Summary
Minimally invasive esophagectomy is increasingly performed for the treatment of esophageal cancer, but it is unclear whether hybrid minimally invasive esophagectomy (HMIE) or totally minimally invasive esophagectomy (TMIE) should be preferred. The objective of this study was to perform a meta-analysis of studies comparing HMIE with TMIE. A systematic literature search was performed in MEDLINE, Embase, and the Cochrane Library. Articles comparing HMIE and TMIE were included. The Newcastle–Ottawa scale was used for critical appraisal of methodological quality. The primary outcome was pneumonia. Sensitivity analysis was performed by analyzing outcome for open chest hybrid MIE versus total TMIE and open abdomen MIE versus TMIE separately. Therefore, subgroup analysis was performed for laparoscopy-assisted HMIE versus TMIE, thoracoscopy-assisted HMIE versus TMIE, Ivor Lewis HMIE versus Ivor Lewis TMIE, and McKeown HMIE versus McKeown TMIE. There were no randomized controlled trials. Twenty-nine studies with a total of 3732 patients were included. Studies had a low to moderate risk of bias. In the main analysis, the pooled incidence of pneumonia was 19.0% after HMIE and 9.8% after TMIE which was not significantly different between the groups (RR: 1.46, 95% CI: 0.97–2.20). TMIE was associated with a lower incidence of wound infections (RR: 1.81, 95% CI: 1.13–2.90) and less blood loss (SMD: 0.78, 95% CI: 0.34–1.22) but with longer operative time (SMD:-0.33, 95% CI: −0.59—-0.08). In subgroup analysis, laparoscopy-assisted HMIE was associated with a higher lymph node count than TMIE, and Ivor Lewis HMIE was associated with a lower anastomotic leakage rate than Ivor Lewis TMIE. In general, TMIE was associated with moderately lower morbidity compared to HMIE, but randomized controlled evidence is lacking. The higher leakage rate and lower lymph node count that was found after TMIE in sensitivity analysis indicate that TMIE can also have disadvantages. The findings of this meta-analysis should be considered carefully by surgeons when moving from HMIE to TMIE.
Keywords: esophageal cancer, totally minimally invasive esophagectomy, hybrid minimally invasive esophagectomy
INTRODUCTION
Esophagectomy is the cornerstone for curative treatment of esophageal cancer. Open esophagectomy is increasingly being replaced by minimally invasive esophagectomy (MIE). Currently it is estimated that nearly 45% of patients are operated using a minimally invasive approach worldwide.1 MIE can be performed by hybrid minimally invasive esophagectomy (HMIE, laparotomy and thoracoscopy or laparoscopy and thoracotomy) or totally minimally invasive esophagectomy (TMIE, laparoscopy and thoracoscopy). In the Western world, laparoscopy-assisted HMIE is increasingly replaced by TMIE, in an attempt to further decrease postoperative morbidity without compromising patients’ safety.1
Systematic reviews of retrospective studies comparing the results of open esophagectomy to TMIE and open esophagectomy to HMIE have found that both HMIE and TMIE have advantages over the open approach in terms of blood loss, length of stay, and pulmonary complications.2,3 In addition, these positive effects of MIE have been shown in a randomized controlled trial for HMIE4 and for TMIE.5 Because these beneficial effects seem to be comparable between HMIE and TMIE in these randomized controlled trials, HMIE and TMIE are currently considered to be surgical techniques with equivalently beneficial outcomes. However, no randomized controlled trials have compared HMIE and TMIE and no meta-analysis comparing HMIE and TMIE have been performed.
Therefore, the aim of this article was to perform a systematic review and meta-analysis of studies comparing HMIE with TMIE in patients undergoing esophagectomy.
MATERIALS AND METHODS
Literature search
The review protocol is registered in the PROSPERO international prospective register of systematic reviews (number CRD 42016043291).6 PRISMA guidelines for systematic reviews were followed, and the PRISMA checklist is available in online Appendix I.7
The electronic databases of MEDLINE, Embase, and the Cochrane central register of controlled trials were systematically searched. The search strategy was composed in collaboration with a medical librarian, and the exact (MEDLINE) search strategy was (minimal invasive[tiab] OR minimally invasive[tiab] OR laparo-thoracoscop*[tiab] OR laparothoracoscop*[tiab] OR thoracolaparoscop*[tiab] OR thoraco-laparoscop*[tiab] OR laparoscop*[tiab] OR hybrid[tiab] OR VATS[tiab] OR video-assisted[tiab] OR video assisted[tiab] OR thoracoscop*[tiab]) AND (esophagectom*[tiab] OR oesophagectom*[tiab] OR (resection*[tiab] AND (oesophagus[tiab] OR oesophageal[tiab] OR oesophagal[tiab] OR esophagus[tiab] OR esophageal[tiab] OR esophagal[tiab]))). A cited reference search and hand search were additionally performed. No language restrictions were applied and all results up to April 2019 were included.
Criteria for selecting studies for this review
Comparative cohort studies or randomized controlled trials comparing patients undergoing HMIE versus TMIE were included. We suspected that articles on ‘outcome after MIE’ could contain data on both HMIE and TMIE without this being explicitly described in the abstract. Therefore, we liberally included abstracts that contained outcome data after any form of MIE for full text screening.
Exclusion criteria were less than 10 patients per treatment arm and unclear description of operative technique rendering classification into HMIE or TMIE impossible. Studies that incorporated results of a transhiatal approach in the TMIE group were also excluded, because transhiatal resection cannot be performed as a hybrid procedure and inclusion would therefore be a source of selection bias. Video-assisted thoracic surgery (VATS) procedures and hand-assisted laparoscopic surgery (HALS) procedures were classified as minimally invasive and were also included.
Articles were selected for inclusion using a three-step review process. First, the titles and abstracts of all identified studies were examined by three reviewers (FvW, BK, and NB) independently, and studies that failed to meet the inclusion criteria were excluded. Second, reviewers (FvW, BK, and NB) independently examined the full text of potentially relevant articles. In the event of disagreement regarding the eligibility of a study during this phase, the opinion of a fourth reviewer (CR) was sought, and the parameters of the study’s inclusion were discussed until consensus was reached. Third, all articles cited in and cited by the remaining eligible and relevant articles were independently assessed for inclusion.
Quality assessment
The Newcastle–Ottawa quality assessment scale was used to assess bias in studies included in this review.8 This scale rates studies on three sources of bias based on eight criteria. Each criterion is worth one star except confounding, which is worth two stars. For this systematic review, studies scoring seven to nine stars were considered to be of high methodological quality, studies scoring four to six stars were considered to be of moderate methodological quality, and studies scoring one to three stars were considered to be of low methodological quality.
Outcome parameters and data extraction
The primary outcome parameter was pneumonia. Secondary outcome parameters were all complications, severe complications (Clavien–Dindo>2),9 pulmonary complications, anastomotic leakage, chyle leakage, RLN palsy, wound infection, reoperation, hospital length of stay, ICU length of stay, postoperative mortality, operating time, blood loss, R0 resection rate, number of lymph nodes, and quality of life. Data was extracted and was entered into Review Manager (version 5.3).
In case continuous variables were expressed as median and interquartile range or range, the mean and SD were estimated from the available data by methods described elsewhere.10,11
Analysis
Since studies were homogeneous enough to pool, meta-analyses were performed, and statistical heterogeneity was assessed. The Mantel–Haenszel method was used for dichotomous data, presented as relative risks (RR) with 95% confidence intervals (CIs). The inverse variance method was used for meta-analysis of continuous data; results are presented as standardized mean difference (SMD) with 95% CIs. A random effects model was used for all analyses. The statistical heterogeneity was assessed with I2. A funnel plot with the effect measures on the x-axis and standard error of the log for the effect measures on the y-axis was created for the primary outcome parameter in order to assess publication bias.
In addition to comparing all articles reporting on outcome of patients undergoing HMIE versus TMIE, subgroup and sensitivity analyses were performed for (i) laparoscopy-assisted HMIE (minimally invasive abdominal phase and open thoracic phase) versus TMIE; (ii) thoracoscopy-assisted HMIE (minimally invasive thoracic phase and open abdominal phase) versus TMIE; (iii) Ivor Lewis HMIE versus Ivor Lewis TMIE; and (iv) McKeown HMIE versus McKeown TMIE. For the Ivor Lewis HMIE group, we decided to only include the Ivor Lewis laparoscopy-assisted HMIE (therefore excluding one study that compared Ivor Lewis thoracoscopy-assisted HMIE with Ivor Lewis TMIE), since this reflects the predominant change of practice that is currently taking place in the Western world.
