Abstract
We present a novel treatment for removal of SL that is efficient and enables removal or lesions not immediately visible. Kleresca® FLE technology combined with picosecond laser treatment removes SL lesions and improves skin quality and appearance.
Keywords: biophotonic platform, fluorescent light energy, healing, picosecond laser, pigmentation
We present a novel treatment for removal of SL that is efficient and enables removal or lesions not immediately visible. Kleresca® FLE technology combined with picosecond laser treatment removes SL lesions and improves skin quality and appearance.
1. INTRODUCTION
The Kleresca® biophotonic platform that generates fluorescent light energy (FLE) was used before and after the picosecond laser targeting of solar lentigines (SL). Pretreatment unmasked and intensified underlying SL, which were successfully removed with only one laser session, post‐treatment, supported a healing response to improve the overall appearance of the skin.
The aging process has significant effects on the skin and support structures of the face. Dermal changes are driven by both intrinsic (genetic) and extrinsic factors (mainly ultraviolet (UV) irradiation). 1 Melanin, an endogenous light absorbing chromophore, is the main color‐determining pigment of the skin. In melanocytes, its synthesis occurs within the melanosome, through a series of complex enzymatic steps. Skin pigmentation is typically determined by the function of melanocytes and their interaction with other key skin cells, primarily, keratinocytes and fibroblasts. 2 Dysregulation of these mechanisms can give rise to pigmentary disorders. 2
Solar/senile lentigines (age spots) are benign hyperpigmented macules typically occurring in elderly individuals. 3 Associated with acute or chronic UV radiation–induced photodamage, they present in areas of high sun exposure, for example, the face and dorsal hands. While not precancerous, these macules are aesthetically displeasing to patients who choose to have them removed. There are various treatment approaches that can be applied, including cryotherapy, chemical peeling, bleaching, and topical retinoids. 4 However, recent advances support the selective disruption of the melanin pigment using laser techniques. 5 Various modalities exist, including long‐pulse duration and short‐pulse duration Q‐switched nanosecond lasers, which induce photothermolysis of the melanin target. Initially used for tattoo removal, picosecond lasers have emerged as a new treatment approach for targeting SL. 5 , 6 , 7 , 8 Picosecond lasers offer the advantage of inducing a photomechanical effect (photoacoustic) with higher compartmentalized peak temperature generation and typically fewer side effects. 5 Common side effects of picosecond laser treatment can include pain, erythema, crusting, blistering, scarring, and postinflammatory hyperpigmentation, which are more severe with higher fluences. 9
A recent report described the successful eradication of facial SL with two 532‐nm picosecond laser treatments. Following the laser targeting, patients also received a biophotonic treatment which generates fluorescent light energy (FLE) to induce a novel form of photobiomodulation, activating the skin and enabling a healing response. The combined effect was the removal of the SL and an improvement in the overall appearance of the skin. 6 A common observation with the Kleresca® biophotonic treatment is the transient emergence of hyperpigmented spots. This was recently exploited to unmask and intensify the underlying SL prior to 694‐nm QS ruby laser targetting. 10
The aim of the current case study was to combine the Kleresca® biophotonic treatment before (pre) and after (post) picosecond laser treatment to intensify and unmask the underlying SL for more effective laser targeting and to support a healing response post–laser treatment.
2. CASE REPORT
All procedures were carried out with prior, informed patient consent.
A 56‐year‐old woman with facial SL received a single 9‐minute biophotonic treatment (Kleresca® skin rejuvenation (SKR), Klox Technologies, Inc) as per the instructions for use. Two day later, visible SL spots were treated with a 532‐nm picosecond laser using a 4‐mm Zoom handpiece and an energy output of 0.60 joules at 2 Hz (PicoWay, Syneron‐Candela). 6 Two weeks later the patient received another biophotonic treatment.
Standardized pictures were obtained using a VISIA camera system (Canfield), before the Kleresca® treatment (Figure 1A), 2 days after the Kleresca® treatment and immediately prior to the treatment with the 532‐nm PicoWay laser (Figure 1B), and 2 weeks following the second and final Kleresca® treatment (Figure 1C). Pictures were analyzed using ImageJ (NIH); the percentage area of pigmentation was measured and, from this, the percentage change in pigmentation between treatments was calculated (Figure 1D).
