Abstract
The present research describes the synthesis of cadmium sulfide (CdS) nanoparticles from Escherichia coli under the influence of bacterial enzyme sulphate reductase and study on their cytotoxicity for applications in cancer therapy. Escherichia coli cells were used to synthesize CdS nanoparticles under different concentrations of cadmium chloride and sodium sulfide. The morphology of the nanoparticles was analysed using scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) was used for elemental analysis of nanoparticles. Fourier-transform infrared spectroscopy analysis (FTIR) was performed to assess the functional groups of the nanoparticles. Crystalline nature of nanoparticles was assessed using powder X-ray diffraction (XRD). Antibacterial studies of CdS nanoparticles were carried out on foodborne pathogens and cytotoxicity studies were carried out on Mus musculus skin melanoma (B16F10) and human epidermoid carcinoma (A431) cell lines. CdS nanoparticle showed more cytotoxic effect on cancer cells compared with standard 5-aminolevulinic acid (5-ALA). The Escherichia coli-synthesized CdS nanoparticles showed highest zone of inhibition in the ratio 4:1 of cadmium chloride and sodium sulfide on all tested bacterial strains. The nanoparticles were also tested for haemolytic activity on RBC cells, which exhibited lower cytotoxicity than sodium dodecyl sulphate which was used as positive control. The cytotoxicity of CdS nanoparticles assessed on A431 cells showed an inhibition of 81.53% at 100 μM concentration while the cytotoxicity assessed on B16F10 cells showed an inhibition of 75.71% at 200 μM concentration which was much efficient than 5-ALA which showed an inhibition of 31.95% at a concentration against B16F10 cells and 33.45% against A431 cells at a concentration of 1 mM. Cadmium sulfide nanoparticles were thus found to be highly toxic on cancer cells compare with standard anticancerous drug 5-ALA.
Electronic supplementary material
The online version of this article (10.1007/s42770-020-00238-9) contains supplementary material, which is available to authorized users.
Keywords: Cadmium sulfide nanoparticles, 5-Aminolevulinic acid, Cytotoxicity, Antimicrobial activity
Introduction
The use of microorganisms and plants in the synthesis of nanoparticles paved a new way for eco-friendly synthesis of nanoparticle [1]. Various nanoparticles like gold, silver, cadmium, platinum, zinc, copper and iron have been successfully synthesized using bacteria, yeast, algae and plants [2]. The bacterial synthesized nanoparticles are either extracellular or intracellular depending on the type of organism and nanoparticles [3]. The biological synthesis gave a high control over the size distribution of nanoparticles and did not require the use of stabilizing agents and also the nanoparticles were highly stable for over 6 months [4, 5].
CdS nanoparticles or quantum dots are extensively used in solar batteries, cells and various electronic devices. Cadmium nanocrystals are used as fluorophores and bio-labelling agents because of their unique character of exhibiting fluorescence [6]. However the use of these quantum dots in biological applications was limited because of tedious synthesis process and use of various toxic chemicals. The development of biological synthesis of quantum dots reduced the toxic chemicals used before and extended the horizon to biological applications [7]. Many bacteria like Escherichia coli, Bacillus subtilis and Lactobacillus acidophilus, have shown to synthesize cadmium nanoparticles either extracellular or intracellularly [8, 9]. As far as the biosynthesis of cadmium sulfide (CdS) nanoparticles is concerned, a number of biosynthesis methods have been reported. For example, CdS nanoparticles can be synthesized intracellularly by the yeasts Schizosaccharomyces pombe [10]. However, intracellular synthesis of CdS nanoparticles makes downstream processing difficult and beats the purpose of developing a simple and economical process. The extracellular enzyme secreted by the fungus Fusarium oxysporum can mediate extracellular synthesis of CdS nanoparticles [11]. On this context, this research work describes synthesis of CdS nanoparticles from Escherichia coli under the influence of bacterial enzymes sulphate reductase. Four different ratios of cadmium chloride and sodium sulfide were used to synthesize CdS nanoparticles and study was carried out on antimicrobial activity, haemolytic activity and cytotoxicity of CdS nanoparticles.