RESULTS
Studies
Twenty-nine studies, including a total of 3,732 patients, met the inclusion criteria of this systematic review.12–40 A summary of the screening and selection process is shown in Figure 1. The individual studies included 29–445 patients. In 14 studies (n = 1,631) laparoscopy-assisted HMIE was compared to TMIE; in 12 (n = 1,522) studies, thoracoscopy-assisted HMIE was compared to TMIE and 3 studies (n = 579) included both laparoscopy-assisted HMIE and thoracoscopy-assisted HMIE in the HMIE arm. Seven studies (n = 723) compared Ivor Lewis laparoscopy-assisted HMIE with Ivor Lewis TMIE, 15 studies (n = 2142) compared McKeown HMIE versus McKeown TMIE, and 7 studies (n = 867) used different or multiple surgical techniques of HMIE or TMIE and were therefore ineligible for subgroup analysis. These and other characteristics of the included studies are summarized in Table 1.
Fig. 1.
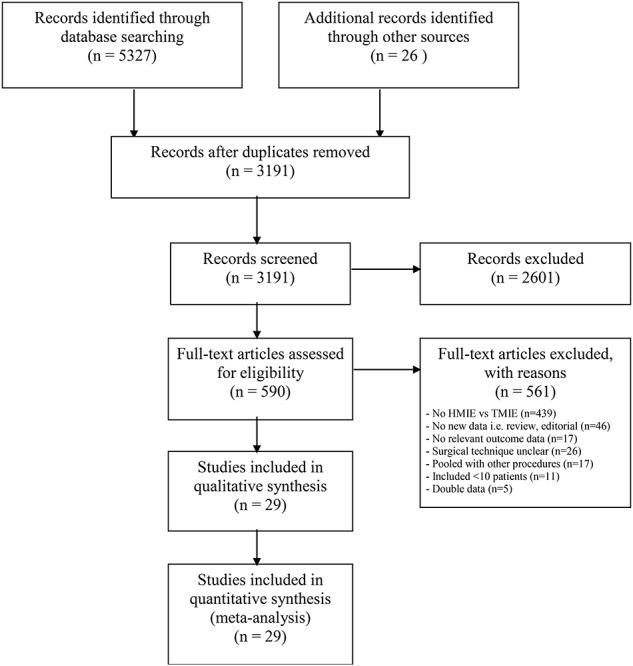
Summary of screening and selection process—PRISMA diagram.
Table 1.
Characteristics of included studies
| Study | Study design | N | Type of HMIO | Type of TMIO | Surgery type HMIO | Surgery type TMIO | Outcome parameters |
|---|---|---|---|---|---|---|---|
| Berlth 2018 | Retrospective cohort | 60 | LA; laparoscopy | Laparoscopy and thoracoscopy | Ivor Lewis | Ivor Lewis | Pneumonia, pulm complications, AL, severe compl, all compl, RLN palsy, mortality, R0, WI, ICU LOS, hosp LOS, LN, OT, blood loss |
| Bizekis 2006 | Retrospective cohort | 50 | LA; laparoscopy | Laparoscopy and thoracoscopy | Ivor Lewis | Ivor Lewis | Pneumonia, pulm compl, AL, chyle leak, mortality |
| Blazeby 2011 | Prospective cohort | 124 | LA; laparoscopy | Laparoscopy and thoracoscopy | Ivor Lewis | Ivor Lewis & McKeown | Severe compl, reoperation, mortality, hosp LOS, LN, OT, blood loss |
| Bonavina 2016 | Retrospective cohort, PSMA | 160 | LA; laparoscopy | Laparoscopy and thoracoscopy | Ivor Lewis | McKeown | Severe compl, pulm compl, AL, reoperation, chyle leak, RLN palsy, mortality, R0, WI, ICU LOS, hosp LOS, LN, OT, blood loss |
| Daiko 2015 | Cohort (not specified) | 64 | TA; thoracoscopy | Laparoscopy and thoracoscopy | McKeown | McKeown | All compl, pneumonia, pulm compl, AL, chyle leak, RLN palsy, mortality, R0, WI, hospital LOS, LN, OT, blood loss. |
| Elshaer 2017 | Prospective cohort | 26 | LA; laparoscopy | Laparoscopy and thoracoscopy | Ivor Lewis | Ivor Lewis | AL, chyle leak, mortality, hosp LOS, LN, OT. |
| Findlay 2017 | Prospective cohort | 162 | LA; laparoscopy | Laparoscopy and thoracoscopy | Ivor Lewis | McKeown | AL, mortality, hosp LOS, LN. |
| Fumagalli 2019 | Prospective cohort | 349 | LA; laparoscopy | Laparoscopy and thoracoscopy | Ivor Lewis | Ivor Lewis | AL. |
| Grimminger 2018 | Prospective cohort | 50 | LA; laparoscopy | Laparoscopy and thoracoscopy | Ivor Lewis | Ivor Lewis | Pneumonia, AL, reoperation, chyle leakage, mortality, R0, WI, ICU LOS, hosp LOS, LN, OT |
| Hamouda 2010 | Prospective cohort | 51 | LA; laparoscopy | Laparoscopy and VATS | Ivor Lewis | Ivor Lewis | Pulm compl, AL, reoperation, chyle leak, R0 |
| Ichikawa 2013 | Prospective cohort | 315 | TA; thoracoscopy | HALS and thoracoscopy | McKeown | McKeown | All compl, pulm compl, AL, chyle leak, RLN palsy, mortality, R0, ICU LOS, LN, OT, blood loss |
| Kinjo 2012 | Cohort (not specified) | 106 | TA; thoracoscopy | HALS or laparoscopy and thoracoscopy | McKeown | McKeown | All compl, pneumonia, pulm compl, AL, reoperation, chyle leak, RLN palsy, mortality, R0, WI, ICU LOS, hosp LOS, blood loss |
| Kitagawa 2016 | Retrospective cohort | 105 | LA, laparoscopy | Laparoscopy and thoracoscopy | McKeown | McKeown | Pneumonia, AL, RLN palsy, mortality, WI, ICU LOS, hosp LOS, LN, blood loss. |
| Kubo 2014 | Cohort (not specified) | 135 | LA; HALS | HALS and VATS | McKeown | McKeown | All compl, pneumonia, pulm compl, AL, chyle leak, RLN palsy, mortality, ICU LOS, hosp LOS, OT, blood loss |
| Lee 2011 | Prospective cohort | 74 | TA; VATS | HALS and VATS | McKeown | McKeown | Pulm compl, AL, mortality, ICU LOS, hosp LOS, LN, OT, blood loss |
| Lee 2015 | Cohort (not specified) | 98 | TA; VATS | Laparoscopy and VATS | Ivor Lewis | Ivor Lewis | Pneumonia, pulm compl, AL, mortality, hosp LOS, LN, OT, blood loss |
| Li 2018 | Retrospective cohort | 172 | TA; thoracoscopy | Laparoscopy and thoracoscopy | McKeown | McKeown | Pneumonia, pulm compl, AL, chyle leak, RLN palsy, WI, hosp LOS, LN, OT, blood loss |
| Mao 2015 | Retrospective cohort | 59 | LA and TA; laparoscopy and thoracoscopy | Laparoscopy and thoracoscopy | McKeown | McKeown | AL, mortality |
| Martin 2005 | Prospective cohort | 36 | TA; thoracoscopy | HALS and thoracoscopy | McKeown | McKeown | OT. |
| Mu 2015 | Retrospective cohort | 445 | LA and TA; laparoscopy & thoracoscopy | Laparoscopy and thoracoscopy | McKeown | McKeown | All compl, pulm compl, AL, mortality, R0, hosp LOS, LN, OT, blood loss |
| Nilsson 2017 | Cohort (not specified) | 173 | LA; laparoscopy | Laparoscopy and thoracoscopy | Ivor Lewis | Ivor Lewis and McKeown | Pulm compl, AL, severe compl |
| Nozaki 2017 | Prospective cohort | 101 | TA; thoracoscopy | HALS/laparoscopy and thoracoscopy | Ivor Lewis and McKeown (94% McKeown) | Ivor Lewis and McKeown (94% McKeown) | Pneumonia, pulm compl, AL, RLN palsy, mortality, hosp LOS, LN, OT, blood loss |
| Oshikiri 2016 | Cohort (not specified) | 64 | TA, thoracoscopy | HALS and thoracoscopy | McKeown | McKeown | Pneumonia, AL, RLN palsy, mortality, hosp LOS, OT, blood loss |
