Abstract
Bladder pain syndrome (BPS) is a chronic condition characterized by pelvic pain or pressure which is perceived to be originating from the bladder, accompanied by one or more urinary symptoms, including frequency, urgency and nocturia. The precise etiology of BPS is not fully understood. Chronic bacterial infection, defective glycosaminoglycan (GAG) layer of the bladder urothelium, inappropriate activation of mast cells in the suburothelial layer of the bladder, autoimmune-mediated mechanisms and autonomic nervous system dysfunction have all been implicated. Treatments targeted at each of these mechanisms have been developed with mixed outcomes. High-quality research into the treatment options is lacking and it is difficult to draw definite conclusions. The treatment approach is multimodal and should be patient specific, targeting the symptoms which they find most bothersome. Conservative treatment, including patient education, behavioural modification, dietary advice, stress relief and physical therapy is an essential initial management strategy for all patients. If no response is observed, oral treatments such as amitriptyline are likely to offer the greatest response. Cystoscopy is essential to phenotype patients, and Hunner lesion directed therapy with fulguration or resection can be performed at the same time. Intravesical instillation of DMSO or lidocaine, detrusor injections of botulinum toxin A and neuromodulation can be used if initial management fails to improve symptoms. Oral cyclosporin can be trialled in those experienced with its use; however, it is associated with significant adverse events and requires intense monitoring. Lastly, radical surgery should be reserved for those with severe, unremitting BPS, in which quality of life is severely affected and not improved by previously mentioned interventions. Future work investigating exact aetiological factors will help target the development of efficacious treatment options, and several promising oral and intravesical treatments are emerging.
Keywords: bladder pain syndrome, interstitial cystitis, Hunner lesion, treatment
Introduction
Bladder pain syndrome (BPS) is a chronic condition characterized pelvic pain or pressure which is perceived to be originating from the bladder, accompanied by one or more urinary symptoms, including frequency, urgency and nocturia.1 The diagnosis of BPS is clinical and can be made when a patient is suffering from these typical symptoms and any alternative diagnosis with a similar presentation has been excluded, such as urinary tract infection, neoplasia and bladder calculi. The true prevalence is difficult to define due to heterogeneity in definitions and nomenclature, but studies have estimated a prevalence of 3–7%.2,3
The precise etiology of BPS is not fully understood, and a range of pathophysiological mechanisms have been suggested. Chronic bacterial infection, defective glycosaminoglycan (GAG) layer of the bladder urothelium, inappropriate activation of mast cells in the suburothelial layer of the bladder, autoimmune-mediated mechanisms and autonomic nervous system dysfunction have all been implicated, and treatments targeted at each of these mechanisms have been developed with mixed outcomes. Furthermore, it is evident that BPS is associated with other systemic pain syndromes and somatic disorders, such as irritable bowel syndrome, chronic fatigue syndrome and fibromyalgia, and this may represent a distinct phenotype of this heterogenous condition.4
Confusion around the nomenclature for this condition has contributed to the difficulty in identifying specific phenotypes that would benefit from targeted therapy, resulting in divergent guideline recommendations worldwide.5 The terms interstitial cystitis (IC), hypersensitive bladder, chronic pelvic pain and painful bladder syndrome have all been used to describe this condition. The International Continence Society (ICS) has standardized terminology and identifies hypersensitive bladder, IC/BPS and IC with Hunner lesions as distinct phenotypes.6 IC and BPS are often used interchangeably; however, it is now understood that IC with Hunner lesions likely represents a distinctive disease process and thus needs to be treated as such.
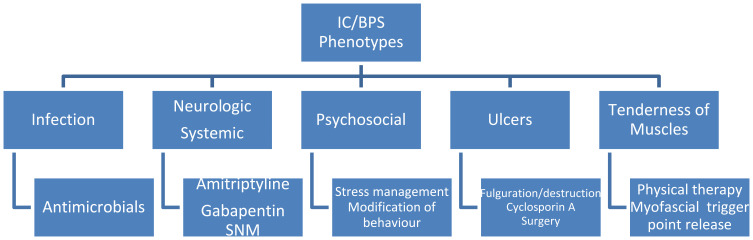
It is important to identify the most well-defined phenotypes of this condition in the initial evaluation so that treatments can be targeted to each aspect simultaneously, thereby improving clinical outcomes. A clinically useful and systematic approach for this evaluation is provided by the INPUT system which evaluates 5 different clinical domains in order to guide multimodal therapy – Infection, Neurologic/systemic, Psychosocial, Ulcers and Tenderness of muscles.7 Initial evaluation of patients with suspected BPS includes a thorough history and examination focussing on each of these domains. There are several validated tools that assess the severity of symptoms and response to treatment, the most commonly utilized being the O’Leary-Sant Interstitial Cystitis Symptom and Problem Index and visual analogue scale (VAS) to evaluate pain. Frequency-volume charts are recommended to assess urinary frequency and functional capacity.
Primary investigations should include urine analysis and culture, testing for sexually transmitted infections, and urine cytology in those considered to be at high risk of urothelial malignancy. Pelvic imaging should be performed if an alternative diagnosis is suspected. Cystoscopy is essential to exclude other pathology and also to aid accurate phenotyping of BPS – being able to evaluate bladder capacity and identify the presence of Hunner lesions for which targeted treatment could be offered. Patients can be classified using the International Society for the Study of BPS criteria (ESSIC) based on cystoscopic and histological.8 For this, patients are required to undergo cystoscopy with hydrodistension and biopsy, with hydrodistension often acting as a therapeutic as well as diagnostic intervention.
This review focuses on current treatment recommendations for patients with BPS and explores the evidence surrounding this. It is important that a multimodal approach targeting the biological, psychological and social aspects of the disease is initiated. It is crucial that patients are educated regarding the condition and its chronic course.
Conservative Management
Almost half of all patients with BPS experience symptom improvement or resolution in the long term, even without regular follow-up or receiving a new treatment.9 Furthermore, pharmacological therapy has variable success rates with high discontinuation rates and minimal long-term efficacy.10 Conservative and behavioural advice is risk-free and relatively inexpensive, and so should be the foundation for long-term management on which all further treatments are built. Options include modifications of behaviour, stress reduction, dietary alteration and physical therapy. Behavioural modifications include timed voiding and bladder training to prolong voiding intervals. The above modifications have been trialled in combination with oral therapies and seen to have a beneficial effect. One study established that a 12-week programme of timed voiding, controlled fluid intake and pelvic floor exercises significantly increased time between voids and decreased urinary frequency, with 88% of patients (37/42) reporting an improvement in symptoms on the global response assessment (GRA) scale.11 A randomized trial evaluating amitriptyline in patients with BPS, whereby all patients received an education and behavioural modification programme consisting of four categories (symptom management, fluid management, modification of diet and bladder training) reported a 45% improvement on the GRA scale with the behavioural programme alone.12
Physiotherapy is recommended for the subset of patients with pelvic floor dysfunction and trigger point or myofascial tenderness on physical examination. This includes soft tissue massage of the muscles of the pelvic floor with myofascial trigger point release. After a period of training, this can be self-performed. Improvements of up to 94% in the O’Leary Sant symptom score have been noted when myofascial release treatment was administered to patients with interstitial cystitis and high-tone pelvic floor dysfunction, with concomitant improvements in urinary frequency and suprapubic pain.13 A randomized trial of 81 women with BPS/IC demonstrated improvements in GRA scores of 59% in those treated with myofascial physical therapy compared to only 26% with global therapeutic massage.14
Over 80% of patients with BPS report sensitivity to certain food groups.15 Frequently reported triggers include caffeine, citrus, spicy foods and carbonated drinks. The patient should be advised to trial elimination of any dietary factors they feel are exacerbating their symptoms.
Oral Pharmacological Treatment
If conservative management does not improve symptoms, oral medications are recommended. Many oral agents have been used in the treatment of BPS and grades of recommendation vary amongst guidelines. The most commonly used medications are discussed here, focussing on evidence from randomized trials where available.