Materials and methods
Synthesis and characterization of CdS nanoparticles
The seed culture of Escherichia coli (MTCC 10312) was prepared by inoculating a loop full of organism into 3 mL of nutrient broth and incubated over night at 37 °C. Seed culture (2%) was transferred to 50 mL of nutrient broth and incubated for 24 h at 37 °C at 160 rpm. The cell biomass was centrifuged and supernatant was used for the synthesis of nanoparticles. The four different ratios of (1:1, 2:1, 3:1 and 4:1) cadmium chloride (0.25 M) and sodium sulfide (0.25 M) respectively were allowed to react in separate test tubes. Following the production of orange-yellow colour of sodium sulfide suspension, equal volume of bacterial supernatant was added and mixed thoroughly. The suspension was kept in water bath at 60 °C for 10–20 min until the formation of fluffy orange-yellow depositions at the bottom of test tube, which indicated the formation of nanoparticles. The suspension was allowed to cool and incubated at room temperature overnight and was observed for formation of coalescent orange-yellow crystals deposited at the bottom of the tube. The sodium chloride formed as a result of the reaction was discarded without disturbing the crystals at the bottom of test tube. The CdS nanoparticles were washed with acetone followed by sterile distilled water, air dried at 45 °C and stored at 6 °C [10–12].
Characterization of nanoparticles was carried out according to our previous studies [10]. The absorbance spectrum (300 to 650 nm) of the CdS nanoparticles was recorded by using the Shimazdu UV-2.42 UV-Visible spectrophotometer. The morphology of the nanoparticles were analysed using scanning electron microscopy (SEM). Elemental analysis was performed to check the purity and metallic content of nanoparticles using energy dispersive X-ray spectroscopy (EDX). Fourier-transform infrared spectroscopy analysis (FTIR) was performed in the infrared region of 400–4000 cm−1 to assess the functional groups of the nanoparticles. Powder X-ray diffraction, Rigaku-Smart Lab (XRD) was performed to assess the crystalline nature of nanoparticles. The intensities were recorded from 10 to 80° at 2Ɵ angles.
Antibacterial activity of CdS nanoparticles
The antibacterial activity of CdS nanoparticles was assessed against foodborne pathogens viz., Escherichia coli, Bacillus licheniformis, Pseudomonas aeruginosa, Bacillus cereus and Staphylococcus aureus by well diffusion methods according to CLSI standard method [13]. Mueller-Hinton agar plates were prepared and 100 μl of culture was uniformly spread with L-shape spreader. Six-millimeter diameter wells were made in each plate with a sterile cork borer. CdS nanoparticles synthesized with different ratios (1:1, 2:1, 3:1 and 4:1) of cadmium chloride and sodium sulfide were added to wells (20 μl of 40 mg/mL stock solution) and incubated at 37 °C for 24 h [14]. The zone of inhibition was measured and the results were compared against set of standard antibiotics; ampicillin (A25), norfloxin (NX10), trimethoprim (COT25), ceftazimidime (CA30) and cephatoxime (CTX30).
Antifungal activity of cadmium sulfide nanoparticles
Antifungal activity of cadmium sulfide nanoparticles was tested against 3 fungal species Fusarium oxysporum, Aspergillus flavus and Penicillium expansum. Fungal spores were prepared by dispersing a loop full of spores in sterile distilled water and mixing thoroughly. Potato dextrose agar was used for culturing Fusarium oxysporum and Aspergillus flavus, maltose dextrose agar was used for culturing of Penicillium expansum. A 100-μl fungal spore suspension was spread evenly on the plate and wells of 6 mm diameter were made using a sterile cork borer and 20 μl (66 mg/mL) CdS nanoparticle samples synthesized using different ratios (1:1, 2:1, 3:1 and 4:1) of cadmium chloride and sodium sulfide were added into wells and the plates were incubated at 28 °C for 72 h. The zone of inhibition was measured and compared with standard antifungal agent fluconazole.