| Safranek 2010 | Prospective cohort | 75 | LA & TA; laparoscopy and thoracoscopy | Laparoscopy and thoracoscopy | McKeown | McKeown | Pneumonia AL, reoperation, RLN palsy, mortality, R0, ICU LOS, hosp LOS, LN, OT |
| Smithers 2007 | Prospective cohort | 332 | TA; thoracoscopy | Laparoscopy and thoracoscopy | McKeown | McKeown | All compl, pneumonia, pulm compl, AL, chyle leak, RLN palsy, mortality, R0, ICU LOS, hosp LOS, LN, OT, blood loss |
| Souche 2019 | Prospective cohort | 137 | LA; laparoscopy | Laparoscopy and thoracoscopy | Ivor Lewis | Ivor Lewis | Pneurmonia, pulm compl, AL, severe compl, reoperation, all compl, RLN palsy, mortality, R0, WI, hosp LOS, LN, OT, blood loss |
| Tsujimoto 2012 | Retrospective cohort | 49 | LA; laparoscopy | Laparoscopy and thoracoscopy | Ivor Lewis | Ivor Lewis & McKeown | All compl, pulm compl, AL, chyle leak, RLN palsy, mortality, WI, ICU LOS, hosp LOS, OT, blood loss |
| Yanasoot 2017 | Cohort (not specified) | 29 | TA; thoracoscopy | Laparoscopy and thoracoscopy | McKeown | McKeown | Pneumonia, AL, RLN palsy, mortality, WI, ICU LOS, Hosp LOS, OT, blood loss |
| Yao 2017 | Prospective cohort | 131 | TA; thoracoscopy | Laparoscopy and thoracoscopy | McKeown | McKeown | Pulm compl, AL, chyle leak, RLN palsy, mortality, R0, WI, hosp LOS, LN, OT, blood loss |
TMIE, totally minimally invasive esophagectomy; HMIE, hybrid minimally invasive esophagectomy; LA, laparoscopy assisted (thus: minimally invasive abdominal and open thoracic stage); TA, thoracoscopy assisted (thus: minimally invasive thoracic and open abdominal stage); PSMA, propensity score matched analysis; VATS, video-assisted thoracic surgery; HALS, hand-assisted laparoscopic surgery; compl, complications; pulm compl, pulmonary complications; AL, anastomotic leakage; RLN, recurrent laryngeal nerve; R0, R0 resection rate; WI, wound infection; ICU, intensive care unit; LOS, length of stay; LN, lymph nodes examined; OT, operating time
Quality and publication bias assessment
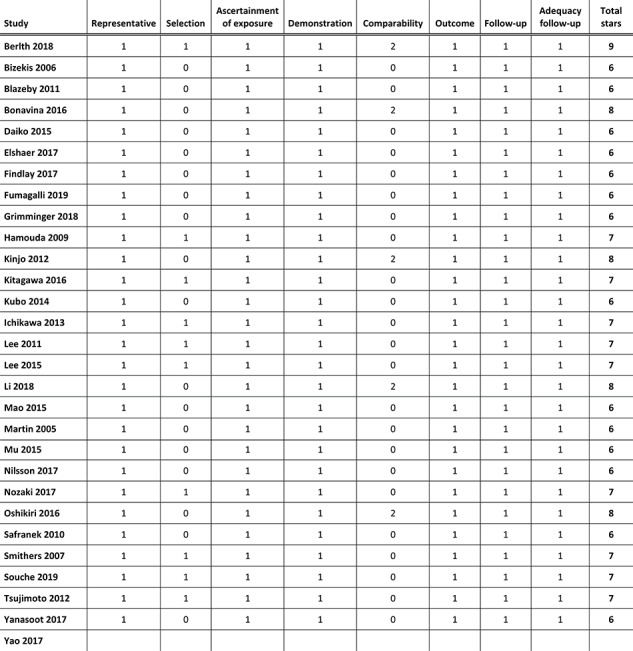
There were no randomized controlled trials. Studies scored six to nine stars out of nine according to the Newcastle–Ottawa rating scale, corresponding to a moderate to low risk of bias for non-randomized studies. The results of the quality assessment of the included studies are shown in online Appendix II.
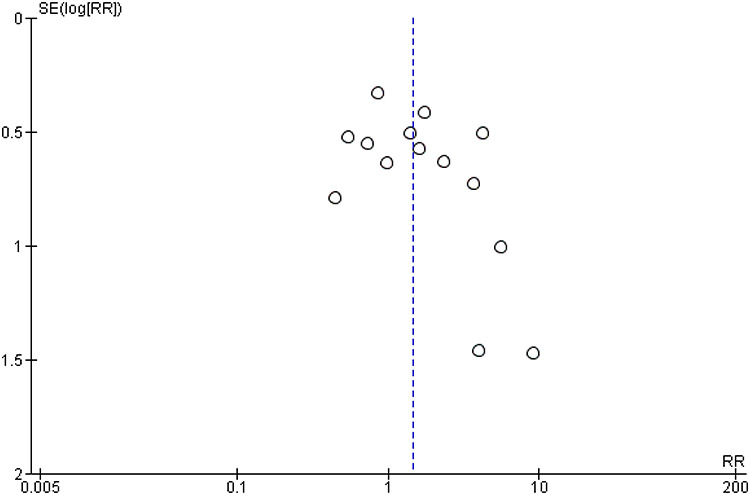
By observation of the funnel plot for the primary outcome parameter in the main analysis, we concluded that publication bias may have been present because there appears to be a gap in the lower left quadrant of the funnel plot. However, the limited number of studies (n = 15) that reported the primary outcome parameter limits reliability of the plot [Online Appendix III].
Meta-analysis of all included studies comparing all HMIE with TMIE
A total of 15 studies including 1,492 patients reported the incidence of the primary outcome parameter. The pooled incidence of pneumonia was 19.0% after HMIE and 9.8% after TMIE which was not significantly different between the groups (RR: 1.46, 95% CI: 0.97–2.20). In a post hoc sensitivity analysis in which we excluded studies that included patients with HALS or VATS, these results remained similar (RR: 1.26, 95% CI: 0.85–1.89). Compared to HMIE, TMIE was associated with a lower incidence of wound infections (RR: 1.81, 95% CI: 1.13–2.90) and less blood loss (SMD: 0.78, 95% CI: 0.34–1.22) but with a longer operative time (SMD: -0.33, 95% CI: −0.59—-0.08) (Appendix IV-a). The other parameters were not statistically different between the groups (Table 2).
Table 2.
All hybrid minimally invasive esophagectomy versus totally minimally invasive esophagectomy
| No of studies | No of patients | RR/SMD (95% CI) | I 2 (%) | |
|---|---|---|---|---|
| Pneumonia (RR) | 15 | 1492 | 1.46 (0.97–2.20) | 39 |
| Pulmonary complications (RR) | 18 | 2653 | 1.24 (0.97–1.58) | 31 |
| Anastomotic leakage (RR) | 27 | 3572 | 0.94 (0.73–1.21) | 32 |
| Chyle leakage (RR) | 13 | 1641 | 1.13 (0.62–2.04) | 0 |
| RLN palsy (RR) | 16 | 2035 | 0.90 (0.65–1.25) | 22 |
| Wound infection (RR) | 11 | 1003 | 1.81 (1.13–2.90) | 0 |
| Severe complications (RR) | 5 | 654 | 0.95 (0.72–1.25) | 24 |
| All complications (RR) | 9 | 1643 | 1.10 (0.99–1.23) | 0 |
| Reoperation (RR) | 7 | 703 | 0.86 (0.51–1.46) | 0 |
| Postoperative mortality (RR) | 24 | 2951 | 1.33 (0.73–2.41) | 0 |
| Irradical resection (RR) | 13 | 2066 | 1.22 (0.93–1.60) | 0 |
| Intensive care LOS (SMD) | 12 | 1490 | 0.19 (0.00–0.38) | 59 |
| Hospital LOS (SMD) | 23 | 2699 | 0.19 (0.00–0.39) | 79 |
| Extracted lymph nodes (SMD) | 19 | 2630 | −0.01 (−0.24–0.22) | 85 |
| Operating time (SMD) | 23 | 2782 | −0.33 (−0.59–−0.08) | 88 |
| Blood loss (SMD) | 71 | 2701 | 0.78 (0.34–1.22) | 96 |
RR, relative risk; SMD, standardized mean difference; CI, confidence interval. For dichotomous parameters, RR > 1 favors TMIE and RR < 1 favors HMIE. For continuous parameters, SMD >0 favors TMIE and SMD <0 favors HMIE, except for the parameter ‘Extracted lymph nodes’, in which SMD >0 favors HMIE and SMD <0 favors TMIE
Subgroup analyses per HMIE type
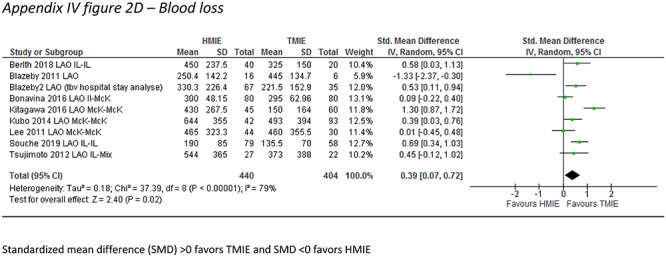
In the laparoscopy-assisted HMIE versus TMIE subgroup, the incidence of pneumonia was described by 6 studies which included 451 patients. The incidence of pneumonia was 17.1% after laparoscopy-assisted HMIE and 8.5% after TMIE (RR: 1.68, 95% CI: 1.03–3.37). In addition, TMIE was associated with less blood loss (SMD: 0.39, 95% CI: 0.07–0.72) but with longer operative times (SMD: −0.50, 95%CI: −0.74–−0.25) and less extracted lymph nodes (SMD 0.29, 95%CI: 0.29–0.49) (Table 3) (Appendix IV-b).