Figure 1.
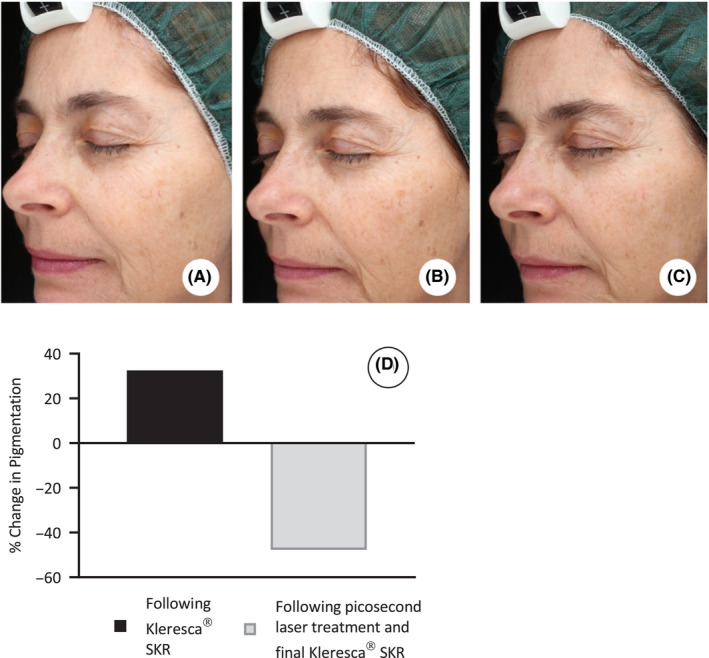
Fluorescent light energy (FLE) pretreatment intensifies underlying solar lentigines for effective laser targeting. Standardized pictures obtained using a Visia camera system (Canfield); before the Kleresca® biophotonic treatment (A), 2 d after the Kleresca® treatment and immediately prior to the treatment with the 532‐nm PicoWay laser (B), and 2 wk following the second and final Kleresca® treatment (C). D, Shows the percentage change in pigmentation calculated following the Kleresca® skin rejuvenation (SKR) treatment compared with baseline (before any treatment) and following the subsequent laser treatment and final SKR treatment compared with baseline
Following the Kleresca® treatment, there was a 33% increase in the percentage area of pigmentation (Figure 1B, D) compared with the baseline (before any treatment) (Figure 1A, D). Following the subsequent PicoWay laser treatment, the pigmentation decreased by 48% (Figure 1C, D).
The patient also received a Kleresca® SKR treatment on their hands 2 days before picosecond laser treatment (Figure 2).
Figure 2.
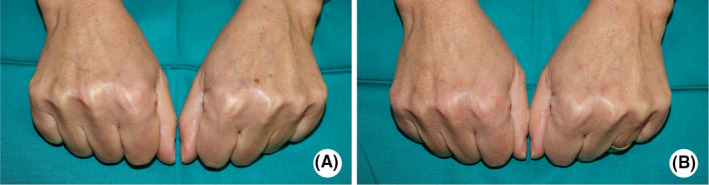
Fluorescent light energy (FLE) and laser treatment of the hands. Patient's hands before the Kleresca skin rejuvenation treatment (A) and 19 days following the 532‐nm PicoWay laser treatment. The combination of FLE and laser successfully removed solar lentigines
3. DISCUSSION
Laser targeting of the melanin pigment responsible for the emergence of solar lentigo is commonly applied in cosmetic dermatology. 1 Effective laser techniques can be associated with significant down time and side effects such as, pain, erythema, blistering, crusting, scarring, and postinflammatory hyperpigmentation. 11
The ability to effectively and efficiently target SL with fewer laser sessions and lower fluencies to reduce the risk of side effects is most favorable. The Kleresca® biophotonic platform which generates FLE to induce a novel form of photobiomodulation (PBM) is used clinically to treat an array of inflammatory skin conditions, including acne and rosacea, 10 , 12 , 13 , 14 , 15 , 16 , 17 as well as rejuvenating the skin. 18 Due to its anti‐inflammatory and healing properties, Kleresca® is often used before, to prepare the skin, and after more invasive treatments, supporting a balanced healing response. Indeed, Kleresca® has previously been used following the laser treatment for SL, which improved the overall texture of the skin. 6
A known side effect of the Kleresca® treatment is the transient emergence of hyperpigmented spots. This has recently been exploited to intensify and unmask underlying SL for treatment with a QS‐ruby switched laser, supporting their effective removal. 19 This study used the Kleresca® treatment both before and after the laser treatment.