RBC lysis assay of CdS nanoparticles
The RBC lysis assay was carried out according to Vives et al. [15]. The cytotoxicity was measured by the amount of haemoglobin released due to rupture of RBC’s using spectrophotometer. Five milliliters of blood was collected from healthy human donor and added to a test tube containing EDTA to prevent coagulation. The blood was centrifuged at 84g for 10 min and the yellow plasma and white fluffy layer containing white blood cells and platelets was removed carefully without disturbing the RBCs. The RBCs were washed thrice with 20-mM phosphate buffer saline (pH 7.4) at room temperature and subsequently resuspended again in phosphate buffer saline (nine times its volume). CdS nanoparticles in concentration ranges of 0.006, 0.012, 0.025, 0.05, 0.1 and 0.2 mM were added to 200 μL of RBCs and the total volume was made up to 1 mL by adding phosphate buffer saline. The mixture was incubated for 20 min at 37 °C and centrifuged at 2000 rpm for 3 min. The haemoglobin in the supernatant was collected and absorbance was measured at 540 nm using UV-Visible spectrophotometer. PBS was used as negative control and 1% SDS was used as positive controls. Percent haemolysis was calculated using the formula
In vitro cytotoxic analysis of cadmium sulfide nanoparticles against B16F10 and A431 cell lines
The cytotoxicity of CdS nanoparticles was tested against Mus musculus skin melanoma (B16F10) and human epidermoid carcinoma (A431) cell lines obtained from ATCC, USA. The cells were split and cultured in 75-cm2 plastic tissue culture flask supplemented with Dulbecco’s Modified Eagle medium (DMEM), 10% FBS and 1% penicillin/streptomycin. The flasks were incubated at humidified atmosphere of 37 °C with 5% CO2 in a CO2 incubator for 24 h. The cells were sub-cultured every 72 h. Before testing for cytotoxicity, the viability of the cells was checked using tryphan blue and the cells which had more than 90% viability were chosen for cytotoxicity assay. Trypsinization was performed on 80% confluent cells and centrifuged at 3020g. B16F10 and A431 cells at a concentration of 105 cells/well were seeded into a 96-well plate and incubated for 24 h at 37 °C in a CO2 incubator with the supply of 5% CO2. All the compounds to be tested were diluted for the required concentration in DMEM without FBS.
The cells were treated with different concentrations (0.006, 0.012, 0.050, 0.1 and 0.2 mM) of CdS nanoparticles in serum-free medium for 4 h at 37 °C. 5-Amino levulinic acid (5-ALA) was used as positive control at concentrations of 0.1, 0.5 and 1 mM. After treatment, the media containing nanoparticles were removed and replaced with 100-μl pure DMEM and incubated for 20 h in CO2 incubator. After incubation, the media components were removed and 100 μl of MTT (3-[4, 5- dimethylthiazol-2-yl]-2, 5- diphenyl tetrazolium bromide) solution (5 mg/10 mL of 1X PBS buffer) filtered using 0.22-μm syringe filter was added into each well and incubated for 4 h at 37 °C in a dark condition. The MTT solution was removed after incubation and 100 μl of dimethyl sulfoxide (DMSO) was added immediately to solubilize the formazan. The absorbance of the extract was measured at 590 nm with a microplate reader and images were captured using fluorescence microscope. Statistical assessment was performed using Graph Pad Prism® Version 6.01 between different concentrations using one-way ANOVA and comparison was performed by t test.