Table 3.
Laparoscopy-assisted hybrid minimally invasive esophagectomy versus totally minimally invasive esophagectomy
| No of studies | No of patients | RR/SMD (95% CI) | I 2 (%) | |
|---|---|---|---|---|
| Pneumonia (RR) | 6 | 451 | 1.86 (1.03–3.37) | 9 |
| Pulmonary complications (RR) | 9 | 889 | 1.15 (0.78–1.71) | 44 |
| Anastomotic leakage (RR) | 14 | 1581 | 0.79 (0.57–1.11) | 30 |
| Chyle leakage (RR) | 5 | 521 | 1.10 (0.48–2.53) | 0 |
| RLN palsy (RR) | 6 | 646 | 0.68 (0.35–1.35) | 23 |
| Wound infection (RR) | 5 | 501 | 1.69 (0.96–2.96) | 0 |
| Severe complications (RR) | 5 | 654 | 0.95 (0.72–1.25) | 24 |
| All complications (RR) | 4 | 381 | 1.00 (0.82–1.22) | 0 |
| Reoperation (RR) | 5 | 522 | 0.79 (0.43–1.46) | 0 |
| Postoperative mortality (RR) | 12 | 1132 | 1.28 (0.61–2.67) | 0 |
| Irradical resection (RR) | 6 | 620 | 1.44 (0.91–2.29) | 0 |
| Intensive care LOS (SMD) | 7 | 633 | 0.28 (−0.06–0.61) | 75 |
| Hospital LOS (SMD) | 12 | 1082 | 0.16 (−0.08–0.39) | 69 |
| Extracted lymph nodes (SMD) | 10 | 898 | 0.29 (0.10–0.49) | 47 |
| Operating time (SMD) | 11 | 920 | −0.50 (−0.74–−0.25) | 65 |
| Blood loss (SMD) | 9 | 844 | 0.39 (0.07–0.72) | 79 |
RR, relative risk; SMD, standardized mean difference; CI, confidence interval. For dichotomous parameters, RR > 1 favors TMIE and RR < 1 favors HMIE. For continuous parameters, SMD >0 favors TMIE and SMD <0 favors HMIE, except for the parameter ‘Extracted lymph nodes’, in which SMD >0 favors HMIE and SMD <0 favors TMIE
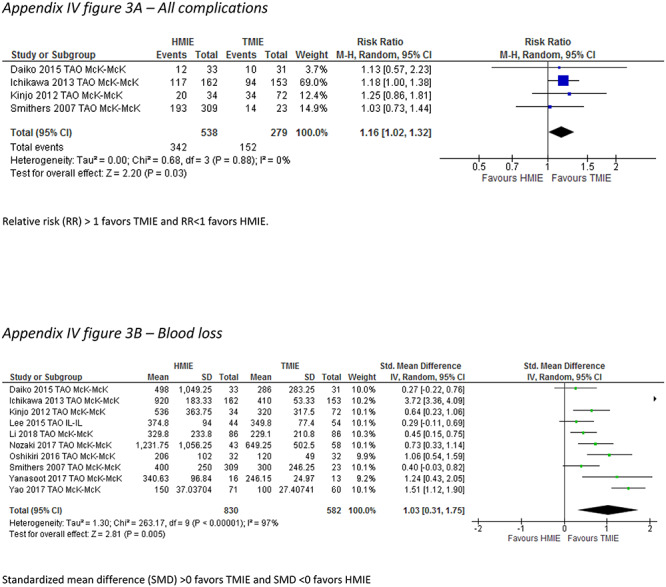
In the thoracoscopy-assisted HMIE versus TMIE subgroup, the incidence of pneumonia was reported by 8 studies which included 966 patients. The incidence of pneumonia did not differ between the groups (RR: 1.24, 95% CI: 0.66–2.34). The overall complication rate was lower after TMIE compared to thoracoscopy-assisted HMIE (RR: 1.16, 95% CI: 1.02–1.32), and there was less blood loss after TMIE compared to thoracoscopy-assisted HMIE (SMD 1.03, 95%CI: 0.31–1.75) (Table 4) (Appendix IV-c).
Table 4.
Thoracoscopy-assisted hybrid minimally invasive esophagectomy versus totally minimally invasive esophagectomy
| No of studies | No of patients | RR/SMD (95% CI) | I 2 (%) | |
|---|---|---|---|---|
| Pneumonia (RR) | 8 | 966 | 1.24 (0.66–2.34) | 57 |
| Pulmonary complications (RR) | 8 | 1319 | 1.33 (0.95–1.86) | 30 |
| Anastomotic leakage (RR) | 10 | 1412 | 1.28 (0.81–2.03) | 29 |
| Chyle leakage (RR) | 6 | 1120 | 1.16 (0.50–2.69) | 0 |
| RLN palsy (RR) | 9 | 1314 | 1.16 (0.92–1.45) | 0 |
| Wound infection (RR) | 5 | 502 | 2.13 (0.88–5.14) | 0 |
| Severe complications (RR) | 0 | 0 | N/A | N/A |
| All complications (RR) | 4 | 817 | 1.16 (1.02–1.32) | 0 |
| Reoperation (RR) | 1 | 106 | 3.18 (0.56–18.14) | N/A |
| Postoperative mortality (RR) | 9 | 1240 | 1.34 (0.36–5.08) | 12 |
| Irradical resection (RR) | 5 | 926 | 0.90 (0.57–1.42) | 0 |
| Intensive care LOS (SMD) | 4 | 782 | 0.17 (0.00–0.34) | 0 |
| Hospital LOS (SMD) | 9 | 1097 | 0.31 (−0.12–0.74) | 88 |
| Extracted lymph nodes (SMD) | 7 | 1212 | −0.37 (−0.81–0.07) | 91 |
| Operating time (SMD) | 10 | 1342 | 0.21 (−0.65–0.23) | 91 |
| Blood loss (SMD) | 10 | 1412 | 1.03 (0.31–1.75) | 97 |
RR, relative risk; SMD, standardized mean difference; CI, confidence interval. For dichotomous parameters, RR > 1 favors TMIE and RR < 1 favors HMIE. For continuous parameters, SMD >0 favors TMIE and SMD <0 favors HMIE, except for the parameter ‘Extracted lymph nodes’, in which SMD >0 favors HMIE and SMD <0 favors TMIE
Subgroup analyses per resection type
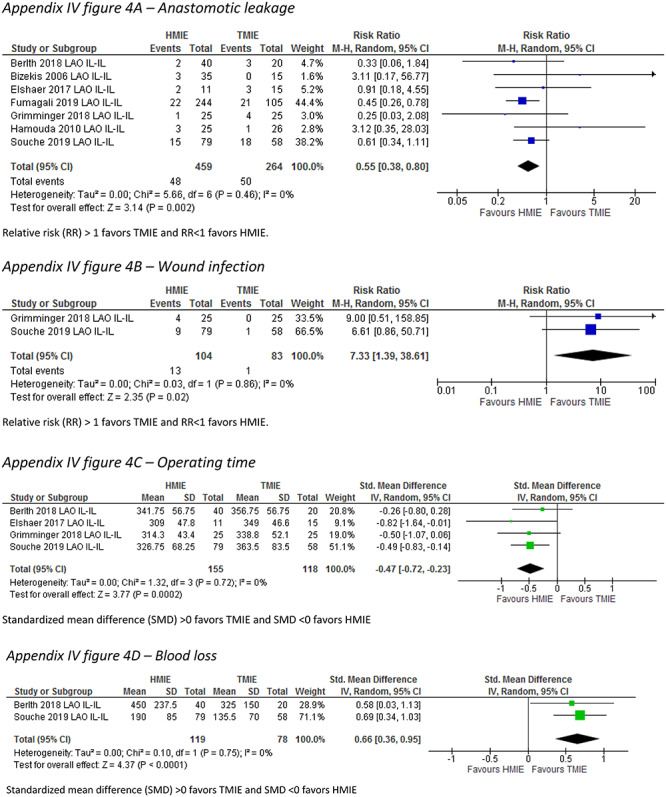
In the Ivor Lewis HMIE versus TMIE subgroup, the incidence of pneumonia was described by four studies (n = 297) and was not statistically different between the groups (RR: 1.83, 95% CI: 0.71–4.71). Compared to Ivor Lewis HMIE, Ivor Lewis TMIE was associated with a lower incidence of wound infections (RR: 7.33, 95% CI: 1.39–38.61) and less blood loss (SMD: 0.66, 95% CI: 0.36–0.95), but with a longer operative time (SMD: −0.47, 95% CI: −0.72–−0.0.23). Anastomotic leakage was reported in seven studies (n = 723), and the pooled incidence was 10.0% after Ivor Lewis HMIE compared to 18.9% after Ivor Lewis TMIE (RR: 0.55, 95% CI: 0.38–0.80) (Table 5) (Appendix IV-d).