Following the Kleresca® treatment, there was an increase in the % area of the pigmented spots on the face. Following this, only a single laser session was required to effectively remove the visible SL. In a previous study using the same laser to target SL, two laser sessions were required to achieve a satisfactory removal of the visible SL. 6 This was carried out of a time frame of weeks. In the current study, single laser treatment was followed 2 weeks later by a single biophotonic treatment which enhanced the patients’ experience with reports of an added boost in the skin's complexion and overall appearance.
The emergence of transient hyperpigmentation following the Kleresca® light treatment is not completely understood. However, this is possibly an oxidation of the melanin already present in the skin as the effect is immediate and emerges within the 9‐minute treatment cycle and the effect is transient. In this instance, the intensification of the SL spots was utilized to enhance the overall laser treatment experience and the patient did not experience any adverse effects following their single picolaser treatment. Interestingly, there is no emergence of pigmentation following the post–laser biophotonic treatment supporting the idea that the hyperpigmentation that can be experienced following Kleresca® is acting on the melanin that is present in the skin since the laser treatment has already ablated the current melanin.
This report also shows the capability of the biophotonic platform in treating other areas of the body, for example, the hands, which also highly implicated in SL.
The ability to target the melanin pigment responsible for the SL and, indeed, responsible for many pigmentary disorder with a single laser session following a prebiophotonic treatment has relevance for laser treatment of darker skin types which can often present as a challenge, especially regarding side effects. 7 Moreover, the post‐treatment has been shown to improve the overall complexion of the skin, likely by increasing collagen production. 6 , 17 , 18 , 20
To conclude, utilizing the Kleresca biophotonic treatment prior to the laser targeting of SL aided in the successful removal of the pigmented spots with one single laser session. The application of FLE following the laser therapy gave the skin an extra boost, supporting a healing response to improve the overall feeling and appearance of the skin. The Kleresca® biophotonic light therapy is a useful adjunct tool preparing and repairing the skin before and after more invasive procedures.
CONFLICT OF INTEREST
G. Scarcella and P.A Gerber have served as investigators, consultants, and speakers for FB Dermatology/Kleresca®. They have received honoraria for membership of advisory board, travel, and research funding. MCE. Nielsen and D. Edge are employees of FB Dermatology/Kleresca®.
AUTHOR CONTRIBUTIONS
GS and PAG: contributed to study design, patient selection, and clinical assessment; DE and MCEN: contributed to study design and drafting of the original manuscript. All the authors listed have reviewed and approved the final version of the manuscript.
ACKNOWLEDGMENTS
Published with written consent of the patient.