Results and discussion
Synthesis and characterization of Escherichia coli-synthesized CdS nanoparticles
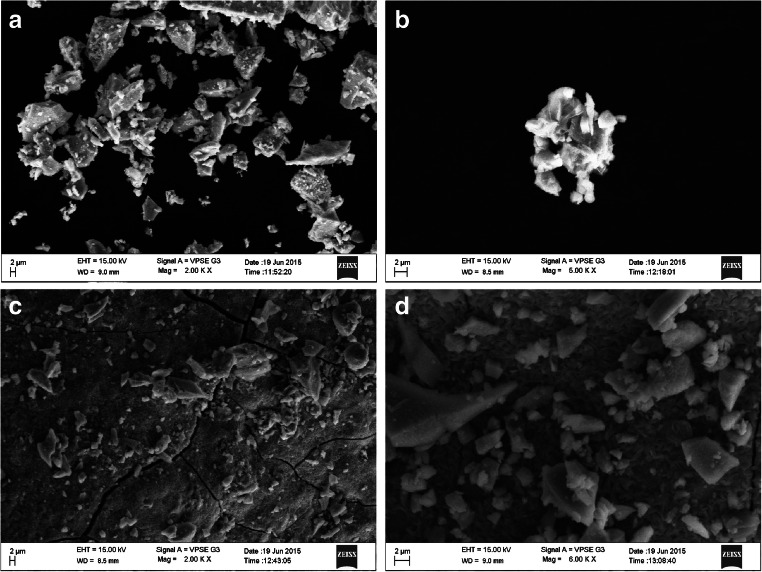
The formation of coalescent orange-yellow clusters at the bottom of the tube indicated the synthesis of CdS nanoparticles. The results showed the highest precipitation in 1:1 ratio and lowest in 4:1 ratio of cadmium chloride and sodium sulfide respectively indicating that the highest synthesis of CdS nanoparticles was found in the 4:1 ratio. This has also been confirmed by earlier studies conducted by Bai and Mousavi et al. which showed that formation of precipitate is inversely proportional to the formation of nanocrystal [12, 16]. Our results also correlate with the studies conducted previously which showed that the cells obtained from the stationary face had maximum synthesis of nanoparticles than cells of the logarithmic phase [17]. Our results can be correlated with the above findings as the cells used for the synthesis of nanoparticles were from stationary phase. The UV-Visible absorption spectrum showed strong absorption peaks from 420 to 440 nm (refer supplementary material 1). The broad peaks indicate the surface plasmon resonance exhibited and the varying size of the nanoparticles. The increase in the intensity of the peak at 3:1 ratio of cadmium chloride and sodium sulfide nanoparticles can be attributed to the increase in the number of nanoparticles in the solution. The decrease in the intensity of the peak or the shift of the optical absorption indicates the decreasing size of the nanoparticles [18, 19]. The SEM observation showed that the nanoparticles appeared triangular in shape with the length varying from 600 nm to 1 μm and the average size of the nanoparticles ranging from 40 to 80 nm taken at a scale bar of 2 μm. The size of the nanoparticles was cross verified using Image J software which confirmed the same [20]. A few aggregates were seen but the absence of internal agglomeration in nanoparticles showed the proteins played a crucial role in stabilizing and providing specific structure to the nanoparticles (Fig. 1). The stabilizing agents for nanoparticles are either present as free amino groups or residues which bind to the surface of nanoparticles by electrostatic interactions. It is noted that the binding of the proteins to nanoparticles did not alter the secondary structures of proteins. This was confirmed by using the FTIR analysis which showed the presence of various functional groups [21, 22]. The FTIR analysis showed various absorption bands from 4000 to 400 cm−1 (refer supplementary material 2). Peaks at 3437.49 to 3666.02 correspond to the O-H stretching vibrations and N-H stretching vibrations due to the primary and secondary amine linkages of proteins and amino acids [23]. The peaks occurring at 2924, 2923, 2855, 2854, 2853 correspond to C-H stretching vibrations of alkanes. The peaks at 1645, 1630, 1652 and 1632 correspond to N-H bending vibrations of amide I and amide II proteins [24]. Similarly peak at 1537.9 was due to the C=C bending vibration of the aromatics. The peaks at 1403, 1389 and 1385 correspond to the presence of aromatic and aliphatic alkane groups [25]. Seven hundred ten to seven hundred eighty peaks showed the phenyl ring substitution and 624, 625 peaks were due to S-S disulfide stretching. Peaks at 483 represent C-S stretching vibrations. The elemental analysis of CdS nanoparticles by EDX showed the presence of metals, cadmium and sulfide. The graph plotted between energy KeV and X-ray counts showed peaks 3–4 KeV which is the typical absorption peak for the metallic CdS nanoparticles due to surface plasmon resonance. The studies conducted by Pandian et al. and Rajeshkumar et al. also showed similar results [20, 26]. The graphs plotted for all ratios showed that the weight percentage of the cadmium gradually increased and showed the highest concentration at 4:1 ratio (refer supplementary material 3).
Fig. 1.