Table 5.
Laparoscopy-assisted hybrid minimally invasive Ivor Lewis esophagectomy versus totally minimally invasive Ivor Lewis esophagectomy
| No of studies | No of patients | RR/SMD (95% CI) | I 2 (%) | |
|---|---|---|---|---|
| Pneumonia (RR) | 4 | 297 | 1.83 (0.71–4.71) | 32 |
| Pulmonary complications (RR) | 4 | 298 | 1.45 (0.98–2.15) | 4 |
| Anastomotic leakage (RR) | 7 | 723 | 0.55 (0.38–0.80) | 0 |
| Chyle leakage (RR) | 4 | 177 | 1.05 (0.21–5.28) | 0 |
| RLN palsy (RR) | 2 | 197 | 4.18 (0.52–33.57) | 0 |
| Wound infection (RR) | 2 | 187 | 7.33 (1.39–38.61) | 0 |
| Severe complications (RR) | 2 | 197 | 0.85 (0.57–1.27) | 0 |
| All complications (RR) | 2 | 197 | 1.02 (0.79–1.32) | 0 |
| Reoperation (RR) | 3 | 238 | 2.21 (0.44–11.06 | 0 |
| Postoperative mortality (RR) | 5 | 323 | 0.85 (0.17–4.19) | 0 |
| Irradical resection (RR) | 4 | 298 | 1.63 (0.39–6.73) | 0 |
| Intensive care LOS (SMD) | 2 | 110 | 0.45 (−0.77–1.67) | 89 |
| Hospital LOS (SMD) | 4 | 273 | −0.05 (−0.31–0.21) | 9 |
| Extracted lymph nodes (SMD) | 4 | 273 | 0.17 (−0.09–0.42) | 6 |
| Operating time (SMD) | 4 | 273 | −0.47 (−0.72–-0.23) | 0 |
| Blood loss (SMD) | 2 | 197 | 0.66 (0.36–0.95 | 0 |
RR, relative risk; SMD, standardized mean difference; CI, confidence interval. For dichotomous parameters, RR > 1 favors TMIE and RR < 1 favors HMIE. For continuous parameters, SMD >0 favors TMIE and SMD <0 favors HMIE, except for the parameter ‘Extracted lymph nodes’, in which SMD >0 favors HMIE and SMD <0 favors TMIE
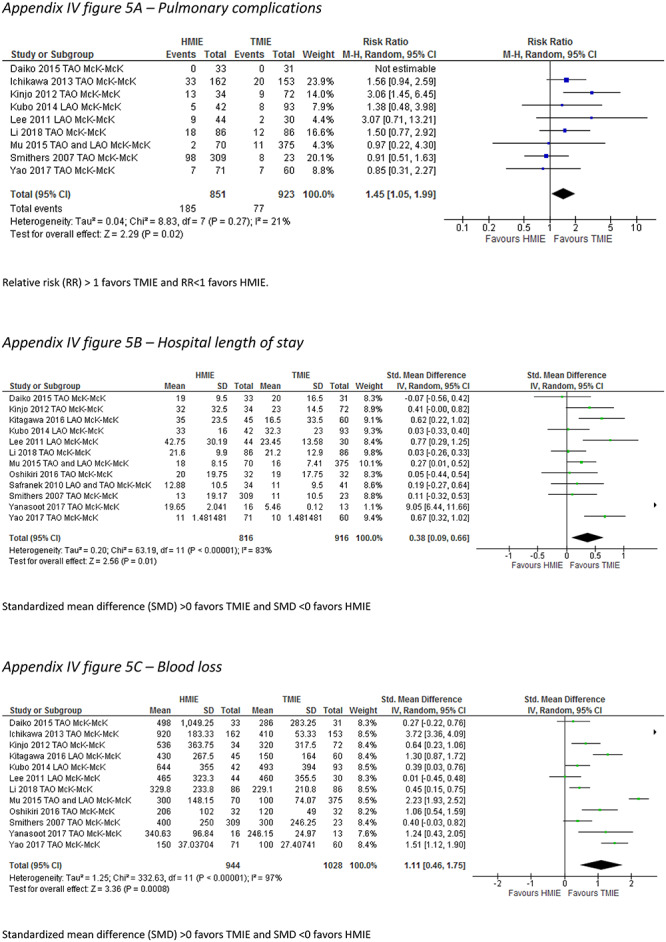
In the McKeown HMIE versus McKeown TMIE subgroup, the incidence of pneumonia was reported by 8 studies which included 947 patients. The incidence of pneumonia did not differ between the groups (RR: 1.45, 95% CI: 0.84–2.54). Compared to McKeown HMIE, McKeown TMIE was associated with a lower incidence of pulmonary complications (RR: 1.45, 95% CI: 1.05–1.99), less blood loss (SMD: 1.11, 95% CI: 0.46–1.75), and a shorter hospital length of stay (SMD: 0.38, 95% CI: 0.09–0.66) (Table 6) (Appendix IV-e).
Table 6.
Hybrid minimally invasive McKeown esophagectomy versus totally minimally invasive McKeown esophagectomy
| No of studies | No of patients | RR/SMD (95% CI) | I 2 (%) | |
|---|---|---|---|---|
| Pneumonia (RR) | 8 | 947 | 1.46 (0.84–2.54) | 52 |
| Pulmonary complications (RR) | 9 | 1774 | 1.45 (1.05–1.99) | 21 |
| Anastomotic leakage (RR) | 14 | 2106 | 1.26 (0.93–1.72) | 19 |
| Chyle leakage (RR) | 7 | 1255 | 1.14 (0.58–2.25) | 0 |
| RLN palsy (RR) | 11 | 1528 | 0.82 (0.56–1.22) | 36 |
| Wound infection (RR) | 6 | 607 | 1.65 (0.98–2.78) | 0 |
| Severe complications (RR) | 0 | 0 | N/A | N/A |
| All complications (RR) | 6 | 1397 | 1.13 (1.00–1.27) | 0 |
| Reoperation (RR) | 2 | 181 | 1.25 (0.25–6.28) | 55 |
| Postoperative mortality (RR) | 13 | 1934 | 1.74 (0.68–4.48) | 0 |
| Irradical resection (RR) | 7 | 1446 | 1.12 (0.81–1.57) | 0 |
| Intensive care LOS (SMD) | 8 | 1171 | 0.12 (−0.02–0.26) | 7 |
| Hospital LOS (SMD) | 12 | 1732 | 0.38 (0.09–0.66) | 83 |
| Extracted lymph nodes (SMD) | 9 | 1712 | −0.18 (−0.46–0.10) | 83 |
| Operating time (SMD) | 13 | 1977 | −0.26 (−0.62–0.10) | 91 |
| Blood loss (SMD) | 12 | 1972 | 1.11 (0.46–1.75) | 83 |
RR, relative risk; SMD, standardized mean difference; CI, confidence interval. For dichotomous parameters, RR > 1 favors TMIE and RR < 1 favors HMIE. For continuous parameters, SMD >0 favors TMIE and SMD <0 favors HMIE, except for the parameter ‘Extracted lymph nodes’, in which SMD >0 favors HMIE and SMD <0 favors TMIE
DISCUSSION
This meta-analysis showed that a clinically relevant difference in the incidence of pneumonia between HMIE and TMIE might exist, but we were unable to demonstrate this since this difference did not reach statistical significance. Interestingly, in the subgroup analysis in which different types of HMIE were compared to TMIE, the incidence of pneumonia was lower after TMIE when it was compared with laparoscopy-assisted HMIE but not when it was compared with thoracoscopy-assisted HMIE. Although laparoscopy-assisted HMIE has clearly been shown to reduce pulmonary complications,4 this finding may implicate that a further reduction of postoperative pneumonia is possible by moving from laparoscopy-assisted HMIE to TMIE. In general, parameters regarding postoperative morbidity showed moderately improved outcome after TMIE compared to HMIE. However, we additionally found that anastomotic leakage was higher after Ivor Lewis TMIE compared to Ivor Lewis HMIE and that laparoscopy-assisted HMIE was associated with higher numbers of extracted lymph nodes compared to TMIE, and this suggests that TMIE can also have disadvantages regarding clinically important outcome parameters.