Amitriptyline
Amitriptyline is a tricyclic anti-depressant which acts to block the reuptake of the neurotransmitters serotonin and noradrenaline. Although not licensed for use in BPS, it is commonly used to treat neuropathic pain and as such has demonstrated efficacy in this patient population in two randomized trials. A multi-centre, randomized controlled trial found that treatment-naïve patients who were able to tolerate a dose of at least 50 mg of amitriptyline reported significantly greater improvements in symptoms in comparison to placebo.12 Sixty-six per cent of patients in the amitriptyline group versus 47% in the placebo group reported a moderate or marked improvement in their symptoms from baseline. It should be noted that this improvement was non-significant when all doses were collectively analysed. Well-recognised side effects associated with amitriptyline include blurred vision, dry mouth and constipation, and in this trial, less than half of the patients could tolerate a dose of 50 mg or higher. Similarly, another randomized trial reported a 63% improvement in O’Leary Sant scores at 4-month follow-up compared to 4% with placebo, but 92% reported at least one side effect.16
Other oral agents have been studied in combination with amitriptyline. A statistically significant reduction in the O’Leary Sant symptom score and visual analogue scale (VAS) for pain were observed at 1 month when amitriptyline was combined with a non-steroidal anti-inflammatory and an additional neuropathic agent, gabapentin.17 The reductions were observed until the end of follow up at 6 months; however, results did not reach statistical significance.
Pentosan Polysulphate
Pentosan polysulphate (PPS) is a semi-synthetic drug which has been used orally and instilled via the bladder for the treatment of BPS. Although licenced for the treatment of BPS/IC, the evidence is conflicting and recent reports of ophthalmic adverse events with long-term exposure may limit its use. A meta-analysis of 4 randomized placebo-controlled trials of oral PPS with 448 patients reported significant improvements in pain, urgency and frequency (success defined as >50% reduction in symptoms) compared to placebo.18 Nocturia was not found to be significantly reduced. However, more recent randomized trials have reported mixed results. A randomized trial of PPS and oral cyclosporin A (CyA) in 64 patients with IC revealed significantly greater efficacy for CyA based on GRA score (75% vs. 19%).19 Furthermore, a recent large multi-centre randomized controlled trial of 368 patients randomized to receive either 100 mg of PPS once per day, three times per day or placebo, reported no significant difference between any group (success defined as a 30% reduction in baseline interstitial cystitis symptom index (ICSI) score).20 Observational studies have reported mixed mid-term success rates, with one study of 271 patients demonstrating persistent efficacy (>50% improvement of GRA) in 54% at a mean 22-month follow-up,21 and a smaller study of 97 patients reporting only 11% were still taking PPS after 18 months of treatment.22 Furthermore, there have been increasing recent reports of pigmentary maculopathy leading to visual disturbance with long-term use (median 186 months) in up to 16% of patients, and this is likely to reduce the use of PPS for BPS/IC in the future.23,24
Interestingly, it has been reported that subcutaneous heparin may enhance the beneficial effects of oral PPS.25 Subcutaneous heparin was found to be most efficacious in patients who had an initial “minor” response to oral PPS. At 3 months, 31.8% (7/22) in the minor response group reported a statistically significant overall improvement in their well-being compared with 12.5% (1/8, P ≤0.001) patients in the intermediate group and 18.2% (2/11, P ≤0.001) patients in the major response group. This improvement was witnessed through to 6 months at study end but this finding has not been replicated in other studies.
Antihistamines
Antihistamines are thought to prevent the effects on the bladder of increased histamine release from mast cells in some patients with BPS/IC, but evidence of efficacy is conflicting and based on low-quality studies. Cimetidine has demonstrated significant improvement in symptoms (especially suprapubic pain and nocturia) compared to placebo in one small randomized placebo-controlled trial of 36 patients.26 Hydroxyzine has also only been studied in one small randomized trial, either alone or in combination with pentosan polysulphate, compared to placebo. There was no significant benefit of hydroxyzine alone over placebo (23% vs. 13%), but hydroxyzine in combination with PPS had greater short-term efficacy than PPS alone (40% vs. 28%).27 Evidence is therefore mixed, and antihistamines require further study in larger randomized trials, with subgroup analysis of those with elevated mast cells on bladder biopsy, to determine their optimal place in the management pathway of BPS/IC.
Oral Cyclosporin A
Cyclosporin A (CyA) is an immunosuppressive medication which modulates T cells and often used in transplant recipients and patients with Crohn’s disease. It is emerging as a treatment option and has been seen to have beneficial effects in select BPS patients. It presents its own risks and patients can experience severe side effects. It is therefore reserved for patients with refractory BPS who have not seen improvements with other oral/intravesical agents. It should only be administered by those well accustomed to its use with close monitoring of blood pressure.
A randomized trial comparing CyA to PPS found greater efficacy with CyA with regards to improving frequency, O’Leary Sant symptom score and VAS pains scores.19 However, a high number of adverse events were noted in the CyA group (94%), including nephrotoxicity, hair loss, hypertension and immunosuppression.
Patients with Hunner lesions have been demonstrated to have a better response to CyA than those without (85% vs. 30%).28 If a positive effect was seen, this was within 4 months. However, adverse effects were common and led to discontinuation of treatment in 21%. Therefore, this treatment should only be considered as a last resort in those with Hunner lesion IC refractory to all other therapies and should be administered by a specialist with experience in the use of this medication.
Intravesical Treatment
Intravesical agents are instilled directly into the bladder, thereby minimising the systemic side-effects seen with oral therapies. Intravesical glycosaminoglycan (GAG) layer treatments have been investigated in numerous randomized trials and reported mechanisms of actions include repair of the defective GAG layer and reduction of neurogenic inflammation/hypersensitivity in the bladder. Variable clinical improvements have been seen with different agents, including dimethyl sulfoxide (DMSO), hyaluronic acid (HA), chondroitin sulphate (CS), heparin, PPS and lidocaine. Furthermore, several “cocktails” with various combinations of these agents with steroid or sodium bicarbonate have also been studied (Table 1).
Table 1.
Common Intravesical “Cocktails” for BPS/IC51
| 0.5% Marcaine + 2% lidocaine jelly Heparin sulphate 10,000 IU Triamcinolone 40mg Gentamicin 80mg |
Moldwin |
| 8 mL 2% lidocaine 4 mL 8.4% NaHCO3 20,000 IU heparin |
Welk and Teichman |
| 300 mg pentosan polysulfate sodium 10 mL 2% lidocaine 10 mL 4.2% NaHCO3 |
Bade |
| 40,000 IU heparin 8 mL 1% (80 mg) or 2% lidocaine (160 mg) 3 mL 8.4% NaHCO3 |
Parson |
| Heparin 10,000 units/mL–2mL Hydrocortisone 125 mg Gentamicin 80mg/2mL–2mL Sodium Bicarbonate 8.4%–50mL Marcaine 0.5%–50 mL |
Whitmore |
Dimethyl Sulfoxide (DMSO)
DMSO remains the sole intravesical agent approved by the Food and Drug Administration (FDA) for the treatment of interstitial cystitis and is often used in combination with other agents. It is also recommended by the European Association of Urology (EAU) for the treatment of BPS. However, these recommendations are based mostly on small randomized or non-placebo-controlled studies. The precise mechanism of action of DMSO is unknown; however, it is hypothesised to exert its clinical action by decreasing inflammation, triggering relaxation of bladder muscles and influencing inflammatory mast cells.29 Typically, 50 mL of a 50% DMSO solution is instilled into the bladder via a temporary catheter. There is no standardized treatment regime, but it is typically administered once a week for 6 weeks. If a good response is achieved with this initial trial, a further 6-week course can be provided, followed by monthly maintenance.