SEM image of CdS nanoparticles synthesized using different ratios of cadmium chloride and sodium sulfide (a 1:1, b 2:1, c 3:1, d 4:1)
The X-ray diffraction pattern measured at 2Ɵ angles from 10° ≤ 2Ɵ ≤ 80° showed sharp diffraction peaks for the ratio 1:1 of CdCl2 and Na2S. The peaks were seen at 27.50°, 31.76°, 43.56° and 51.80° which correspond to (111), (200), (220), (311) and the size varied from 1 to 30 nm. For 2:1 ratio of CdCl2 and Na2S, the peaks were observed at 27.49°, 31.22°, 43.56° and 52.10° which correspond to (111), (200), (220) and (311) with size varying from 20 to 70 nm. Similarly the peaks for 3:1 of CdCl2 and Na2S ratio were observed at 26.81°, 31.16°, 43.60° and 51.92° corresponding to (111), (200), (220) and (311) with size varying with 15–30 nm. The peaks for 4:1 ratio of CdCl2 and Na2S were observed at 26.71°, 31.13°, 43.94° and 52.26° corresponding to (111), (200), (220) and (311) with size varying from 2 to 10 nm (refer supplementary material 4). The lattice plane of CdS nanoparticles 1:1, 2:1 and 3:1 ratios showed spacing (d) of 3.2 Å corresponding to the strongest peak in the plane (111) and the lattice plane for 4:1 ratio nanoparticles showed (d) of 2.8 Å corresponding to the strongest peak (200) of face-centered crystalline nanoparticles which correlates to the results obtained by Malarkodi et al. and Rodriguez-Fragoso et al. [24, 27]. The peaks obtained corresponds to the data published by the International Centre for Diffraction Data (ICDD,) JCPDS (Joint Committee for Powder Diffraction Standards) file number 10-454 for CdS nanoparticles. The average particle size was determined by full width half maximum (FWHM) of the strongest peak by Scherrer’s equation.
where λ is the wavelength of X-ray radiation, β is the full width at half maximum (FWHM) in radians, θ is the Bragg angle of diffraction and K is the shape factor. The particle size correlated with the results obtained by using SEM analyses. The particle size usually depends on the reaction time, as the reaction time increases, the particle size also increases; however, the particle size is restricted to nanoscale due to agglomerations of the neighbouring crystals. And therefore the intensity of the XRD peak gradually decreases and the width of the peak increases with decreasing crystallite size [6].
Antibacterial and antifungal activity of cadmium sulfide nanoparticles
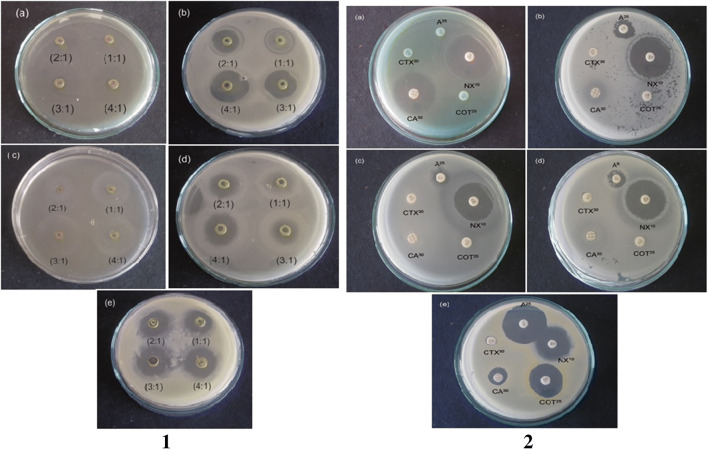
Antimicrobial activity against foodborne pathogens was performed using Escherichia coli-synthesized CdS nanoparticles which showed the highest inhibition in a ratio of 4:1 of cadmium chloride and sodium sulfide respectively on all tested bacterial strains (Fig. 2). The highest inhibition zone of 25.1 ± 0.7 mm was observed in Pseudomonas aeruginosa followed by Bacillus licheniformis (23.5 ± 0.5 mm), Escherichia coli (20.6 ± 0.5 mm), Bacillus cereus (13.6 ± 1.5 mm) and Staphylococcus aureus (18.5 ± 0.5 mm) (Table 1). CdS nanoparticles showed better antibacterial activity with the maximum inhibition on Pseudomonas aeruginosa which may be due to the electrostatic interaction of positively charged CdS nanoparticles with negatively charged protein surface. The nanoparticles release ions which interact with the thiol groups of the proteins resulting in the production of reactive oxygen