The major strength of this systematic review and meta- analysis is that this is the first study that directly compares the effectiveness of HMIE with TMIE. Some possible limitations should also be discussed. First, although statistical heterogeneity was limited, supporting our decision to pool results of the included studies in a meta-analysis, clinical heterogeneity (i.e. variations in surgical technique of HMIE and TMIE) was present. The variations in surgical technique of the included studies reflect the current lack of robust evidence on the optimal surgical technique for resection of esophageal cancer.1 In order to address this, we performed subgroup-and sensitivity analyses for which we included studies that only compared similar types of surgery, and this indeed resulted in lower heterogeneity for most parameters. Additionally, there was heterogeneity in our primary outcome parameter definition across studies. Second, selection bias could not be excluded since TMIE was most frequently implemented after HMIE and compared retrospectively, possibly favoring outcome in the TMIE group. However, the fact that the anastomotic leakage rate was higher after TMIE cannot be explained by this type of selection bias since TMIE cases were generally operated on in later time frames. In addition, TMIE has been described to be associated with a significant learning curve, and this might favor outcome in the HMIE group.41–44 Finally, the Newcastle–Ottawa rating scale was used because high-quality randomized studies were absent. Although this score gives a relevant indication of the quality of non-randomized studies, it generally results in an overestimation of the quality of the included studies, and this should be taken into account when interpreting the results of this study.
Currently, no RCTs have been performed that compared the effectiveness of HMIE versus TMIE, and as far as we are aware, no RCTs are currently being performed on this subject. Although the ROMIO feasibility trial has randomized between open, laparoscopy-assisted HMIE and TMIE, this feasibility study was not designed to identify a difference between HMIE and TMIE,45 and the definitive ROMIO trial does not randomize patients between open esophagectomy and laparoscopy-assisted HMIE.46 Therefore, surgeons will have to rely on non-randomized data when making decisions regarding whether to use HMIE or TMIE for surgical resection of esophageal cancer. The current meta-analysis provides an overview of the best available evidence on differences in outcome of HMIE compared to TMIE. From our data, TMIE was generally associated with a (trend towards) lower postoperative morbidity compared to HMIE. This suggests that TMIE has potential benefits over HMIE regarding morbidity. However, anastomotic leakage was higher after Ivor Lewis TMIE compared to Ivor Lewis HMIE. This may be explained by a surgical learning curve, which has been described to be long for Ivor Lewis TMIE (>100 cases, which can correspond to years of practice),41,43,44 since intrathoracic anastomosis can be difficult to perform safely with minimally invasive techniques. Although no studies have been published that directly compare learning curves of HMIE and TMIE, it is assumed that HMIE is associated with a shorter learning curve and less associated morbidity because it is technically less complex. However, literature also shows that favorable results of TMIE can be achieved after the learning curve has been completed,43,44 but this might not have been the case in most included studies. Another important finding is that laparoscopy-assisted HMIE was associated with higher numbers of extracted lymph nodes compared to TMIE. This suggests that surgeons performing thoracoscopic instead of open thoracic resection performed a more limited lymph node dissection, although other factors (e.g. pathology department related) may have also influenced lymph node count. In general, higher lymph node count is associated with improved survival after esophagectomy, and this is therefore an important finding.47 However, similar rates of extracted lymph nodes after minimally invasive versus open surgery and even higher numbers of extracted lymph nodes after thoracoscopic versus open transthoracic resection have been reported.48,49
Fig. 2.
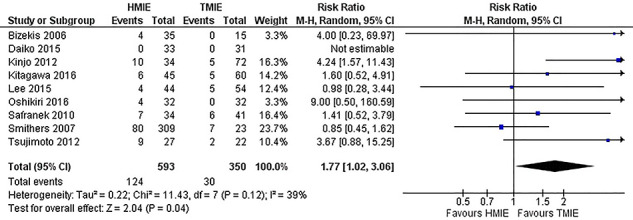
All hybrid minimally invasive esophagectomy (HMIE) versus totally minimally invasive esophagectomy (TMIE) for primary outcome parameter pneumonia.
Clinical implications
Currently, HMIE and TMIE are regarded as equally effective, and safe surgical approaches and both procedures are used to treat patients with esophageal cancer worldwide. In this meta-analysis, a moderate benefit for TMIE regarding morbidity was found. Because outcomes between HMIE and TMIE are only moderately different, the learning curve of HMIE and TMIE procedures and its associated morbidity may also be important arguments in choosing which type of procedure to implement.50 Therefore, surgeons moving from HMIE to TMIE should carefully consider this, since this study showed that TMIE can also have disadvantageous effects and randomized controlled evidence supporting the benefits of TMIE over HMIE is lacking.
CONCLUSIONS
In general, TMIE was associated with moderately lower morbidity compared to HMIE, but randomized controlled evidence is lacking. The higher leakage rate and lower lymph node count that was found after TMIE in sensitivity analysis indicate that TMIE can also have disadvantages. The findings of this meta-analysis should be considered carefully by surgeons when moving from HMIE to TMIE.
Details of contributions
All authors contributed to the design of the work; F. Van Workum and B.R. Klarenbeek and N. Baranov were involved in acquisition of the data. Analysis was performed by F. van Workum, and all other authors were involved in interpretation of the work. F. Van Workum and B.R. Klarenbeek were involved in drafting the manuscript. All other authors were involved in critically revising the manuscript for intellectual content. All authors approve of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
None.
No funds were received in support of this study.