Scarcella G, Gerber PA, Edge D, Nielsen MCE. Effective removal of solar lentigines by combination of pre– And post–fluorescent light energy treatment with picosecond laser treatment. Clin Case Rep. 2020;8:1429–1432. 10.1002/ccr3.2839
REFERENCES
- 1. Sadick NS, Karcher C, Palmisano L. Cosmetic dermatology of the aging face. Clin Dermatol. 2009;27(3 suppl):S3‐S12. [Google Scholar]
- 2. Bastonini E, Kovacs D, Picardo M. Skin pigmentation and pigmentary disorders: Focus on epidermal/dermal cross‐talk. Ann Dermatol. 2016;28(3):279‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nam JH, Kim HS, Lee GY, Kim WS. Beneficial effect of low fluence 1,064 nm Q‐Switched neodymium:Yttrium‐Aluminum‐Garnet laser in the treatment of senile lentigo. Ann Dermatol. 2017;29(4):427‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Plensdorf S, Livieratos M, Dada N. Pigmentation disorders: diagnosis and management. Am Fam Physician. 2017;96(12):797‐804. [PubMed] [Google Scholar]
- 5. Kung KY, Shek SYN, Yeung CK, Chan HHL. Evaluation of the safety and efficacy of the dual wavelength picosecond laser for the treatment of benign pigmented lesions in Asians. Lasers Surg Med. 2019;51(1):14‐22. [DOI] [PubMed] [Google Scholar]
- 6. Scarcella G, Dethlefsen M, Nielsen M. Treatment of solar lentigines using a combination of picosecond laser and biophotonic treatment. Clin Case Reports. 2018;00:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kono T, Shek SY, Chan HHL, Groff WF, Imagawa K, Akamatsu T. Theoretical review of the treatment of pigmented lesions in Asian skin. Laser Ther. 2016;25(3):179‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ross V, Naseef G, Lin G, et al. Comparison of responses of tattoos to picosecond and nanosecond Q‐switched neodymium: YAG lasers. Arch Dermatol. 1998;134(2):167‐171. [DOI] [PubMed] [Google Scholar]
- 9. Ali RF, Al‐Niaimi F. Picosecond laser. DermNet Nz.
- 10. Braun SA, Gerber PA. A photoconverter gel‐assisted blue light therapy for the treatment of rosacea. Int J Dermatol. 2017;56(12):1489‐1490. [DOI] [PubMed] [Google Scholar]
- 11. Patil UA, Dhami LD. Overview of lasers. Indian J Plast Surg. 2008;41:S101‐S113. [PMC free article] [PubMed] [Google Scholar]
- 12. Antoniou C, Dessinioti C, Sotiriadis D, et al. A multicenter, randomized, split‐face clinical trial evaluating the efficacy and safety of chromophore gel‐assisted blue light phototherapy for the treatment of acne. Int J Dermatol. 2016;55(12):1321‐1328. [DOI] [PubMed] [Google Scholar]
- 13. Nikolis A, Fauverghe S, Scapagnini G, et al. An extension of a multicenter, randomized, split‐face clinical trial evaluating the efficacy and safety of chromophore gel‐assisted blue light phototherapy for the treatment of acne. Int J Dermatol. 2018;57(1):94‐103. [DOI] [PubMed] [Google Scholar]
- 14. Piccolo D, Crisman G, Kostaki D, Cannarozzo G, Sannino M, Chimenti S. Rhodamine intense pulsed light versus conventional intense pulsed light for facial telangiectasias. J Cosmet Laser Ther. 2016;18(2):80‐85. [DOI] [PubMed] [Google Scholar]
- 15. Mahendran A, Wong XL, Kao S. Sebaratnam DF. Treatment of erlotinib‐induced acneiform eruption with chromophore gel‐assisted phototherapy. Photodermatol Photoimmunol Photomed. 2019;35(3):190‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu RC, Makhija M, Wong XL, Sebaratnam DF. Treatment of granulomatous rosacea with chromophore gel‐assisted phototherapy. Photodermatol Photoimmunol Photomed. 2019;1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koceva I, Rümmelein B, Gerber PA, Edge D, Nielsen MCE. Fluorescent light energy a new therapeutic approach to effectively treating acne conglobata and hidradenitis suppurativa. Clin Case Reports. 2019;00:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nikolis A, Bernstein S, Kinney B, Scuderi N, Rastogi S, Sampalis JS. A randomized, placebo‐controlled, single‐blinded, split‐faced clinical trial evaluating the efficacy and safety of KLOX‐001 gel formulation with KLOX light‐emitting diode light on facial rejuvenation. Clin Cosmet Investig Dermatol. 2016;9:115‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerber PA, Scarcella G, Edge D, Nielsen MCE. Biophotonic pretreatment enhances the targeting of senile lentigines with a 694 nm QS‐ruby laser. Photodermatol Photoimmunol Photomed. 2020;36(2):159‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edge D, Mellergaard M, Dam‐Hansen C, et al. Fluorescent light energy: the future for treatment of inflammatory skin conditions? J Clin Aesthet Dermatol. 2019;12(5):E61‐E68. [PMC free article] [PubMed] [Google Scholar]