species resulting in the disruption of cells [21, 28, 29]. The binding of the nanoparticles to the protein layer causes inhibition of active transport, dehydrogenase and the enzymatic activity in the periplasmic region and thus inhibits the synthesis of DNA, RNA and proteins leading to cell lysis [30, 31]. In the present work, we see high inhibition in the Gram-negative bacteria which has a slender peptidoglycan layer when compared with Gram-positive bacteria which has thick peptidoglycan layer. The nanoparticles can thus easily penetrate the cell wall of the Gram-negative bacteria. The inhibition also depends on the size of the nanoparticles. Generally smaller nanoparticles have more surface atoms which give them large surface area for interaction with bacterial cell. The smaller nanoparticles also have larger fractions of atoms on low coordination and high energy sites like corners, edges and steps which make them more active than larger molecules [32]. The inhibition of CdS nanoparticles compared with standard antibiotics indicated that Pseudomonas aeruginosa had maximum zone of inhibition (34.75 ± 0.35 mm) to norfloxin and (23.5 ± 0.70) to ceftazimidime (Fig. 2). Bacillus licheniformis showed the highest zone of inhibition to norfloxin (30.5 ± 0.70 mm), moderate inhibition to ampicillin (20 ± 2.8 mm) and trimethoprim (18.5 ± 0.70 mm) and low zone of inhibition to ceftazimidime (13.5 ± 0.71 mm). Bacillus cereus showed moderate zone of inhibition to norfloxin (22 ± 2.12 mm) and low zone of inhibition to ampicillin (8.5 ± 0.70 mm). Escherichia coli had the highest zone of inhibition to norfloxin (26.5 ± 2.12 mm) and low zone of inhibition for ampicillin (12.75 ± 0.35 mm) and ceftazimidime (8.25 ± 0.35 mm). Staphylococcus aureus showed the highest zone of inhibition in ampicillin (28.5 ± 2.12 mm) followed by norfloxin (25.5 ± 0.70 mm), trimethoprim (23.75 ± 0.35 mm), ceftazimidime (12.5 ± 0.70 mm) and cephatoxime (7.75 ± 0.35 mm) (Table 2). Therefore, it was clear from the studies that CdS nanoparticles had better antimicrobial activity against Pseudomonas aeruginosa, Bacillus cereus and Escherichia coli which did not show inhibition with standard antibiotics ampicillin, trimethoprim and cephatoxime.
Fig. 2.
Antibacterial activity of Escherichia coli-synthesized CdS nanoparticles against 1(a) Pseudomonas aeruginosa, 1(b) Bacillus licheniformis, 1(c) Bacillus cereus, 1(d) Escherichia coli and 1(e) Staphylococcus aureus; Antibacterial activity of standard antibiotics against food borne pathogens. 2(a) Pseudomonas aeruginosa, 2(b) Bacillus licheniformis, 2(c) Bacillus cereus, 2(d) Escherichia coli and 2(e) Staphylococcus aureus
Table 1.
Antibacterial activity of Escherichia coli-synthesized CdS nanoparticles
| Bacteria | Concentration of CdS NPs (mg/mL) | CdS nanoparticles in four different ratios Zone of inhibition (mm in diameter) | |||
|---|---|---|---|---|---|
| 1:1 | 2:1 | 3:1 | 4:1 | ||
| P. aeruginosa | 40 | 22.0 ± 1.0 | 24.3 ± 0.5 | 24.2 ± 0.6 | 25.1 ± 0.7 |
| B. licheniformis | 40 | 16.0 ± 0.5 | 18.2 ± 0.6 | 19.6 ± 0.5 | 23.5 ± 0.5 |
| B. cereus | 40 | 8.0 ± 1.0 | 11.3 ± 1.1 | 12.2 ± 1.1 | 13.6 ± 1.5 |
| E. coli | 40 | 15.8 ± 0.7 | 18.3 ± 1.1 | 20.3 ± 0.5 | 20.6 ± 0.5 |
| S. aureus | 40 | 16.4 ± 0.5 | 17.3 ± 0.5 | 18.3 ± 0.7 | 18.5 ± 0.5 |
± Standard deviation
Table 2.
