitor brain injury and associated pathologic processes, as well as the development of mTBI-related behavioral symptoms, even remotely after injury. Nevertheless, this study has limitations, such as a relatively small sample size and variability in the number of years from the first and most recent TBIs. Further, there was variability in mechanisms of injury, as we included participants with deployment-associated TBI, which includes but it is not restricted to blast-related TBI, as well as TBIs sustained before military service.
Our findings demonstrate the potential of these easily accessible biomarkers as predictors of behavioral alterations in veterans years after mTBI and provide insights into potential underlying pathologic mechanisms. The elevated levels of NfL among veterans and SM with repetitive mTBI suggests that chronic axonal degeneration, dysregulation, or remodeling persists for years after sustaining repetitive head injuries. The increase in inflammatory biomarkers may suggest an associated chronic inflammatory process underlying this persistent axonal degeneration. Future studies including additional time points, serial biomarker assays, advanced imaging correlates, and cognitive evaluations are required to explore the predictive value of NfL and TNF-α, and the relationship between elevated concentration of these TBI biomarkers and chronic TBI-associated pathologic processes.
Acknowledgment
The authors thank the military servicemembers and veterans who participated in this study. The CENC Observational Study site PIs or co-PIs include Heather Belanger, PhD (Tampa), Carlos Jaramillo, MD (San Antonio), Ajit Pai, MD (Richmond), Melissa Guerra, MD (Fort Belvoir), Randall Scheibel, PhD (Houston), Terri Pogoda, PhD (Boston), Scott Sponheim, PhD (Minneapolis), and Kathleen Carlson, PhD (Portland). The authors thank the CENC Observational Study Leadership Working Group and Core Team members who besides the authors also include Justin Alicea, Jessica Berumen, Cody Blankenship, Jennifer Boyce, Linda Brunson, Katrina Burson, Julia Christensen, Margaret Clarke, Doreen Collins, Sureyya Dikmen, Esra Doud, Connie Duncan, Stephanie Edmunds, Robyn Endsley, Elizabeth Fogleman, Laura M. Franke, Katelyn Gormley, Brenda Hair, Jim Henry, Nancy Hsu, Cheryl Ford-Smith, George Gitchel, Col. Sidney Hinds (Consortium Co-PI), Caitlin Jones, Sunchai Khemalaap, Valerie Larson, Tiffany Lewis, Scott McDonald, Tamara McKenzie-Hartman, Frank Mierzwa, Alison Molitor, Joe Montanari, Johnnie Mortenson, Nicholas Pastorek, Judy Pulliam, Risa Richardson, Callie Riggs, Rachel Rosenfield, Sara Salkind, James K. Sickinger, Taylor Swankie, Nancy Temkin, Doug Theriaque, Maya Troyanskaya, Rodney Vanderploeg, and Carmen Vasquez. This material is based upon work supported with resources and the use of facilities at Hunter Holmes McGuire Veterans Affairs Medical Center in Richmond, Virginia; James A. Haley Veterans Hospital, Tampa, Florida; and Audie L. Murphy Memorial Veterans Hospital, San Antonio, Texas; and is based upon work supported in part by the Defense and Veterans Brain Injury Center, US Army Medical Research and Material Command.
Glossary
- AOC
alteration of consciousness
- CENC
Chronic Effects of Neurotrauma Consortium
- DoD
Department of Defense
- DRRI-2
Deployment Risk and Resiliency Inventory–2
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, 5th edition
- GLM
generalized linear model
- IL
interleukin
- IQR
interquartile range
- IRB
institutional review board
- LOC
loss of consciousness
- mTBI
mild traumatic brain injury
- NfL
neurofilament light
- NSI
Neurobehavioral Symptom Inventory
- OEF
Operation Enduring Freedom
- OIF
Operation Iraqi Freedom
- OND
Operation New Dawn
- PCE
possible concussive event
- PCL-M
PTSD Checklist Military Version
- PCS
postconcussive syndrome
- PHQ-9
9-item Patient Health Questionnaire Depression Scale
- PTA
posttraumatic amnesia
- PTSD
posttraumatic stress disorder
- SM
service members
- TBI
traumatic brain injury
- TNF
tumor necrosis factor
- VA
Veterans Affairs
- VCU rCDI
Virginia Commonwealth University Retrospective Concussion Diagnostic Interview
- VEGF
vascular endothelial growth factor
Appendix 1. Authors
Appendix 2. Coinvestigators

See page 1017
Podcast: NPub.org/7mc8bk
Study funding
This work was supported by grant funding from the Department of Defense Chronic Effects of Neurotrauma Consortium (CENC) Award W81XWH-13-2-0095 and Department of Veterans Affairs CENC Award I01 CX001135, NIH, National Institute of Nursing Research Intramural Research Program.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Mac Donald CL, Barber J, Jordan M, et al. Early clinical predictors of 5-year outcome after concussive blast traumatic brain injury. JAMA Neurol 2017;74:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pattinson CL, Shahim P, Taylor P, et al. Elevated tau in military personnel relates to chronic symptoms following traumatic brain injury. J Head Trauma Rehabil 2020;35:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radhakrishnan R, Garakani A, Gross LS, et al. Neuropsychiatric aspects of concussion. Lancet Psychiatry 2016;3:1166–1175. [DOI] [PubMed] [Google Scholar]
- 4.Yee MK, Janulewicz PA, Seichepine DR, Sullivan KA, Proctor SP, Krengel MH. Multiple mild traumatic brain injuries are associated with increased rates of health symptoms and Gulf War illness in a cohort of 1990-1991 Gulf War veterans. Brain Sci 2017;7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manley G, Gardner AJ, Schneider KJ, et al. A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med 2017;51:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 2005;57:719–726. [DOI] [PubMed] [Google Scholar]