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
APPENDIX I—PRISMA checklist
|
1. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097
APPENDIX II—Risk of bias assessment
APPENDIX III—Funnel plot for primary outcome parameter pneumonia
APPENDIX IV. Forest plots for parameters showing significant differences between hybrid and total MIE groups
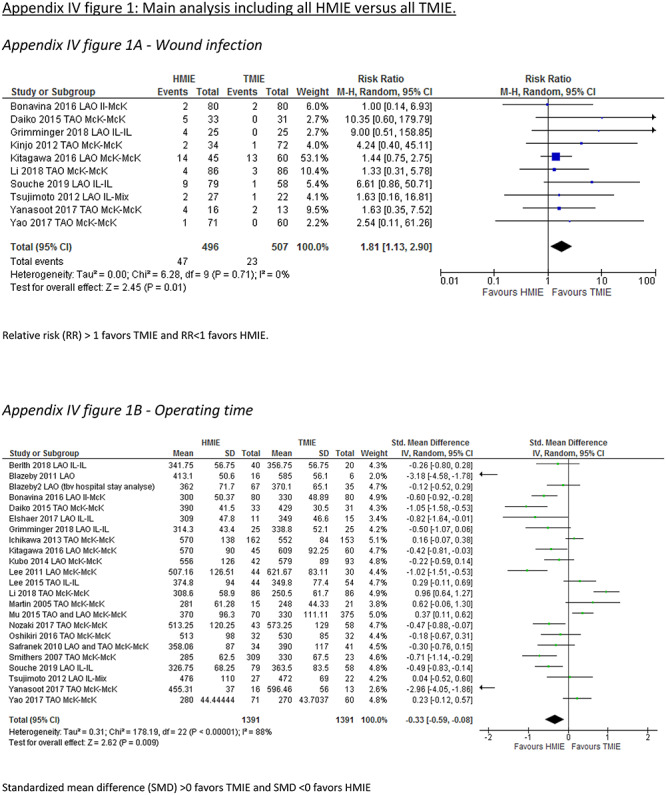
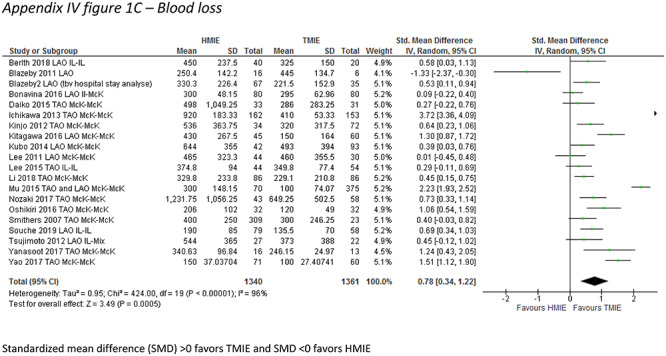
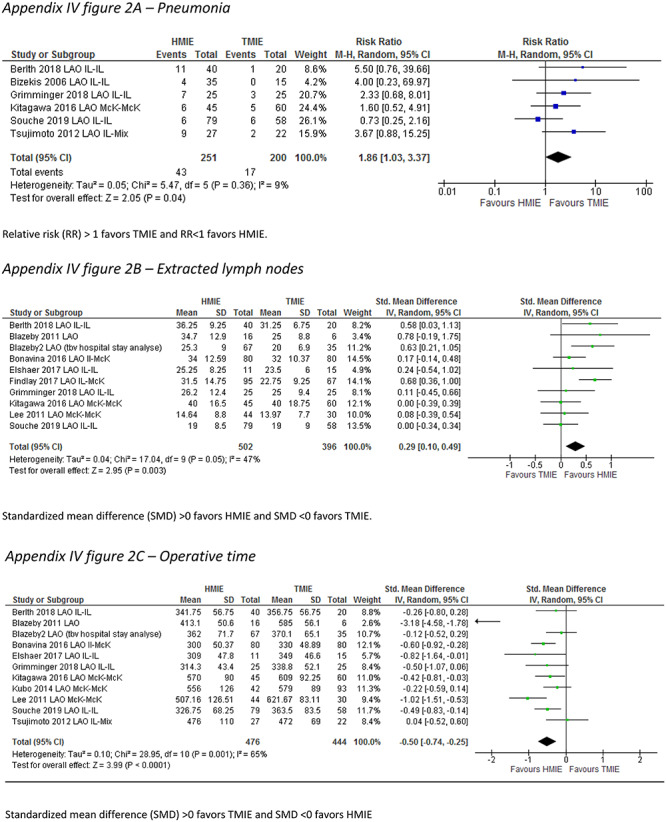
Appendix IV-a: Main analysis including all HMIE versus all TMIE
Wound infection
Relative risk (RR) > 1 favors TMIE and RR < 1 favors HMIE.
Operating time
Standardized mean difference (SMD) >0 favors TMIE and SMD <0 favors HMIE
Blood loss
Standardized mean difference (SMD) >0 favors TMIE and SMD <0 favors HMIE.
Appendix IV-b: Subgroup analysis including laparoscopy-assisted hybrid minimally invasive esophagectomy versus totally minimally invasive esophagectomy
Pneumonia
Relative risk (RR) > 1 favors TMIE and RR < 1 favors HMIE.
Extracted lymph nodes
Standardized mean difference (SMD) >0 favors HMIE and SMD <0 favors TMIE.
Operative time
Standardized mean difference (SMD) >0 favors TMIE and SMD <0 favors HMIE
Blood loss
Standardized mean difference (SMD) >0 favors TMIE and SMD <0 favors HMIE.
Appendix IV-c: Subgroup analysis including thoracoscopy-assisted hybrid minimally invasive esophagectomy versus totally minimally invasive esophagectomy
All complications
Relative risk (RR) > 1 favors TMIE and RR < 1 favors HMIE.
Blood loss
Standardized mean difference (SMD) >0 favors TMIE and SMD <0 favors HMIE.
Appendix IV-d: Subgroup analysis including laparoscopy-assisted hybrid minimally invasive Ivor Lewis esophagectomy versus totally minimally invasive Ivor Lewis esophagectomy
Anastomotic leakage
Relative risk (RR) > 1 favors TMIE and RR < 1 favors HMIE.
Wound infection
Relative risk (RR) > 1 favors TMIE and RR < 1 favors HMIE.
Operating time
Standardized mean difference (SMD) >0 favors TMIE and SMD <0 favors HMIE
Blood loss
Standardized mean difference (SMD) >0 favors TMIE and SMD <0 favors HMIE.
Appendix IV-e: Subgroup analysis including hybrid minimally invasive McKeown esophagectomy versus totally minimally invasive McKeown esophagectomy
Pulmonary complications
Relative risk (RR) > 1 favors TMIE and RR < 1 favors HMIE.
Hospital length of stay
Standardized mean difference (SMD) >0 favors TMIE and SMD <0 favors HMIE
Blood loss
Standardized mean difference (SMD) >0 favors TMIE and SMD <0 favors HMIE.
References
- 1. Haverkamp L, Seesing M F, Ruurda J P et al. . Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017; 30: 1–7. [DOI] [PubMed] [Google Scholar]
- 2. Nagpal K, Ahmed K, Vats A et al. . Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010; 24: 1621–9. [DOI] [PubMed] [Google Scholar]
- 3. Xiong W L, Li R, Lei H K et al. . Comparison of outcomes between minimally invasive oesophagectomy and open oesophagectomy for oesophageal cancer. ANZ J Surg 2017; 87: 165–70. [DOI] [PubMed] [Google Scholar]
- 4. Mariette C, Markar S R, Dabakuyo-Yonli T S et al. . Hybrid minimally invasive Esophagectomy for Esophageal cancer. N Engl J Med 2019; 380: 152–62. doi: 10.1056/NEJMoa1805101. [DOI] [PubMed] [Google Scholar]
- 5. Biere S S, Berge Henegouwen M I, Maas K W et al. . Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012; 379: 1887–92. [DOI] [PubMed] [Google Scholar]
- 6. PROSPERO international prospective register of systematic reviews http://www.crd.york.ac.uk/PROSPERO (accessed May 30th 2017).
- 7. Liberati A, Altman D G, Tetzlaff J et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wells G, Shea B, O’Connell D et al. . The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed July 27th 2017).
- 9. Dindo D, Demartines N, Clavien P A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. http://handbook.cochrane.org (accessed May 30th 2017).