Antibacterial activity of standards antibiotics against foodborne pathogens
| Bacteria | Zone of inhibition (mm in diameter) | ||||
|---|---|---|---|---|---|
| Ampicillin (A25) ZOI (mm) |
Norfloxin (NX10) ZOI (mm) |
Trimethoprim (COT25) ZOI (mm) |
Ceftazimidime (CA30) ZOI (mm) |
Cephatoxime (CTX30) ZOI (mm) |
|
| P. aeruginosa | – | 34.75 ± 0.35 | – | 23.5 ± 0.70 | – |
| B. licheniformis | 20 ± 2.8 | 30.5 ± 0.70 | 18.5 ± 0.70 | 13.5 ± 0.71 | – |
| B. cereus | 8.5 ± 0.70 | 22 ± 2.12 | – | – | – |
| E. coli | 12.75 ± 0.35 | 26.5 ± 2.12 | 8.25 ± 0.35 | ||
| S. aureus | 28.5 ± 2.12 | 25.5 ± 0.70 | 23.75 ± 0.35 | 12.5 ± 0.70 | 7.75 ± 0.35 |
± Standard deviation
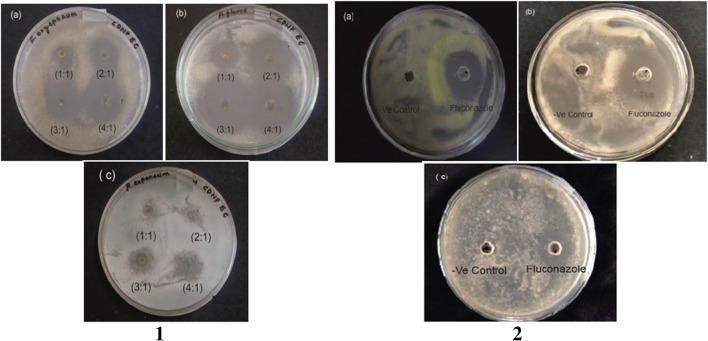
CdS nanoparticles efficiently inhibited the fungal growth showing effective antifungal activity (Fig. 3). The Escherichia coli-synthesized CdS nanoparticles showed maximum inhibition zone on Aspergillus flavus (29.7 ± 0.6 mm) followed by Fusarium oxysporum (23.0 ± 1.0 mm) and Penicillium expansum (18.6 ± 1.5 mm) (Table 3). Antifungal activity was maximum on Aspergillus flavus with minimum inhibitory concentration of 26 mg/mL but Fusarium oxysporum and Penicillium expansum showed no inhibition at this concentration and therefore a higher concentration of 66 mg/mL was used for efficient inhibition. An earlier study conducted by He et al. [33] showed that the antifungal activity depends on the growth morphology of the fungi. The fungi that grow densely on the surface show more inhibition as they are exposed more to nanoparticles. This increases the nucleic acid content due to the stress response of fungal hyphae. Consistent exposure of fungi to nanoparticles results in the formation of the ‘pits’ on the surface of cell resulting in the formation of pores and cell death [33, 34]. Flow cytometric analysis conducted by Kim et al. showed that the nanoparticles inhibited the cellular process involved in budding through destruction of membrane integrity [35]. The fungal antibiotic fluconazole showed maximum inhibition of (25.5 ± 0.5 mm) on Fusarium oxysporum, (17.7 ± 0.6 mm) in Aspergillus flavus and (12.2 ± 0.8 mm) in Penicillium expansum thus indicating the CdS nanoparticles had better activity (Fig. 3) (Table 4).
Fig. 3.
Antifungal activity of Escherichia coli-synthesized CdS nanoparticles against 1(a) Fusarium oxysporum, 1(b) Aspergillus flavus and 1(c) Penicillum expansum; Antifungal activity of standard fluconazole against 2(a) Fusarium oxysporum, 2(b) Aspergillus flavus and 2(c) Penecillium expansum
Table 3.