- 7.Kerr ZY, Zuckerman SL, Wasserman EB, Covassin T, Djoko A, Dompier TP. Concussion symptoms and return to play time in youth, high school, and college American football athletes. JAMA Pediatr 2016;170:647–653. [DOI] [PubMed] [Google Scholar]
- 8.Mckee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol 2015;127:45–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron 2012;76:886–899. [DOI] [PubMed] [Google Scholar]
- 10.Taylor DD, Gercel-Taylor C. Exosome platform for diagnosis and monitoring of traumatic brain injury. Philos Trans R Soc Lond B Biol Sci 2014;369:20130503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menke RAL, Gray E, Lu CH, et al. CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol 2015;2:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahim P, Tegner Y, Gustafsson B, et al. Neurochemical aftermath of repetitive mild traumatic brain injury. JAMA Neurol 2016;73:1308. [DOI] [PubMed] [Google Scholar]
- 13.Kim YK, Amidfar M, Won E. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry 2019;91:103–112. [DOI] [PubMed] [Google Scholar]
- 14.Rodney T, Osier N, Gill J. Pro- and anti-inflammatory biomarkers and traumatic brain injury outcomes: a review. Cytokine 2018;110:248–256. [DOI] [PubMed] [Google Scholar]
- 15.Vempati P, Popel AS, Mac Gabhann F. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev 2014;25:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee C, Agoston DV. Inhibition of VEGF receptor 2 increased cell death of dentate hilar neurons after traumatic brain injury. Exp Neurol 2009;220:400–403. [DOI] [PubMed] [Google Scholar]
- 17.Walker WC, Carne W, Franke LM, et al. The Chronic Effects of Neurotrauma Consortium (CENC) multi-centre observational study: description of study and characteristics of early participants. Brain Inj 2016;30:1469–1480. [DOI] [PubMed] [Google Scholar]
- 18.VA/DoD Clinical Practice Guideline for the Management of Concussion: Mild Traumatic Brain Injury: The Management of Concussion–Mild Traumatic Brain Injury Working Group; 2016. Available at: healthquality.va.gov/guidelines/Rehab/mtbi/mTBICPGFullCPG50821816.pdf. Accessed October 30, 2018. [Google Scholar]
- 19.Vanderploeg RD, Cooper DB, Belanger HG, et al. Screening for postdeployment conditions. J Head Trauma Rehabil 2014;29:1–10. [DOI] [PubMed] [Google Scholar]
- 20.Caplan LJ, Ivins B, Poole JH, Vanderploeg RD, Jaffee MS, Schwab K. The structure of postconcussive symptoms in 3 US military samples. J Head Trauma Rehabil 2010;25:447–458. [Google Scholar]
- 21.Lange RT, Brickell TA, French LM. Examination of the Mild Brain Injury Atypical Symptom Scale and the Validity-10 Scale to detect symptom exaggeration in US military service members. J Clin Exp Neuropsychol 2015;37:325–337. [DOI] [PubMed] [Google Scholar]
- 22.Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety 2011;28:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meeter LH, Dopper EG, Jiskoot LC, et al. Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann Clin Transl Neurol 2016;3:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernick C, Zetterberg H, Shan G, Banks S, Blennow K. Longitudinal performance of plasma neurofilament light and tau in professional fighters: the Professional Fighters Brain Health study. J Neurotrauma 2018;35:2351–2356. [DOI] [PubMed] [Google Scholar]
- 26.Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017;88:1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res 2010;87:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKee CA, Lukens JR. Emerging roles for the immune system in traumatic brain injury. Front Immunol 2016;7:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fehily B, Fitzgerald M. Repeated mild traumatic brain injury. Cell Transpl 2017;26:1131–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polinder S, Cnossen MC, Real RGL, et al. A multidimensional approach to post-concussion symptoms in mild traumatic brain injury. Front Neurol 2018;9:1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 2015;66:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahim P, Tegner Y, Wilson DH, et al. Blood biomarkers for brain injury in concussed professional ice hockey players. JAMA Neurol 2014;71:684–692. [DOI] [PubMed] [Google Scholar]
- 33.Oliver JM, Jones MT, Kirk KM, et al. Serum neurofilament light in American football athletes over the course of a season. J Neurotrauma 2016;33:1784–1789. [DOI] [PubMed] [Google Scholar]
- 34.Devoto C, Arcurio L, Fetta J, et al. Inflammation relates to chronic behavioral and neurological symptoms in military personnel with traumatic brain injuries. Cell Transpl 2017;26:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Passos IC, Vasconcelos-Moreno MP, Costa LG, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015;2:1002–1012. [DOI] [PubMed] [Google Scholar]
- 36.Maes M, Lin AH, Delmeire L, et al. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry 1999;45:833–839. [DOI] [PubMed] [Google Scholar]
- 37.Speer K, Upton D, Semple S, McKune A. Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. J Inflamm Res 2018;11:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gill J, Latour L, Diaz-Arrastia R, et al. Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI. Neurology 2018;91:e1385–e1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindqvist D, Wolkowitz OM, Mellon S, et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun 2014;42:81–88. [DOI] [PubMed] [Google Scholar]
- 41.Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry 2008;64:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Donovan A, Chao LL, Paulson J, et al. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology 2015;51:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request to the corresponding author.