Abstract
Pneumothorax is a potentially life‐threatening complication of neonatal respiratory distress syndrome (RDS). We describe a case of a tension pneumothorax that occurred during neurally adjusted ventilatory assist (NAVA) in a preterm infant suffering from RDS. The infant was included in a multicenter study examining the role of electrical impedance tomography (EIT) in intensive care and therefore continuously monitored with this imaging method. The attending physicians were blinded for EIT findings but offline analysis revealed the potential of EIT to clarify the underlying cause of this complication, which in this case was heterogeneous lung disease resulting in uneven ventilation distribution. Instantaneous increase in end‐expiratory lung impedance on the affected side was observed at time of the air leak. Real‐time bedside availability of EIT data could have modified the treatment decisions made.
Keywords: electrical impedance tomography (EIT), neonate, neurally adjusted ventilatory assist, pneumothorax, respiratory distress syndrome (RDS)
Real‐time bedside availability of EIT for continuous monitoring of aeration and ventilation distribution in neonatal RDS might help to recognize patients at risk of complications, allow timely treatment decisions and early detection of undesired events.
1. CASE REPORT
A male infant, born at 29 + 3 weeks of gestation (birth weight 1360 g), required nasal CPAP, and supplemental oxygen (FiO2 0.28) due to respiratory distress syndrome (RDS). After written informed consent from the parents, he was included in a blinded observational electrical impedance tomography (EIT) study (‘Continuous Regional Analysis Device for neonate Lung (CRADL)’ (ClinicalTrials.gov: NCT02962505)) with continuous EIT monitoring (SenTec, Landquart, Switzerland) started at 3 hours of age.
The patient needed intubation for increasing oxygen requirements at 5 hours of age and pressure‐controlled ventilation (PCV) was started. After confirming correct tube position with chest radiography (CXR) (Figure 1A), he received a first dose of exogenous surfactant resulting in rapid improvement in oxygenation. The patient could be rapidly weaned to room air and the ventilation mode was switched to neurally adjusted ventilatory assist (NAVA) at 6 hours of age. During the next day, the patient was relatively stable, although a slight increase in oxygen requirements was observed.
Figure 1.
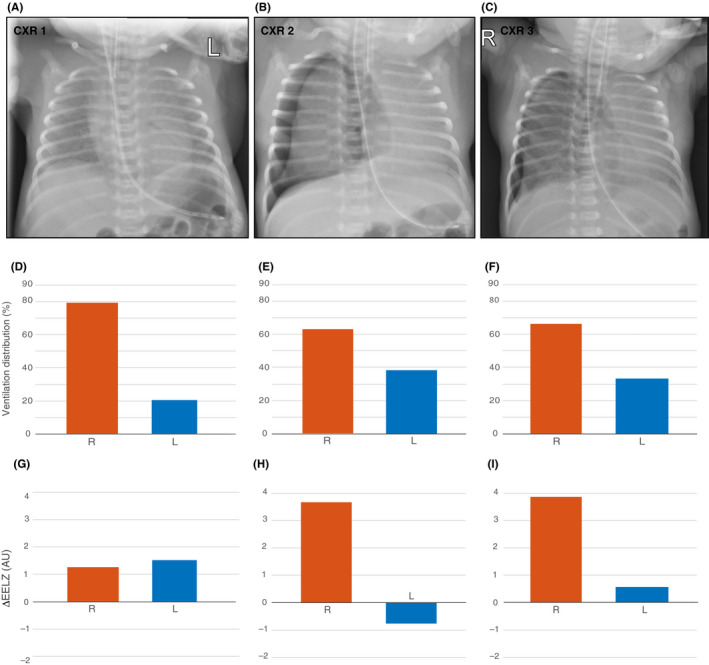
Chest X‐rays (CXR) taken during the case study with corresponding EIT findings at the same time points. A, Correct positions of the endotracheal tube and nasogastric tube were confirmed. B, Right‐sided tension pneumothorax was observed. C, Right‐sided pneumothorax remained until the end of EIT recording and insertion of a chest tube. D‐F, Relative distribution of ventilation between the right and left chest sides based on tidal impedance variation (ΔZ). G‐I, Changes in regional aeration at the right and left chest sides in arbitrary units (AU). The values show differences in end‐expiratory lung impedance (EELZ) compared with the respective values before intubation. Positive values imply aeration increase
Oxygenation suddenly deteriorated at 36 hours of age during intravenous cannulation, which was accompanied by crying. The attending physician decided to switch the ventilation mode back to PCV and administer a second dose of surfactant due to suspected worsening of the RDS. Although FiO2 could briefly be weaned from 0.72 to 0.34, oxygenation deteriorated again 3 hours later, and CXR revealed a right‐sided tension pneumothorax (Figure 1B), treated with a single needle aspiration. The ventilation mode was changed back to NAVA. Patient's condition improved, but 10 hours later oxygenation deteriorated again with the recurrence of the pneumothorax (Figure 1C). A chest drain was inserted, which prevented further recording with EIT. The patient stabilized, the pneumothorax resolved and the chest drain could successfully be removed 3 days later.