An early randomized placebo-controlled trial of 33 patients investigated the use of intravesical DMSO versus saline placebo, measuring outcomes including urodynamic measures and symptom scores. DMSO demonstrated an improvement in subjective symptoms (53% vs 18% in placebo) and this was also duplicated with improvements objectively based on urodynamic and voiding diary changes following treatment (93% vs 35% in placebo).30
A more recent study of 55 females, with median 5-year follow-up, combined DMSO with heparin, hydrocortisone and bupivacaine.31 Statistically significant improvements of 23–47% were observed in the O’Leary-Sant and pain scores. 21.8% of the women required oral medication for the management of their symptoms at the end of follow up and 34.5% reported resolution of their symptoms, with no further treatment requirement. Patients whose main complaints were urinary frequency (15 or more episodes per day), nocturia and a bladder capacity of <500ml when measured under general anaesthetic were found to be more likely to experience treatment failure.
DMSO has also been compared with intravesical CS, with CS demonstrating superior effects.32 This randomized controlled, multicentre trial included 36 patients diagnosed with BPS, and they had either DMSO or CS instilled weekly for 6 weeks. The DMSO group saw a high withdrawal rate of patients (57%), largely as the result of pain on instillation, insufferable garlic smell and lack of effect. CS was found to be better tolerated with a 27% drop out rate. In the remaining patients, CS was found to have superior outcomes in comparison to DMSO. More patients in the CS group reported moderate or marked improvement (72.7% vs 14%, P=0.002) and a reduction in VAS scores (20% vs 8.3%). CS groups performed significantly better in pain reduction (−1.2 vs −0.6) and nocturia (−2.4 vs −0.7). Due to the lack of large, randomized controlled trials with sufficient follow-up, DMSO remains unlicensed in the UK.33
Glycosaminoglycan Layer Treatments
A range of intravesical GAG layer therapies have been studied, each with similar mechanisms of action. The best-studied are intravesical heparin, HA, CS, and PPS.
Intravesical heparin is most commonly used in combination with anaesthetic agents (eg lidocaine) or sodium bicarbonate, with good short-term success rates of 56–73% reported in cohort series.34,35 Furthermore, the combination of heparin and alkalinised lidocaine has been reported to provide immediate relief of acute flares of pain and urgency for up to 12 hours compared to placebo (50% vs. 13%).36 However, randomized placebo-controlled trials for heparin as a sole agent are lacking.
Intravesical HA has been studied in several observational trials with efficacy rates of 66–87%.37,38 In the largest study of 121 women with a mean duration of symptoms of BPS/IC of 6 years, and who had a positive modified potassium test indicating a urothelial barrier defect, 40 mg hyaluronic acid administered weekly resulted in symptomatic improvement in 85%.37 A more recent study compared 120 mg hyaluronic acid to chondroitin sulphate, with instillations administered weekly for the first month followed by a reducing regime over a total of 4 months.39 Significant improvements were noted in pain scores in both arms but intravesical CS was superior to intravesical HA in terms of frequency, nocturia and visual analogue scale (VAS) pain scores (strong benefit 38% HA vs. 52% CS). However, there have been no published randomized trials for its use, and three unpublished randomized trials reported lack of efficacy compared to placebo.40 Similarly, intravesical CS as monotherapy has been studied in small cohort series with promising efficacy rates of approximately 60%, but adequately powered randomized trials are lacking, and the small number of randomized trials that have been published report less satisfactory effect sizes.41,42 The combination of HA and CS has demonstrated encouraging results in small non-controlled studies, and a recent randomized trial suggested the combination of HA and CS was as effective as DMSO but with a more favourable side-effect profile.43,44 Furthermore, the combination of HA/CS was found to be superior to intravesical CS alone in terms of improvements in pain scores and female sexual function.45 A systematic review and meta-analysis that included 10 studies with a total of 390 patients revealed significant improvements in pain scores (O’Leary-Sant and VAS scores) with both intravesical HA and combined HA/CS.46 The EAU guidelines, therefore, provide a weak recommendation that intravesical HA or CS be offered before more invasive measures. However, there is a lack of high-quality evidence with clinically significant effects sizes for intravesical HA or CS, and further well-powered randomized trials are required to determine whether they provide clinically significant efficacy compared to placebo.
PPS instillations have been studied as an alternative to oral administration. Two small randomized trials have demonstrated short-term efficacy of 40–62% compared to placebo with minimal adverse events.47,48 A recent systematic review and meta-analysis of intravesical therapies for BPS/IC have shown that effect sizes for overall response rates were similar between HA, CS and PPS.49
Anaesthetic Agents
Intravesical lidocaine is recommended by the EAU for the short-term relief of acute symptom flares. Furthermore, rapid improvement in symptoms was observed in a prospective study of 102 patients randomized to receive intravesical alkalinised lidocaine (lidocaine with sodium bicarbonate) or a placebo with a 5-day treatment course.50 Significantly more patients reported improvements in the GRA scale 3 days after completing treatment if they received alkalinized lidocaine vs placebo (30% vs 9.6%, respectively), and these effects were maintained beyond the end of treatment.
Intravesical “Cocktails”
Intravesical “cocktails” containing different combinations of alkalinized anaesthetic agents, antibiotics, steroids, and GAG-layer therapies have been widely used with the aim of improving efficacy through additive effects (Table 1).51 A single instillation of the combination of heparin and alkalinised 2% lidocaine was found to result in significant and immediate relief in pain and urgency in 94%, with effects lasting up to 48 hours.34 Another study reported complete resolution of dyspareunia in 57% of women treated with this combination.52 These cocktails are often therefore considered as treatments for acute symptomatic flares. A recent study comparing intravesical GAG-layer therapies with Whitmore’s cocktail reported that the cocktail was the most cost-effective option.53 Although commonly used, the evidence for these combination therapies is limited to small cohort studies and so randomized placebo-controlled trials are required to prove the efficacy of these agents due to the high placebo effect in studies of BPS treatments.54
Endoscopic Treatment
Hydrodistention
Bladder distention has been used in patients with BPS/IC for many years, as both a diagnostic tool and for treatment. Variable techniques have been utilized between centres and a standardized protocol for therapeutic hydrodistention has not been devised. Studies evaluating hydrodistention in BPS tend to be small and uncontrolled and the evidence base is therefore weak with regards to its efficacy. A systematic review of 17 trials reported efficacy rates of up to 56% in the short term (2–3 months). However, there was considerable heterogeneity in patient selection and hydrodistension technique, as well as the use of non-validated outcome measures in most studies, and therefore no firm conclusions can be reached regarding its therapeutic efficacy.55 Furthermore, acute flare of symptoms occurs in 9%.56 It is not currently recommended as a treatment modality by the EAU.
Transurethral Treatment of Hunner Lesions
Hydrodistention is often combined with transurethral destruction of Hunner lesions if they are present, be this with resection, fulguration or laser coagulation. High success rates have been reported with the destruction of Hunner lesions in the short term, although the majority require repeat treatments to maintain longer-term efficacy. In a large study of 103 patients, 89% experienced symptom relief with transurethral resection, with 40% reporting long-term efficacy (more than 3 years). However, the majority required 2–4 repeat resections, with some having undergone 16 resections. Similar efficacy has been noted with fulguration.57,58 A recent randomized-controlled trial between transurethral resection and transurethral coagulation in 126 patients with Hunner lesions reported good symptomatic efficacy in both groups with no significant difference between the groups.59 The Nd:YAG laser has also been utilized without complication by Rofiem et al to ablate Hunner lesions in 24 patients.60 Symptomatic improvement was experienced by all patients within 3 days. This included decreases in pain scores (9.1 to 1.2, P<0.003), urgency (8.2 to 1.9 P<0.003) and nocturia (7.9 voids per night to 2.9 P<0.0001). In addition, patients experienced an increase in the time between voids, from every 30 minutes to over 100 minutes (P<0.0001). Forty-six per cent of patients experienced a relapse in symptoms, however, experienced similar benefits with subsequent re-treatment. With a lesser degree of bladder penetration than resection or coagulation, laser ablation may prevent long-term bladder contracture with repeated treatments, but this remains to be determined.
The injection of the synthetic steroid, triamcinolone, directly into Hunner lesions has also demonstrated similar short-term symptomatic improvement in 70–74% of patients, with effects lasting up to 12 months.61,62 This modality requires study in randomized placebo-controlled trials.
