A 77-year-old nonsmoker man was admitted to the intensive care unit of Padova University Hospital for pneumonia with severe respiratory failure. He had been complaining of cough, dyspnea, and shortness of breath for 19 days, and empirical antibiotics had been previously administered. His medical history revealed a kidney transplantation, type 2 diabetes, hypertension, and ischemic heart disease. A nasopharyngeal swab was positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Chest computed tomography scan, performed before admission to the intensive care unit, revealed a bilateral pleural effusion, patchy areas of ground glass, reticular thickening of the interstitium, and right hilar lymphadenopathy (Fig. 1 A and B). Laboratory findings revealed lymphopenia and slightly increased D-dimer (500 μg/liter). Despite receiving 15 liter/min of supplemental oxygen through a facial mask with a reservoir bag, the patient had severe hypoxemia (partial pressure of oxygen <55 mmHg) and dyspnea (respiratory rate >30/min). The patient promptly underwent noninvasive mechanical ventilation through helmet interface. Pressure support ventilation was instituted with an initial fraction of inspired oxygen of 100% and both inspiratory pressure support and positive end-expiratory pressure ranging between 10 and 12 cmH2O. Subsequently, his respiratory rate decreased below 20 breaths per minute but arterial oxygen pressure (partial pressure of oxygen) remained constant below 80. Despite supportive and anticoagulant therapies, respiratory and hemodynamic functions continued with severe multiorgan failure characteristics, and the patient died 25 days after hospital admission. A full postmortem examination, according to the National Health Service guidelines, was performed. The lungs weighed 1800 g and had an increased consistency with diffuse micronodularities and lardaceous thickening of 2 cm (largest dimension) of the right main bronchus. At histology, the bronchus had a full-thickness infiltration of a poorly differentiated solid tumor with morphologic and immunophenotypical features (p40 positive and thyroid transcription factor 1 negative) of nonkeratinizing squamous cell carcinoma. Occasional neoplastic thrombi in both lymphatic and vascular vessels were found (Fig. 2 A and C). Hilar lymph node metastasis (1 of 3 examined) was also evident. The remaining parenchyma of both lungs had diffuse chronic lymphocytic infiltration in the alveolar wall. Foci of acute lung injury with diffuse alveolar damage/hyaline membranes, reactive pneumocytes, and organizing pneumonia were detected only in the left lower lobe. Mild to moderate chronic cell infiltration was detected in small and large airways. Furthermore, the vascular bed revealed the following several intriguing changes: different grades of capillary/small vessel inflammation in more severe form with endothelialitis and microthrombi (Fig. 2 A and B). Thus, in addition to neoplastic microthrombi, other types were detected with features of fibrin clots in small- and medium-sized vessels. The ultrastructural examination of nonneoplastic lung parenchyma highlighted the findings of severe endothelial injury with diffuse reduplication of the basement membrane. The endothelial cells seemed swollen with numerous instances of cytoplasmic vacuolization (Fig. 2 D). In addition, aggregates of viral-like particles were evident in both type 2 pneumocytes and endothelial cells (Fig. 2 E). Examination of the other organs was unremarkable for any kind of acute virus-associated pathologic processes. The final diagnosis was nonkeratinizing squamous cell carcinoma, pT2 N1a, with neoplastic thrombi associated to pneumonia with SARS-CoV-2–related vessel injury. Coronavirus disease 2019 (COVID-19) is a pandemic infectious disease mainly affecting the respiratory tract1 with a large variability of clinical manifestations and symptom severity. The highest morbidity and mortality rates are detected in elderly men with chronic diseases, such as diabetes or hypertension.2 Pathogenetic substrates are not completely understood. Growing clinicopathologic3 and experimental evidence4 support the hypothesis of a viral endothelial cell other than epithelial tropism. Thus, endothelialitis, as a direct SARS-CoV-2 infection or related to host inflammatory response, could explain the systemic impaired microcirculatory function with the occurrence of small-/medium-sized vessel microthrombi. In our case, the vascular injury was evident only in the lungs. The presence of concomitant or superimposed diseases, as infections or tumors, is an important aspect that should be considered for an appropriate patient management and more precise epidemiologic information on COVID-19. A careful investigation and report of all lesions detected in COVID-19 lungs of deceased patients is mandatory to consistently prove the microvascular impairment. Tissue analysis with ancillary techniques from patients infected with SARS-CoV-2 represents the best tool to investigate and understand the molecular mechanisms at the basis of the disease alone or associated with others as lung cancer.
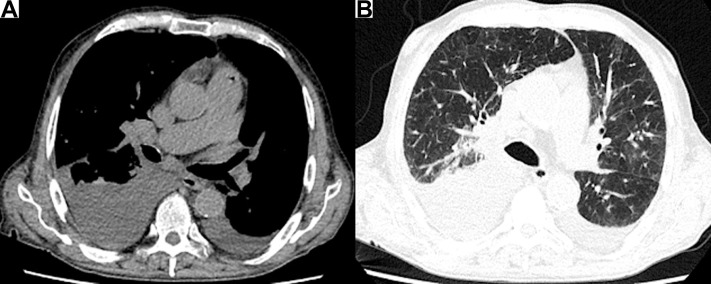
Figure 1.