Retrospective analysis of the EIT data, oxygenation, and ventilator parameters was performed (Figure 2). Improved oxygenation after the first surfactant dose was associated with a gradual increase in end‐expiratory lung impedance (EELZ), especially in the right chest side (Figure 2A). The slow decline in oxygenation over the next 30 hours of age (Figure 2B) was accompanied by a gradual decline in EELZ in both lungs. During this time, peak inspiratory pressure (PIP) during NAVA was 12.9 ± 3.7 cmH2O, occasionally peaking to 37.7 cmH2O for short periods of time. The tidal volume showed a similar pattern with an average volume of 5.2 ± 3.5 mL/kg with peak values up to 27 mL/kg. At the time of rapid deterioration (36 hours), a sudden increase in EELZ was seen in the right chest side, indicating a pneumothorax. 1 This coincided with a sudden increase in PIP and tidal volumes (Figure 2C‐D). Analysis of the video that was continuously captured along with EIT recording showed that the sudden changes in oxygenation and EELZ coincided with patient crying during intravenous cannulation. Following needle aspiration, EELZ in the right lung decreased. With recurrence of the pneumothorax a rapid increase in EELZ in the right chest side was observed again.
Figure 2.
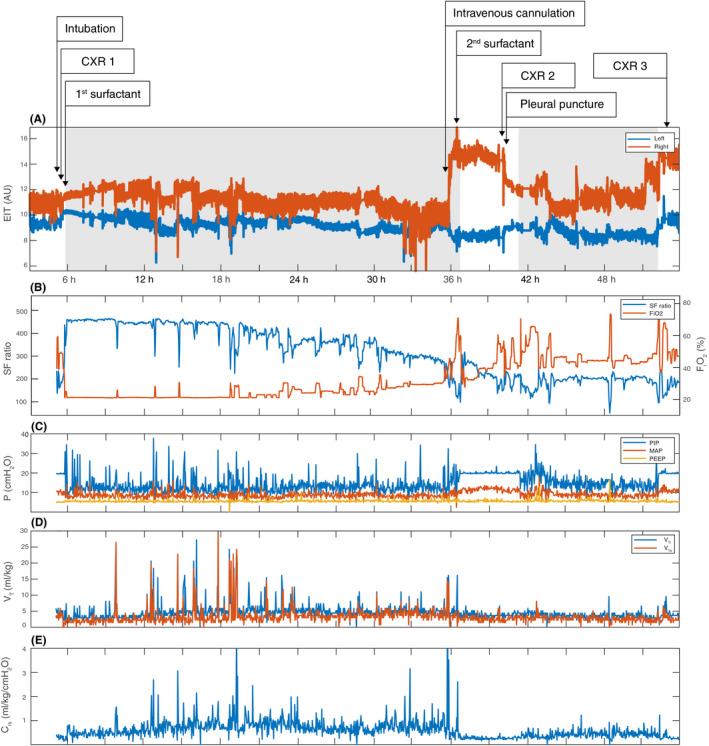
Electrical impedance tomography data, oxygenation and ventilator parameters during the case study. A, Composite electrical impedance tomography (EIT) signal in arbitrary units (AU) for left (blue) and right (orange) chest sides are presented. The level of each curve reflects the regional aeration in terms of end‐expiratory lung impedance (EELZ) and the amplitude of the curve the regional tidal volumes in terms of tidal impedance variation (∆Z) at each point in time. Postnatal age of the patient is presented on the X axis. The exact time points of selected interventions are marked with arrows. Gray background highlights the time on NAVA, white on PCV. B, Changes in oxygenation over time. Fraction of inspired oxygen (FiO2) in orange, peripheral oxygen saturation/fraction of inspired oxygen (SF) ratio in blue. C, Airway pressures over time. Peak inspiratory pressure (PIP) in blue, mean airway pressure (MPAW) in orange and positive end‐expiratory pressure (PEEP) in yellow. D, Inspiratory (VTi) in blue and expiratory (VTe) tidal volumes in red over time. E, Dynamic respiratory system compliance (Crs) derived from the airway pressures and tidal volumes
Ventilation distribution and changes in EELZ at the times of CXRs are given in Figure 1D‐I, respectively. They highlight the permanent right‐left ventilation asymmetry and massive increase in the right chest side EELZ and loss in the left side aeration during patient's deterioration. These findings suggest that right‐sided tension pneumothorax caused shifting of the heart to the left and led to a further collapse of the left lung.