Botulinum Toxin Type A
Hyper-excitability of afferent nerve fibres in the suburothelial layer of the bladder has been suggested to play a crucial role in the pathogenesis of BPS. It is thought that increased levels of neurotransmitters which act to induce so-called “neuronal hypersensitivity” in the urothelium and suburothelium contribute to the pain perceived in BPS.63 Botulinum toxin type A (BTX-A) has been suggested to inhibit neurotransmitter release from these afferent nerve fibres and thus decrease the sensitivity experienced, in addition to modulating detrusor contractility in BPS.
Kuo et al compared hydrodistention with sub-urothelial BTX-A injections and hydrodistention alone in patients with characteristic symptoms of BPS in addition to cystoscopic changes.63 Patients enrolled remained symptomatic despite being treated with oral PPS or intravesical heparin/hyaluronic acid for at least 6 months, and all patients remained on oral PPS throughout the study. Success was defined as being moderately or markedly improved on global response assessment (GRA) and 72% (21/29) reported success with BTX-A in comparison to 48% (11/23) in the hydrodistention alone group (P<0.05) at 3 months. This decreased to 69% vs 35% and 45% vs 26% at 6 and 12 months, respectively (P<0.05). The only significant reductions in VAS scores and increases in bladder capacity were detected in the BTX-A group at 3 months. A higher dose of 200 units of botox was trialled; however, this was associated with an increase in adverse effects including urinary retention.
The effect of BTX-A alone was explored by Akiyama et al.64 In parallel with the previous study, all patients were deemed to have refractory BPS and had previously received at least one trial of hydrodistention. Thirty-four patients received either immediate injection with 100 units of BTX-A or received the treatment at 1 month (whilst maintaining their present oral medications for BPS). Injection was performed at 30 sites in the trigonal area. Patients reporting slight improvement to marked improvement were deemed responders to treatment. At 1 month, patients who had received BTX-A reported a significantly higher response to treatment according to GRA assessment score (72.2% vs 25% P=0.01), in addition to significant increases in quality of life index scores. At 1 month, cohorts were combined and the response rate was 73.5% decreasing to 20.6% at 12 months. The average length of the effect of BTX-A was 5.4 months. Patients who had received hydrodistention three or more times were more likely to have a sustained positive effect with BTX-A.
Notably, BTX-A has been compared to placebo in a recent randomized-controlled trial.65 Larger decreases in VAS scores were recorded in patients who received BTX-A injections in comparison to normal saline (2.6 vs 0.9 P=0.021). These results were mirrored in a recent meta-analysis of seven randomized trials.66 Interestingly, a recent study showed no difference between trigone-only or trigone-sparing injections.67 It is unclear whether those with Hunner lesions have a poorer outcome of treatment with BTX-A. A cohort study of 40 patients reported efficacy in 50% of patients without Hunner lesions, but no benefit in any patient with Hunner lesions.68 However, a more recent comparative study of 24 patients revealed no difference in efficacy between the ulcer and non-ulcer groups.69 Long-term studies are required to be able to draw solid conclusions, but BTX-A is an appropriate option for patients who are willing to accept the risk of voiding dysfunction requiring clean intermittent self-catheterisation.
Neuromodulation – Sacral and Pudendal
Sacral neuromodulation (SNM) has been studied for the treatment of symptoms in BPS. The two-stage procedure involves a test phase followed by implantation of the permanent stimulator. The implanted electrode and conducting lead stimulate the afferent sacral nerves as they exit the sacral foramina. Several small studies have investigated the use of SNM in patients with severe BPS, with conflicting results.
The long-term efficacy of SNM was evaluated in patients from a single centre.70 Between 1994 and 2008, 46 out of 78 patients had a positive 1st stage percutaneous stimulation response and subsequently had permanent implantation. All patients had cystoscopic evidence of Hunner lesions or glomerulations. After 5 years, more than 50% improvement in GRA scale was observed in 70% of patients and the average improvement on the GRA scale in these patients was 80%. Of the 28% of patients who had the electrode and leads removed, the chief reason cited was poor outcome. Fifty per cent of all patients underwent device revision at some point during the 5-year follow-up.
These positive outcomes have been confirmed in a recent meta-analysis, including 12 studies and 583 patients.71 SNM was associated with significant reductions in pelvic pain, frequency, nocturia, urgency, and Interstitial Cystitis Problem Score. Overall pooled treatment success rates were 84% (95% CI 76% to 91%).
It has been proposed that stimulation of the pudendal nerve (S2-S4 nerve roots) would offer a more direct afferent stimulation to the micturition centre. One randomized trial recruited 22 patients with refractory interstitial cystitis and evidence of Hunner lesions or petechial haemorrhage on cystoscopy and bladder distention.72 Each patient had a lead placed at the S2 nerve root and also at the pudendal nerve. Each lead was tested blindly and the lead with the best response was implanted into the generator. Seventeen of 22 patients who exhibited a positive response to initial lead implantation had a permanent generator placed. The pudendal nerve was chosen as the superior lead in 77% of patients. A significant overall reduction in symptoms was observed with both the pudendal and sacral nerve root; however, the pudendal nerve offered a greater reduction (59% vs 44%, P=0.05). Mean voided volume was increased by 95% in pudendal nerve stimulation vs 33% when the sacral nerve roots were targeted. Complication rates were low and included two seromas which required needle drainage. This data must be interpreted with caution due to the small sample size, short follow-up period of 6 months and lack of confirmation of these outcomes from other centres. However, this promising study demonstrates that pudendal nerve stimulation is a feasible and effective option for which large comparative trials are required to be able to derive more definitive conclusions.
SNM is a minimally invasive procedure which has demonstrated safety and good long-term efficacy in patients with BPS. Patients need to be aware that the implantation of device may not resolve symptoms and symptoms may worsen, leading to revision or explantation. Randomized trials of SNM are lacking, and further studies regarding the relative efficacy in those with and without Hunner lesions are required to optimally guide treatment selection.
Major Surgery
The last resort option for the treatment of severe, disabling BPS/IC which has not responded to other treatment is radical surgery. The aim is to increase the capacity of the bladder or divert the urinary stream, with options including bladder augmentation cystoplasty, cystoplasty with or without subtrigonal resection, or urinary diversion with or without cystectomy.
Valdemar et al performed major surgery in 41 patients with severe BPS after the failure of other treatment.73 The majority of patients (20) underwent urinary diversion with the bladder remaining in situ and 65% (13) of these patients underwent a subsequent cystectomy 12 months later, with the most common reason for this being persistent pain. Other surgical procedures included initial cystectomy or subtotal cystectomy/bladder augmentation. Median follow up of all patients was 5.5 years, with 75% reporting being pain free and 68% being satisfied with the final outcomes. Patients with the best post-operative resolution of pain were found to have a shorter duration of pre-operative symptoms, and it was therefore concluded that longer pre-operative symptoms were a predictor for persisting post-operative pain.
Rossberger et al also concluded that major reconstructive surgery is mainly suitable for BPS patients with the Hunner subtype of the disease.74 Eighty-two per cent of patients with Hunner-type disease who underwent an initial surgical procedure reported resolution of their symptoms and this was increased to 94% when the patients who remained symptomatic were operated on with cystectomy, diversion or treatment of the trigonal remnant. Only 23% of patients with non-Hunner disease reported symptom resolution after reconstructive procedures. It is therefore important to classify patients and counsel them that pain may persist despite radical treatment.
A recent systematic review of 448 BPS patients from 20 studies, undergoing radical surgery found that 77% of patients reported symptomatic improvement and the highest clinical response was observed in patients who underwent total cystectomy with orthotopic neobladder formation.75 Other procedures performed included subtotal cystectomy with cystoplasty and urinary diversion alone. 6.9% of patients underwent a follow-up surgical procedure (total cystectomy ± ileal conduit) and almost half of those patients subsequently improved. Of note, 23% of patients reported no improvement in symptoms and morbidity was as high as 26.5% with a mortality of 1.3%.