- 11. Hozo S P, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berlth F, Plum P S, Chon S H et al. . Total minimally invasive esophagectomy for esophageal adenocarcinoma reduces postoperative pain and pneumonia compared to hybrid esophagectomy. Surg Endosc 2018; 32: 4957–65. doi: 10.1007/s00464-018-6257-2Epub 2018 Jun 21. [DOI] [PubMed] [Google Scholar]
- 13. Bizekis C, Kent M S, Luketich J D et al. . Initial experience with minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg 20082: 402–6. [DOI] [PubMed] [Google Scholar]
- 14. Blazeby J M, Blencowe N S, Titcomb D R et al. . Demonstration of the IDEAL recommendations for evaluating and reporting surgical innovation in minimally invasive oesophagectomy. Br J Surg 2011; 98: 544–51. [DOI] [PubMed] [Google Scholar]
- 15. Bonavina L, Scolari F, Aiolfi A et al. . Early outcome of thoracoscopic and hybrid esophagectomy: propensity-matched comparative analysis. Surgery 2016; 159: 1073–81. [DOI] [PubMed] [Google Scholar]
- 16. Daiko H, Fujita T. Laparoscopic assisted versus open gastric pull-up following thoracoscopic esophagectomy: a cohort study. Int J Surg 2015; 19: 61–6. [DOI] [PubMed] [Google Scholar]
- 17. Elshaer M, Gravante G, Tang C B, Jayanthi N V. Totally minimally invasive two-stage esophagectomy with intrathoracic hand-sewn anastomosis: short-term clinical and oncological outcomes. Dis Esophagus. 2018; 1: 31. doi: 10.1093/dote/dox150. [DOI] [PubMed] [Google Scholar]
- 18. Findlay L, Yao C, Bennett D H, Byrom R, Davies N. Non-inferiority of minimally invasive oesophagectomy: an 8-year retrospective case series. Surg Endosc. 2017; 31: 3681–9. doi: 10.1007/s00464-016-5406-8Epub 2017 Jan 11. [DOI] [PubMed] [Google Scholar]
- 19. Fumagalli U, Baiocchi G L, Celotti A et al. . Incidence and treatment of mediastinal leakage after esophagectomy: insights from the multicenter study on mediastinal leaks. World J Gastroenterol 2019; 25: 356–66. doi: 10.3748/wjg.v25.i3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grimminger P P, Tagkalos E, Hadzijusufovic E et al. . Change from hybrid to fully minimally invasive and robotic Esophagectomy is possible without compromises. Thorac Cardiovasc Surg. 2018. doi: 10.1055/s-0038-1670664[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Hamouda A H, Forshaw M J, Tsigritis K et al. . Perioperative outcomes after transition from conventional to minimally invasive Ivor-Lewis esophagectomy in a specialized center. Surg Endosc 2010; 24: 865–9. [DOI] [PubMed] [Google Scholar]
- 22. Ichikawa H, Miyata G, Miyazaki S et al. . Esophagectomy using a thoracoscopic approach with an open laparotomic or hand-assisted laparoscopic abdominal stage for esophageal cancer: analysis of survival and prognostic factors in 315 patients. Ann Surg 2013; 257: 873–85. [DOI] [PubMed] [Google Scholar]
- 23. Kinjo Y, Kurita N, Nakamura F et al. . Effectiveness of combined thoracoscopic-laparoscopic esophagectomy: comparison of postoperative complications and midterm oncological outcomes in patients with esophageal cancer. Surg Endosc 2012; 26: 381–90. [DOI] [PubMed] [Google Scholar]
- 24. Kitagawa H, Namikawa T, Munekage M et al. . Outcomes of thoracoscopic esophagectomy in prone position with laparoscopic gastric mobilization for esophageal cancer. Langenbecks Arch Surg 2016; 401: 699–705. [DOI] [PubMed] [Google Scholar]
- 25. Kubo N, Ohira M, Yamashita Y et al. . The impact of combined thoracoscopic and laparoscopic surgery on pulmonary complications after radical esophagectomy in patients with resectable esophageal cancer. Anticancer Res 2014; 34: 2399–404. [PubMed] [Google Scholar]
- 26. Lee J M, Cheng J W, Lin M T et al. . Is there any benefit to incorporating a laparoscopic procedure into minimally invasive esophagectomy? The impact on perioperative results in patients with esophageal cancer. World J Surg 2011; 35: 790–7. [DOI] [PubMed] [Google Scholar]
- 27. Lee J W, Sung S W, Park J K et al. . Laparoscopic gastric tube formation with pyloromyotomy for reconstruction in patients with esophageal cancer. Ann Surg Treat Res 2015; 89: 117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li K K, Wang Y J, Liu X H et al. . Propensity-matched analysis comparing survival after hybrid Thoracoscopic-laparotomy Esophagectomy and complete Thoracoscopic-laparoscopic Esophagectomy. World J Surg 2019; 43: 853–61. doi: 10.1007/s00268-018-4843-z. [DOI] [PubMed] [Google Scholar]
- 29. Mao T, Fang W, Gu Z et al. . Comparison of perioperative outcomes between open and minimally invasive esophagectomy for esophageal cancer. Thorac Cancer 2015; 6: 303–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin D J, Bessell J R, Chew A et al. . Thoracoscopic and laparoscopic esophagectomy: initial experience and outcomes. Surg Endosc 2005; 19: 1597–601. [DOI] [PubMed] [Google Scholar]
- 31. Mu J W, Gao S G, Xue Q et al. . Updated experiences with minimally invasive McKeown esophagectomy for esophageal cancer. World J Gastroenterol 2015; 21: 12873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nilsson M, Kamiya S, Lindblad M, Rouvelas I. Implementation of minimally invasive esophagectomy in a tertiary referral center for esophageal cancer. J Thorac Dis 2017; 9: S817–25. doi: 10.21037/jtd.2017.04.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nozaki I, Mizusawa J, Kato K et al. . Impact of laparoscopy on the prevention of pulmonary complications after thoracoscopic esophagectomy using data from JCOG0502: a prospective multicenter study. Surg Endosc 2018; 32: 651–9. doi: 10.1007/s00464-017-5716-5Epub 2017 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oshikiri T, Yasuda T, Kawasaki K et al. . Hand-assisted laparoscopic surgery (HALS) is associated with less-restrictive ventilatory impairment and less risk for pulmonary complication than open laparotomy in thoracoscopic esophagectomy. Surgery 2016; 159: 459–66. [DOI] [PubMed] [Google Scholar]
- 35. Safranek P M, Cubitt J, Booth M I et al. . Review of open and minimal access approaches to oesophagectomy for cancer. Br J Surg 2010; 97: 1845–53. [DOI] [PubMed] [Google Scholar]
- 36. Smithers B M, Gotley D C, Martin I et al. . Comparison of the outcomes between open and minimally invasive esophagectomy. Ann Surg 2007; 245: 232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Souche R, Nayeri M, Chati R et al. . Thoracoscopy in prone position with two-lung ventilation compared to conventional thoracotomy during Ivor Lewis procedure: a multicenter case-control study. Surg Endosc 2019. doi: 10.1007/s00464-019-06742-w[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38. Tsujimoto H, Takahata R, Nomura S et al. . Video-assisted thoracoscopic surgery for esophageal cancer attenuates postoperative systemic responses and pulmonary complications. Surgery 2012; 151: 667–73. [DOI] [PubMed] [Google Scholar]
- 39. Yanasoot A, Yolsuriyanwong K, Ruangsin S, Laohawiriyakamol S, Sunpaweravong S. Costs and benefits of different methods of esophagectomy for esophageal cancer. Asian Cardiovasc Thorac Ann 2017; 25: 513–7. doi: 10.1177/0218492317731389Epub 2017 Sep 5. [DOI] [PubMed] [Google Scholar]
- 40. Yao F, Wang J, Yao J et al. . Is thoracoscopic-laparoscopic esophagectomy a better alternative to thoracoscopic esophagectomy? Int J Surg 2017; 48: 105–9. doi: 10.1016/j.ijsu.2017.10.036Epub 2017 Oct 20. [DOI] [PubMed] [Google Scholar]
- 41. Tapias L F, Morse C R. Minimally invasive Ivor Lewis esophagectomy: description of a learning curve. J Am Coll Surg 2014; 218: 1130–40. [DOI] [PubMed] [Google Scholar]
- 42. Mackenzie H, Markar S R, Askari A et al. . National proficiency-gain curves for minimally invasive gastrointestinal cancer surgery. Br J Surg 2016; 103: 88–96. [DOI] [PubMed] [Google Scholar]
- 43. Workum F, Stenstra M H B C, Berkelmans G H K et al. . Learning curve and associated morbidity of minimally invasive Esophagectomy: a retrospective Multicenter study. Ann Surg 2017. doi: 10.1097/SLA.0000000000002469[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 44. Claassen L, Workum F, Rosman C. Learning curve and postoperative outcomes of minimally invasive esophagectomy. J Thorac Dis 2019; 11: S777–85. doi: 10.21037/jtd.2018.12.54Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Avery K N, Metcalfe C, Berrisford R et al. . The feasibility of a randomized controlled trial of esophagectomy for esophageal cancer--the ROMIO (randomized Oesophagectomy: minimally invasive or open) study: protocol for a randomized controlled trial. Trials 2014; 15: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Metcalfe C, Avery K, Berrisford R et al. . Comparing open and minimally invasive surgical procedures for oesophagectomy in the treatment of cancer: the ROMIO (randomised Oesophagectomy: minimally invasive or open) feasibility study and pilot trial. Health Technol Assess 2016; 20: 1–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Visser E, Markar S R, Ruurda J P, Hanna G B, Hillegersberg R. Prognostic value of lymph node yield on overall survival in Esophageal cancer patients: a systematic review and meta-analysis. Ann Surg 2019; 269: 261–8. doi: 10.1097/SLA.0000000000002824. [DOI] [PubMed] [Google Scholar]
- 48. Yibulayin W, Abulizi S, Lv H, Sun W. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol 2016; 14: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seesing M F J, Gisbertz S S, Goense L et al. . A propensity score matched analysis of open versus minimally invasive transthoracic Esophagectomy in the Netherlands. Ann Surg 2017; 266: 839–46. doi: 10.1097/SLA.0000000000002393. [DOI] [PubMed] [Google Scholar]
- 50. Workum F, Fransen L, Luyer M D, Rosman C. Learning curves in minimally invasive esophagectomy. World J Gastroenterol 2018; 24: 4974–8. doi: 10.3748/wjg.v24.i44.4974Review. [DOI] [PMC free article] [PubMed] [Google Scholar]