2. DISCUSSION
Pneumothorax is a rare but potentially life‐threatening complication of neonatal RDS. 2 Proportional assist ventilation with strong spontaneous breathing efforts can result in high transpulmonary distending pressures and excessive tidal volumes increasing the risk of alveolar overdistension and air leaks. NAVA, giving pressure support proportional to the electrical activity of the diaphragm and improving patient‐ventilator synchrony in comparison to conventional ventilation modes, 3 is believed to provide lung protective ventilation, as intrinsic pulmonary reflexes are expected to restrict both alveolar overinflation and collapse. 4 However, our case demonstrates the potential risk of such synchronized proportional support modes which may result in high transpulmonary distending pressures and large tidal volumes during vigorous inspiration, such as crying.
Early clinical signs of pneumothorax, that is, hypercarbia and increase in oxygen requirement, 2 are often nonspecific. Increased oxygen requirement in our case was first interpreted as worsening of RDS. However, the retrospective data analysis strongly suggests that the pneumothorax occurred at 36 hours of age, indicated by a sudden increase in EELZ on the affected side, as a sign of air leak to pleural space. 1 , 5 This highlights the need for continuous respiratory monitoring of lung aeration, certainly in patients with highly vulnerable lung conditions.
Electrical impedance tomography is an easy to use, radiation free, real‐time bedside imaging method for monitoring of regional lung aeration and tidal volume distribution. With recent technical developments its application has become feasible also in neonates. In our case, EIT revealed a significant underlying pathophysiology of pneumothorax in neonatal RDS, that is, heterogeneous lung disease resulting in uneven ventilation distribution. The event of excessively large tidal volume delivered to heterogeneously and asymmetrically aerated lungs, when the child was crying while on proportional support with NAVA, led to an airleak. Real‐time information on asymmetric ventilation distribution and ultimately the observation of a sudden rise in EELZ could have changed various treatment decisions over time, such as patient positioning, optimization of ventilator settings, timing of surfactant re‐administration, and chest drainage.
Alarm limits should be used to prevent high PIP and/or large tidal volumes in order to minimize the risk of lung injury in the spontaneously breathing patient while on proportional assist ventilation, coined by the adult intensivists as self‐inflicted lung injury. 6 Also, extrapulmonary reasons for high inspiratory drive (ie, crying), which repeatedly occur in neonates, ought to be taken into account when safety limits are adjusted.
3. CONCLUSIONS
Strong spontaneous breathing efforts during proportional assist ventilation may lead to dangerous transpulmonary pressures and high tidal volumes, which subsequently not only worsen lung injury but also may cause air leaks. Real‐time bedside availability of EIT for continuous monitoring of aeration and ventilation distribution in the ventilated infant might help to recognize the patients at risk for treatment complications, allow rapid detection of such undesired events and enable early intervention.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
MK and MR recruited patients in CRADL, collected data and performed primary data analysis and interpretation. They also wrote the first draft of the manuscript. AHK, PCR, RB, and IF designed the CRADL study protocol, supervised the project and participated in writing the manuscript. All authors have read the manuscript, participated in the revision process, and given final approval of the version to be published.
Kallio M, Rahtu M, van Kaam AH, Bayford R, Rimensberger PC, Frerichs I. Electrical impedance tomography reveals pathophysiology of neonatal pneumothorax during NAVA. Clin Case Rep. 2020;8:1574–1578. 10.1002/ccr3.2944
Funding information
The study was funded by the European Union's Horizon 2020 Research and Innovation Program (Project CRADL, grant agreement number 668259) and by the Swiss State Secretariat for education, research and Innovation (SERI) under contract number 15.0342‐1. Dr Kallio was supported by The Finnish Foundation for Pediatric Research. MD Rahtu received a personal research grant from The Alma and KA Snellman Foundation, Oulu, Finland.
REFERENCES
- 1. Rahtu M, Frerichs I, Waldmann AD, et al. Early recognition of pneumothorax in neonatal RDS with electrical impedance tomography. Am J Respir Crit Care Med. 2019;200:1060‐1061. [DOI] [PubMed] [Google Scholar]
- 2. Aly H, Massaro A, Acun C, Ozen M. Pneumothorax in the newborn: clinical presentation, risk factors and outcomes. J Matern Fetal Neonatal Med. 2014;27:402‐406. [DOI] [PubMed] [Google Scholar]
- 3. Alander M, Peltoniemi O, Pokka T, Kontiokari T. Comparison of pressure‐, flow‐, and NAVA‐triggering in pediatric and neonatal ventilatory care. Pediatr Pulmonol. 2012;47:76‐83. [DOI] [PubMed] [Google Scholar]
- 4. Brander L, Moerer O, Hedenstierna G, et al. Neural control of ventilation prevents both over‐distension and de‐recruitment of experimentally injured lungs. Respir Physiol Neurobiol. 2017;237:57‐67. [DOI] [PubMed] [Google Scholar]
- 5. Miedema M, McCall KE, Perkins EJ, et al. First real‐time visualization of a spontaneous pneumothorax developing in a preterm lamb using electrical impedance tomography. Am J Respir Crit Care Med. 2016;194:116‐118. [DOI] [PubMed] [Google Scholar]
- 6. Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438‐442. [DOI] [PubMed] [Google Scholar]