Studies regarding radical surgical intervention in patients with BPS are usually single centre, small and have variable outcome measures, therefore drawing definite conclusions from this data is difficult. Major surgical intervention should remain a last resort, and those with Hunner lesion disease and small anatomic bladder capacity are likely to derive the greatest benefit. There is a need for larger prospective studies to determine which patients would benefit most from major surgical treatment, in view of the significant morbidity and mortality associated with this level of intervention.
Emerging Treatment Options
BPS is a complex disease and the exact etiology remains undiscovered. Further research to discover the exact pathology of the condition will help focus future efforts on novel treatment modalities. Research is being conducted to evaluate the microbiome in patients with BPS along with efforts to discover novel biomarkers which could identify and subtype patients. The precise differences between subtypes of BPS need to be established to be able to stratify patients and target research to ascertain which modalities would be effective against which subtype. Several emerging novel therapies have demonstrated promising results in small randomized trials.
Phosphodiesterase-5 Inhibitors
Phosphodiesterase-5 inhibitors (PDE5-i) inhibit smooth muscle contraction and are thought to prevent mast cell degranulation by inhibiting potassium release. The PDE5-i, Sildenafil, has been studied in a small randomized placebo-controlled trial of 48 women with BPS/IC. Significant improvements in the O’Leary Sant IC symptom and problem scores were seen with low-dose sildenafil at 3 months’ follow-up (success in 63%), with associated improvements in urodynamic bladder capacity and no serious adverse events.76 However, there have been no further published studies replicating these promising findings. Future study of higher doses and careful patient stratification are required.
Monoclonal Antibodies
Adalimumab, a monoclonal antibody against the pro-inflammatory cytokine tumour necrosis factor-alpha, has been studied in a randomized placebo-controlled trial of 43 patients with BPS/IC.77 Although a success rate of 53% was noted at 3-month follow-up, there was no significant difference compared to placebo due to the high placebo response in this study.
A monoclonal antibody against nerve growth factor, tanezumab, has reported more promising results in a small placebo-controlled trial of 34 patients.78 Improvements in pain scores and GRA scores were noted at 6-week follow-up. However, adverse events (including paraesthesia and headaches) occurred in 47%. These findings need to be replicated in larger, longer-term randomized trials.
Cannabinoids
Cannabinoids are known to possess analgesic and anti-inflammatory properties and have been studied in a variety of chronic pain conditions. Activation of the Cannabinoid 2 receptor has been shown to reduce the severity of established cystitis in mice models.79 However, clinical studies have been limited to case reports with promising results and further investigation of these agents is required.80
Enhanced Intravesical Drug Delivery Systems
Novel methods to enhance intravesical drug delivery are likely to considerably change the way in which this condition is managed in the future. The use of electromotive drug administration, liposomes, reverse thermal gelation hydrogel, and the lidocaine-releasing intravesical system (LiRIS) all appear promising in early trials.
Liposomes, spherical phospholipid vesicles, are thought to repair the urothelial lining and reduce its permeability to irritant substances in the urine. Intravesical liposome instillation has been studied in an observational trial of 14 patients with BPS/IC who were administered the treatment once a week for 4 weeks.81 Significant improvements were noted in pain and urgency scores at 4 weeks, but there was no change in urinary frequency. These short-term results warrant further placebo-controlled studies.
Intravesical injection of botulinum toxin A can be painful under local anaesthesia. An alternative is passive instillation, and the use of a reverse thermal gelation hydrogel which is injected as a liquid form, solidifies in the bladder, and then slowly releases botulinum toxin over several hours, represents an attractive alternative to injections.82 A pilot study of 15 patients reported modest improvements in pain scores which were greatest at week 2 (Mean VAS score 4.7 compared to 6.6 at baseline), but then declined by week 12. However, further study to determine the optimal instillation regime, in comparison to intravesical injections, is warranted.
Another slow-releasing drug delivery system, the lidocaine-releasing intravesical system (LiRIS), aims to provide continuous release of lidocaine over a 2-week period. A single-arm pilot study of 16 women with BPS/IC has reported promising short-term efficacy in pain and urinary symptoms, with success rates of 64% based on the GRA.83 Future randomized trials of this device are eagerly awaited.
Hyperbaric Oxygen Therapy
The use of hyperbaric oxygen therapy (HBOT) to increase oxygen delivery to hypoxic urothelial tissues, thereby stimulating healthy granulation and angiogenesis, has been described in radiation cystitis with good results, and small studies have assessed its use in BPS/IC. A randomized trial of 20 women who had undergone treatment with intravesical DMSO were then randomized to HBOT or sham therapy.84 All patients who underwent HBOT therapy had significant and persistent clinical improvement, compared to 40% of those in the sham group, with a mean duration of effect of 9 months (compared to 3 months). This may be a promising option for those who have failed other treatments, but further study is required to determine whether those with end-stage contracted, fibrotic bladders respond poorer than those with less a severe stage of disease.
Extracorporeal Shock-Wave Therapy
The use of extracorporeal shock-wave therapy (ESWT) has been shown to reduce pain and inflammation in preclinical studies. A recent randomized sham-controlled trial of ESWT in 54 patients with BPS/IC administered weekly for 4 weeks reported success rates of 57% vs. 19% in favour of ESWT in terms of VAS pain scores, but there was no change in O’Leary Sant symptom scores.85 This modality requires further studies to determine its efficacy in the treatment of this condition.
Conclusion
Bladder pain syndrome is a complex condition in which the exact etiology has yet to be fully understood. High-quality research into the treatment options is lacking and it is difficult to draw definite conclusions due to heterogeneity between studies in terms of inclusion criteria and outcome measures reported.
There is no treatment for BPS which has been found to help all patients. The treatment approach is multimodal and should be patient specific, targeting the symptoms which they find most bothersome. Clinical phenotyping should be performed at the initial consultation utilising a system such as INPUT, and multimodal treatment should be commenced targeting the relevant phenotypes present (Figure 1). Conservative treatment, including patient education, behavioural modification, dietary advice, stress relief and physical therapy is an essential initial management strategy for all patients. This is the cornerstone of treatment and all further trials of treatment should be based on this foundation. If no response is observed, oral treatments such as amitriptyline are likely to offer the greatest response. Cystoscopy is essential to phenotype patients according to the ESSIC classification, and Hunner-lesion directed therapy with fulguration or resection can be performed at the same time, if appropriate, with good success rates. More invasive treatments include intravesical instillation of DMSO or lidocaine, detrusor injections of botulinum toxin A and neuromodulation, and should be trialled if oral therapies have failed. Oral cyclosporin can be trialled in those experienced with its use; however, this often results in significant adverse events and requires intense monitoring, and so is not widely used. Lastly, radical surgery should be reserved for those with severe, unremitting BPS, with Hunner lesions or reduced anatomic bladder capacity, in which quality of life is severely affected and not improved by previously mentioned interventions. Future work investigating exact aetiological factors will help target the development of efficacious treatment options for this complex condition, and several promising oral and intravesical treatments are emerging.
Figure 1.
A treatment guide based on INPUT patient phenotype.