(A) Unenhanced thoracic CT scan revealing enlarged right hilar lymph nodes and bilateral pleural effusion. (B) In the middle zone of both lungs, patchy areas of ground glass that can be referred to the SARS-CoV-2 infection are evident. CT, computed tomography; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
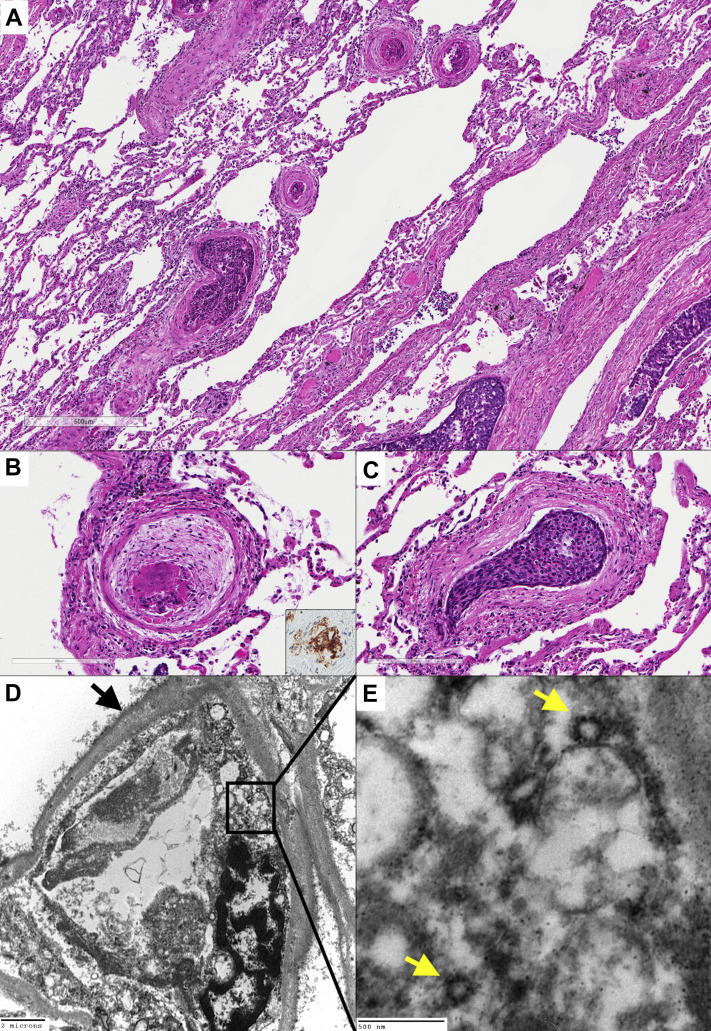
Figure 2.
(A) The lung parenchyma revealing the coexistence of vascular thrombi with (B) peripheral concentric fibrous organization and (C) neoplastic thrombi (hematoxylin and eosin stains, original magnification: ×50 [A], ×200 [B, C]). The CD61 immunostaining highlighted megakaryocytes and platelets within the vascular thrombi (inset, immunohistochemistry, original magnification ×200). The capillary vessel revealed reduplication of the basement membrane (black arrow). The endothelial cell was swollen with cytoplasmic vacuoles. (D) A platelet was also evident in the lumen (transmission electron microscopy, original magnification ×6000). At higher magnification, viral-like particles with electron-dense surfaces and the characteristic projection were evident in the endothelial cell cytoplasm (yellow arrows). (E) The average diameter of the putative virions was approximately 90 nm (transmission electron microscopy, original magnification ×50,000).
Acknowledgments
The authors thank Dr. Mila Della Barbera for electron microscopy technical assistance and Dr. Judith Wilson for English revision. The work was partially supported by a fellowship from the University of Padova (Intesa San Paolo Vita bank). The funding source did not have any role in the study's design, conduct, and reporting. Drs. Calabrese and Navalesi contributed to the conceptualization, writing—reviewing and editing, and supervision of the manuscript. Dr. Fortarezza contributed to the writing—original draft preparation, visualization, and investigation of the manuscript. Dr. Giraudo contributed to the resources, investigation, and visualization of the manuscript. Dr. Pezzuto contributed to the writing—original draft preparation, resources, and investigation of the manuscript. Dr. Faccioli contributed to the resources and investigation of the manuscript. Dr. Rea contributed to the writing—reviewing and editing and supervision of the manuscript. Drs. Pittarello and Correale contributed to the investigation and resources of the manuscript.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J., Zheng Y., Gou X. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monteil V., Kwon H., Prado P. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]