Disclosure
Mr Arun Sahai reports grants, personal fees from Allergan Ltd, non-financial support, funds for a one off meeting to promote neuromodulation service to the region from Medtronic, during the conduct of the study; grants from Boston Scientific, personal fees from Ferring, outside the submitted work. Mr Sachin Malde reports grants from Medtronic, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Fall M, Baranowski AP, Fowler CJ, et al. EAU guidelines on chronic pelvic pain. Eur Urol. 2010;57(1):35–48. doi: 10.1016/j.eururo.2009.08.020 [DOI] [PubMed] [Google Scholar]
- 2.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–544. doi: 10.1016/j.juro.2011.03.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suskind AM, Berry SH, Ewing BA, et al. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND interstitial cystitis epidemiology male study. J Urol. 2013;189(1):141–145. doi: 10.1016/j.juro.2012.08.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afari N, Buchwald D, Clauw D, et al. A MAPP network case-control study of urological chronic pelvic pain compared with nonurological pain conditions. Clin J Pain. 2020;36(1):8–15. doi: 10.1097/AJP.0000000000000769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malde S, Palmisani S, Al-Kaisy A, Sahai A. Guideline of guidelines: bladder pain syndrome. BJU Int. 2018;122(5):729–743. doi: 10.1111/bju.14399 [DOI] [PubMed] [Google Scholar]
- 6.Doggweiler R, Whitmore KE, Meijlink JM, et al. A standard terminology in chronic pelvic pain syndromes: a report from the chronic pelvic pain working group of the international continence society. Neurourol Urodyn. 2017;36(4):984–1008. doi: 10.1002/nau.23072 [DOI] [PubMed] [Google Scholar]
- 7.Crane A, Lloyd J, Shoskes DA. Improving the utility of clinical phenotyping in interstitial cystitis/painful bladder syndrome: from UPOINT to INPUT. Can J Urol. 2018;25(2):9250–9254. [PubMed] [Google Scholar]
- 8.Van de Merwe J, Nordling J, Bouchelouche P, et al. Diagnostic criteria, classification and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol. 2008;53(1):60–67. doi: 10.1016/j.eururo.2007.09.019 [DOI] [PubMed] [Google Scholar]
- 9.Yeh HL, Jhang JF, Kuo YC, Kuo HC. Long-term outcome and symptom improvement in patients with interstitial cystitis/bladder pain syndrome with or without regular follow-up and treatment. Neurourol Urodyn. 2019;38(7):1985–1993. doi: 10.1002/nau.24104 [DOI] [PubMed] [Google Scholar]
- 10.Propert KJ, Schaeffer AJ, Brensinger CM, Kusek JW, Nyberg LM, Landis JR. A prospective study of interstitial cystitis: results of longitudinal follow up of the interstitial cystitis data base cohort. The Interstitial Cystitis Data Base Study Group. J Urol. 2000;163(5):1434–1439. doi: 10.1016/S0022-5347(05)67637-9 [DOI] [PubMed] [Google Scholar]
- 11.Chaiken DC, Blaivas JG, Blaivas ST. Behavioural therapy for the treatment of refractory interstitial cysitits. J Urol. 1993;149(6):1445–1448. doi: 10.1016/S0022-5347(17)36411-X [DOI] [PubMed] [Google Scholar]
- 12.Foster HE, Hanno PM, Nickel C, et al. Effect of Amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183(5):1853–1858. doi: 10.1016/j.juro.2009.12.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukban J, Whitmore K, Kellogg-Spadt S, Bologna A, Lesher A, Fletcher E. The effect of manual physical therapy in patients diagnosed with interstitial cystitis, high tine pelvic floor dysfunction, and sacroiliac dysfunction. Urology. 2001;Jun;57(6 Suppl 1):121–122. doi: 10.1016/S0090-4295(01)01074-3 [DOI] [PubMed] [Google Scholar]
- 14.FitzGerald MP, Payne CK, Lukacz ES, et al. Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. J Urol. 2012;187(6):2113–2118. doi: 10.1016/j.juro.2012.01.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedlander JI, Shorter B, Moldwin RM. Diet and its role in interstitial cystitis/bladder pain syndrome and comorbid conditions. BJU Int. 2012;109(11):1584–1591. doi: 10.1111/j.1464-410X.2011.10860.x [DOI] [PubMed] [Google Scholar]
- 16.van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172:533–536. doi: 10.1097/01.ju.0000132388.54703.4d [DOI] [PubMed] [Google Scholar]
- 17.Lee JW, Han DY, Jeong HJ. Bladder pain syndrome treated with triple therapy with gabapentin, amitriptyline and a nonsteroidal anti-inflammatory drug. Int Neurourol J. 2010;14:256–260. doi: 10.5213/inj.2010.14.4.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang P, Auclair B, Beechinor D, et al. Efficacy of pentosan polysulfate in the treatment of interstitial cystitis: a meta-analysis. Urology. 1997;50(1):39–43. doi: 10.1016/S0090-4295(97)00110-6 [DOI] [PubMed] [Google Scholar]
- 19.Sairanen J, Tammela TLJ, Leppilahti M, et al. Cyclosporine A and pentosan polysulfate sodium for the treatment of interstitial cystitis: a randomized comparative study. J Urol. 2005;174(6):2235–2238. doi: 10.1097/01.ju.0000181808.45786.84 [DOI] [PubMed] [Google Scholar]
- 20.Nickel JC, Herschorn S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo controlled study. J Urol. 2015;193(3):857–862. doi: 10.1016/j.juro.2014.09.036 [DOI] [PubMed] [Google Scholar]
- 21.Al-Zahrani AA, Gajewski JB. Long-term efficacy and tolerability of pentosan polysulphate sodium in the treatment of bladder pain syndrome. Can Urol Assoc J. 2011;5(2):113–118. doi: 10.5489/cuaj.10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jepsen JV, Sall M, Rhodes PR, Schmidt D, Messing E, Bruskewitz RC. Long-term experience with pentosanpolysulfate in interstitial cystitis. Urology. 1998;51(3):381–387. doi: 10.1016/S0090-4295(97)00714-0 [DOI] [PubMed] [Google Scholar]
- 23.Pearce WA, Chen R, Jain N. Pigmentary maculopathy associated with chronic exposure to pentosan polysulfate sodium. Ophthalmology. 2018;125(11):1793–1802. doi: 10.1016/j.ophtha.2018.04.026 [DOI] [PubMed] [Google Scholar]
- 24.Jain N, Li AL, Yu Y, Van der Beek BL. Association of macular disease with long-term use of pentosan polysulfate sodium: findings from a US cohort. Br J Ophthalmol. 2020;104(8):1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Ophoven A, Heinecke A, Hertle L. Safety and efficacy of concurrent application of oral pentosan polysulfate and subcutaneous low-dose heparin for patients with interstitial cystitis. Urology. 2005;66(4):707–711. doi: 10.1016/j.urology.2005.04.056 [DOI] [PubMed] [Google Scholar]
- 26.Thilagarajah R, Witherow RO, Walker MM. Oral cimetidine gives effective symptom relief in painful bladder disease: a prospective, randomized, double-blind placebo-controlled trial. BJU Int. 2001;87(3):207–212. doi: 10.1046/j.1464-410x.2001.02031.x [DOI] [PubMed] [Google Scholar]
- 27.Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170(3):810–815. doi: 10.1097/01.ju.0000083020.06212.3d [DOI] [PubMed] [Google Scholar]
- 28.Forrest JB, Payne CK, Erickson DR. Cyclosporine A for refractory interstitial cystitis/bladder pain syndrome: experience of 3 tertiary centers. J Urol. 2012;188(4):1186–1191. doi: 10.1016/j.juro.2012.06.023 [DOI] [PubMed] [Google Scholar]
- 29.Rawls WF, Cox L, Rovner ES. Dimethyl Sulfoxide (DMSO) as intravesical therapy for interstitial cystitis/bladder pain syndrome: a review. Neurourol Urodyn. 2017;36(7):1677–1684. doi: 10.1002/nau.23204 [DOI] [PubMed] [Google Scholar]
- 30.Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol. 1988;140(1):36–39. doi: 10.1016/S0022-5347(17)41478-9 [DOI] [PubMed] [Google Scholar]
- 31.Lim Y, Dwyer P, Murray CJ, et al. Long-term outcomes of intravesical dimethyl sulfoxide/heparin/hydrocortisone therapy for interstitial cystitis/bladder pain syndrome. Int Urogynecol J. 2017;28(7):1085–1089. doi: 10.1007/s00192-016-3232-0 [DOI] [PubMed] [Google Scholar]
- 32.Tutolo M, Ammirati E, Castagna G, et al. A prospective randomized controlled multicentre trial comparing intravesical DMSO and chondroitin sulphate 2% for painful bladder syndrome/interstitial cystitis. Int Braz J Urol. 2017;43(1):124–141. doi: 10.1590/s1677-5538.ibju.2016.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.NICE. Interstitial cystitis: dimethyl sulfoxide bladder instillation. Evidence summary [ESUOM26]. February 20, 2014.
- 34.Parsons CL. Successful downregulation of bladder sensory nerves with combination of heparin and alkalinized lidocaine in patients with interstitial cystitis. Urology. 2005;65(1):45–48. doi: 10.1016/j.urology.2004.08.056 [DOI] [PubMed] [Google Scholar]
- 35.Kuo HC. Urodynamic results of intravesical heparin therapy for women with frequency urgency syndrome and interstitial cystitis. J Formos Med Assoc. 2001;100(5):309–314. [PubMed] [Google Scholar]
- 36.Parsons CL, Zupkas P, Proctor J, et al. Alkalinized lidocaine and heparin provide immediate relief of pain and urgency in patients with interstitial cystitis. J Sex Med. 2012;9(1):207–212. doi: 10.1111/j.1743-6109.2011.02542.x [DOI] [PubMed] [Google Scholar]
- 37.Riedl CR, Engelhardt PF, Daha KL, Morakis N, Pflüger H. Hyaluronan treatment of interstitial cystitis/painful bladder syndrome. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):717–721. doi: 10.1007/s00192-007-0515-5 [DOI] [PubMed] [Google Scholar]
- 38.Daha LK, Riedl CR, Lazar D, Hohlbrugger G, Pflüger H. Do cystometric findings predict the results of intravesical hyaluronic acid in women with interstitial cystitis? Eur Urol. 2005;47(3):393–397. doi: 10.1016/j.eururo.2004.10.022 [DOI] [PubMed] [Google Scholar]
- 39.Gülpınar Ö, Esen B, Kayış A, Gökçe Mİ, Süer E. Clinical comparison of intravesical hyaluronic acid and chondroitin sulfate therapies in the treatment of bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. 2018;37(1):257–262. doi: 10.1002/nau.23284 [DOI] [PubMed] [Google Scholar]
- 40.Chintea CL, Belal M. Is there enough evidence for the use of intravesical instillations of glycosaminoglycan analogues in interstitial cystitis? BJU Int. 2013;111(2):192–193. doi: 10.1111/j.1464-410X.2012.11635.x [DOI] [PubMed] [Google Scholar]
- 41.Nickel JC, Egerdie B, Downey J, et al. A real-life multicentre clinical practice study to evaluate the efficacy and safety of intravesical chondroitin sulphate for the treatment of interstitial cystitis. BJU Int. 2009;103(1):56–60. doi: 10.1111/j.1464-410X.2008.08028.x [DOI] [PubMed] [Google Scholar]
- 42.Nickel JC, Egerdie RB, Steinhoff G, Palmer B, Hanno P. A multicenter, randomized, double-blind, parallel group pilot evaluation of the efficacy and safety of intravesical sodium chondroitin sulfate versus vehicle control in patients with interstitial cystitis/painful bladder syndrome. Urology. 2010;76(4):804–809. doi: 10.1016/j.urology.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 43.Cervigni M, Natale F, Nasta L, Mako A. Intravesical hyaluronic acid and chondroitin sulphate for bladder pain syndrome/interstitial cystitis: long-term treatment results. Int Urogynecol J. 2012;23(9):1187–1192. doi: 10.1007/s00192-012-1742-y [DOI] [PubMed] [Google Scholar]
- 44.Cervigni M, Sommariva M, Tenaglia R, et al. A randomized, open-label, multicenter study of the efficacy and safety of intravesical hyaluronic acid and chondroitin sulfate versus dimethyl sulfoxide in women with bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. 2017;36(4):1178–1186. doi: 10.1002/nau.23091 [DOI] [PubMed] [Google Scholar]
- 45.Arslan B, Gönültaş S, Gökmen E, Özman O, Avci MA, Özdemir E. Outcomes of intravesical chondroitin-sulfate and combined hyaluronic-acid/chondroitin-sulfate therapy on female sexual function in bladder pain syndrome. Int Urogynecol J. 2019;30(11):1857–1862. doi: 10.1007/s00192-019-04036-2 [DOI] [PubMed] [Google Scholar]
- 46.Pyo JS, Cho WJ. Systematic review and meta-analysis of intravesical hyaluronic acid and hyaluronic acid/chondroitin sulfate instillation for interstitial cystitis/painful bladder syndrome. Cell Physiol Biochem. 2016;39(4):1618–1625. doi: 10.1159/000447863 [DOI] [PubMed] [Google Scholar]
- 47.Bade JJ, Laseur M, Nieuwenburg A, van der Weele LT, Mensink HJ. A placebo-controlled study of intravesical pentosanpolysulphate for the treatment of interstitial cystitis. Br J Urol. 1997;79(2):168–171. doi: 10.1046/j.1464-410X.1997.03384.x [DOI] [PubMed] [Google Scholar]
- 48.Davis EL, El Khoudary SR, Talbott EO, Davis J, Regan LJ. Safety and efficacy of the use of intravesical and oral pentosan polysulfate sodium for interstitial cystitis: a randomized double-blind clinical trial. J Urol. 2008;179(1):177–185. doi: 10.1016/j.juro.2007.08.170 [DOI] [PubMed] [Google Scholar]
- 49.Barua JM, Arance I, Angulo JC, Riedl CR. A systematic review and meta-analysis on the efficacy of intravesical therapy for bladder pain syndrome/interstitial cystitis. Int Urogynecol J. 2016;27(8):1137–1147. doi: 10.1007/s00192-015-2890-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int. 2009;103(7):910–918. doi: 10.1111/j.1464-410X.2008.08162.x [DOI] [PubMed] [Google Scholar]
- 51.International Painful Bladder Foundation. Interstitial cystitis/painful bladder syndrome: anesthetic intravesical cocktails. Available from: https://www.painful-bladder.org/pdf/IPBF.intravesicalcocktails.pdf. Accessed July, 2020.
- 52.Welk BK, Teichman JM. Dyspareunia response in patients with interstitial cystitis treated with intravesical lidocaine, bicarbonate, and heparin. Urology. 2008;71(1):67–70. doi: 10.1016/j.urology.2007.09.067 [DOI] [PubMed] [Google Scholar]
- 53.Cocci A, Alowidah I, Skews R, Hashim H. 421 Comparison of Cystistat®, iAluril®, and Whitmore Cocktail for treatment of patients with bladder pain syndrome/interstitial cystitis. Eur Urol Suppl. 2016;15(3):e421. doi: 10.1016/S1569-9056(16)60423-2 [DOI] [Google Scholar]
- 54.Cox A, Golda N, Nadeau G, et al. CUA guideline: diagnosis and treatment of interstitial cystitis/bladder pain syndrome. Can Urol Assoc J. 2016;10(5–6):E136–E155. doi: 10.5489/cuaj.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olsen LE, Dyer JE, Haq A, Ockrim J, Greenwell TJ. A systematic review on the literature on cystodistention in bladder pain syndrome. Int Urogynaecol J. 2018;29(2):251–257. doi: 10.1007/s00192-017-3355-y [DOI] [PubMed] [Google Scholar]
- 56.Ottem DP, Teichman JM. What is the value of cystoscopy with hydrodistension for interstitial cystitis? Urology. 2005;66(3):494–499. doi: 10.1016/j.urology.2005.04.011 [DOI] [PubMed] [Google Scholar]
- 57.Peeker R, Aldenborg F, Fall M. Complete transurethral resection of ulcers in classic interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11(5):290–295. doi: 10.1007/s001920070019 [DOI] [PubMed] [Google Scholar]
- 58.Ryu J, Pak S, Song M, Chun JY, Hong S, Choo MS. Elimination of hunner’s ulcers by fulguration in patients with interstitial cystitis: is it effective and long lasting? Korean J Urol. 2013;54(11):767–771. doi: 10.4111/kju.2013.54.11.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ko KJ, Cho WJ, Lee YS, Choi J, Byun HJ, Lee KS. Comparison of the efficacy between transurethral coagulation and transurethral resection of hunner lesion in interstitial cystitis/bladder pain syndrome patients: a prospective randomized controlled trial. Eur Urol. 2020;77(5):644–651. doi: 10.1016/j.eururo.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 60.Rofiem O, Hom D, Fried RM, et al. Use of the neodymium: YAG laser for interstitial cystitis: a prospective study. J Urol Assoc J. 2009;3(6):473–477. [PubMed] [Google Scholar]
- 61.Cox M, Klutke JJ, Klutke CG. Assessment of patient outcomes following submucosal injection of triamcinolone for treatment of hunner’s ulcer subtype interstitial cystitis. Can J Urol. 2009;16(2):4536–4540. [PubMed] [Google Scholar]
- 62.Jiang T, Zhou X, Chen Z, et al. Clinical efficacy of submucosal injection of triamcinolone acetonide in the treatment of type II/III interstitial cystitis/bladder pain syndrome. BMC Urol. 2020;20(1):36. doi: 10.1186/s12894-020-00597-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuo HC, Chancellor MB. Comparison of intravesical botulinum toxin A type injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int. 2009;104(5):657–661. doi: 10.1111/j.1464-410X.2009.08495.x [DOI] [PubMed] [Google Scholar]
- 64.Akiyama Y, Nomiya A, Niimi A, et al. Botulinum toxin type A injection for refractory interstitial cystitis: a randomized comparative study and predictors of treatment response. Int J Urol. 2015;22(9):835–841. doi: 10.1111/iju.12833 [DOI] [PubMed] [Google Scholar]
- 65.Kuo HC, Jiang YH, Tsai YC, Kuo YC. Intravesical botulinum toxin-a injections reduce bladder pain of interstitial cystitis/bladder pain syndrome refractory to conventional treatment – a prospective, multicener, randomized, double blind, placebo controlled clinical trial. Neurourol Urodyn. 2016;35(5):609–614. doi: 10.1002/nau.22760 [DOI] [PubMed] [Google Scholar]
- 66.Wang J, Wang Q, Wu Q, Chen Y, Wu P. Intravesical botulinum toxin A injections for bladder pain syndrome/interstitial cystitis: a systematic review and meta-analysis of controlled studies. Med Sci Monit. 2016;14(22):3257–3267. doi: 10.12659/MSM.897350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans RJ, Overholt T, Colaco M, Walker SJ. Injection location does not impact botulinum toxin A efficacy in interstitial cystitis/bladder pain syndrome patients. Can J Urol. 2020;27(1):10125–10129. [PubMed] [Google Scholar]
- 68.Lee C-L, Kuo H-C. Intravesical botulinum toxin a injections do not benefit patients with ulcer type interstitial cystitis. Pain Physician. 2013;16(2):109–116. [PubMed] [Google Scholar]
- 69.Pinto R, Lopes T, Costa D, et al. Ulcerative and nonulcerative forms of bladder pain syndrome/interstitial cystitis do not differ in symptom intensity or response to onabotulinum toxin A. Urology. 2014;83(5):1030–1034. doi: 10.1016/j.urology.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 70.Gajewski JB, Al-Zahrani AA. The long-term efficacy of sacral neuromodulation in the management of intractable cases of bladder pain syndrome: 14 years of experience in one centre. BJU Int. 2011;107(8):1258–1264. doi: 10.1111/j.1464-410X.2010.09697.x [DOI] [PubMed] [Google Scholar]
- 71.Wang J, Chen Y, Chen J, Zhang G, Wu P. Sacral neuromodulation for refractory bladder pain syndrome/interstitial cystitis: a global systematic review and meta-analysis. Sci Rep. 2017;7(1):11031. doi: 10.1038/s41598-017-11062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peters KM, Feber KM, Bennett RC. A prospective, single blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystits. BJU Int. 2007;100(4):835–839. doi: 10.1111/j.1464-410X.2007.07082.x [DOI] [PubMed] [Google Scholar]
- 73.Valdemar A, Granlund P, Schultz A, Talseth T, Hedlund L, Frich L. Long-term experience with surgical treatment of selected patients with bladder pain syndrome/interstitial cystitis. Scand J Urol Nephrol. 2012;46(4):284–289. doi: 10.3109/00365599.2012.669789 [DOI] [PubMed] [Google Scholar]
- 74.Rossberger J, Fall M, Jonsson O, Peeker R. Long-term results of reconstructive surgery in patients with bladder pain syndrome/interstitial cystitis: subtyping is imperative. Urology. 2007;70(4):638–642. doi: 10.1016/j.urology.2007.05.028 [DOI] [PubMed] [Google Scholar]
- 75.Osman NI, Bratt DG, Downey AP, et al. A systematic review of surgical interventions for the treatment of bladder pain syndrome/Interstitial cystitis. Eur Urol Focus. 2020;S2405–4569(20)30071–7. [DOI] [PubMed] [Google Scholar]
- 76.Chen H, Wang F, Chen W, et al. Efficacy of daily low-dose sildenafil for treating interstitial cystitis: results of a randomized, double-blind, placebo-controlled trial—treatment of interstitial cystitis/painful bladder syndrome with low-dose sildenafil. Urology. 2014;84(1):51–56. doi: 10.1016/j.urology.2014.02.050 [DOI] [PubMed] [Google Scholar]
- 77.Bosch PC. A randomized, double-blind, placebo controlled trial of adalimumab for interstitial cystitis/bladder pain syndrome. J Urol. 2014;191(1):77–82. doi: 10.1016/j.juro.2013.06.038 [DOI] [PubMed] [Google Scholar]
- 78.Qu H-C, Zhang W, Yan S, Liu Y-L, Wang P, Hurst R. Urinary nerve growth factor could be a biomarker for interstitial cystitis/painful bladder syndrome: a meta-analysis. PLoS One. 2014;9(9):e106321. doi: 10.1371/journal.pone.0106321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang ZY, Wang P, Bjorling DE. Treatment with a cannabinoid receptor 2 agonist decreases severity of established cystitis. J Urol. 2014;191(4):1153–1158. doi: 10.1016/j.juro.2013.10.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krenn H, Daha LK, Oczenski W, Fitzgerald RD. A case of cannabinoid rotation in a young woman with chronic cystitis. J Pain Symptom Manage. 2003;25(1):3–4. doi: 10.1016/S0885-3924(02)00601-2 [DOI] [PubMed] [Google Scholar]
- 81.Peters KM, Hasenau D, Killinger KA, et al. Liposomal bladder instillations for IC/BPS: an open-label clinical evaluation. Int Urol Nephrol. 2014;46(12):2291–2295. doi: 10.1007/s11255-014-0828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rappaport YH, Zisman A, Jeshurun-Gutshtat M, et al. Safety and feasibility of intravesical instillation of botulinum toxin-A in hydrogel-based slow-release delivery system in patients with interstitial cystitis-bladder pain syndrome: a Pilot Study. Urology. 2018;114:60–65. doi: 10.1016/j.urology.2017.12.028 [DOI] [PubMed] [Google Scholar]
- 83.Nickel JC, Jain P, Shore N, et al. Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: safety and efficacy of a new drug delivery device. Sci Transl Med. 2012;4(143):143ra100. doi: 10.1126/scitranslmed.3003804 [DOI] [PubMed] [Google Scholar]
- 84.Gallego-Vilar D, Garcia-Fadrique G, Povo-Martin I, et al. Maintenance of the response to dimethyl sulfoxide treatment using hyperbaric oxygen in interstitial cystitis/painful bladder syndrome: a prospective, randomized, comparative study. Urol Int. 2013;90:411–416. doi: 10.1159/000343697 [DOI] [PubMed] [Google Scholar]
- 85.Chuang Y-C, Meng E, Chancellor M, Kuo H-C. Pain reduction realized with extracorporeal shock wave therapy for the treatment of symptoms associated with interstitial cystitis/bladder pain syndrome—a prospective, multicenter, randomized, double-blind, placebo-controlled study. Neurourol Urodyn. 2013;39(5):1505–1514. doi: 10.1002/nau.24382 [DOI] [PubMed] [Google Scholar]