Antifungal activity of Escherichia coli-synthesized CdS nanoparticles
| Fungal strain | Concentration of CdS NPs (mg/mL) | CdS nanoparticles in four different ratios Zone of inhibition (mm in diameter) | |||
|---|---|---|---|---|---|
| 1:1 | 2:1 | 3:1 | 4:1 | ||
| F. oxysporum | 66 | 9.2 ± 0.7 | 20.0 ± 1.0 | 20.7 ± 0.6 | 23.0 ± 1.0 |
| A. flavus | 26 | 18.6 ± 0.5 | 27.7 ± 0.6 | 28.9 ± 0.7 | 29.7 ± 0.6 |
| P. expansum | 66 | 10.7 ± 0.6 | 15.8 ± 0.7 | 18.5 ± 0.5 | 18.6 ± 1.5 |
± Standard deviation
Table 4.
Antifungal activity of fluconazole against foodborne pathogens
| Bacteria | Concentration of NPs (mg) | Fluconazole ZOI (mm) |
|---|---|---|
| F. oxysporum | 25 | 25.5 ± 0.5 |
| A. flavus | 25 | 17.7 ± 0.6 |
| P. expansum | 25 | 12.2 ± 0.8 |
± Standard deviation
RBC lysis assay
The CdS nanoparticles had greater haemolytic activity compared with the standard 1% SDS which was used as positive control. A concentration of 0.2 mM CdS nanoparticles showed 33.55% haemolysis of RBC which is lower cytotoxic compare with SDS. The positive charge of the CdS nanoparticles can easily get accumulated in the cell membrane and thus act as a toxic material to cells which may have caused the haemolytic activity (Fig. 4).
Fig. 4.

Haemolytic activity of CdS nanoparticles synthesized from Escherichia coli (CDEC) and Bacillus licheniformis (CDBL)
In vitro cytotoxic analysis of cadmium sulfide nanoparticles against B16F10 and A431 cell lines
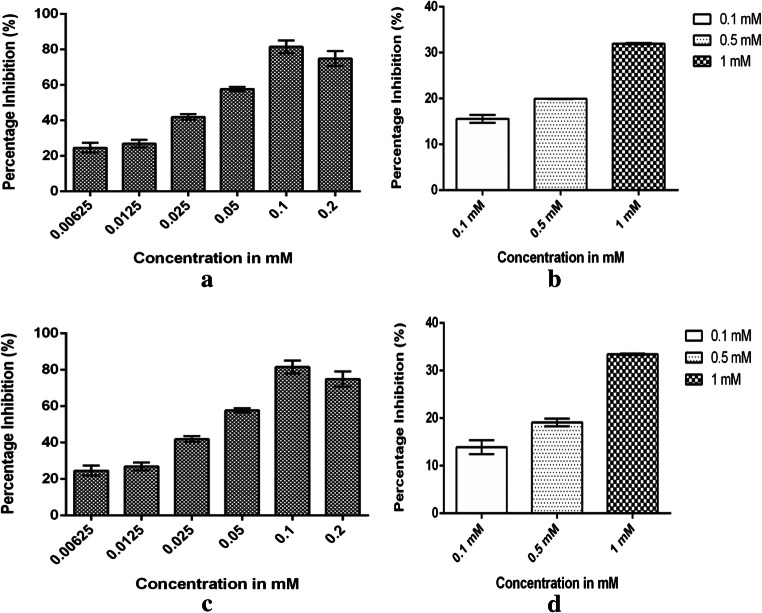
The cytotoxicity of CdS nanoparticles assessed on B16F10 cells showed that at 0.2-mM concentration, an inhibition of 75.71% was observed. The positive control 5-ALA showed a cytotoxicity of 31.95% at a concentration of 1 mM (Fig. 5). When compared with positive control, the CdS nanoparticles proved to be more cytotoxic. One-way ANOVA test performed between different concentrations of CdS nanoparticles showed the result were statistically different with a P value of 0.047* (Table 5) and an IC50 value of 92.2 mM was obtained against the B16F10 cells. The cytotoxicity of CdS nanoparticles assessed on A431 cells showed an inhibition of 81.53% at 0.1-mM concentration. An inhibition of 33.45% was observed in ALA at a concentration of 1 mM (Fig. 5). The ANOVA test performed at different concentrations of CdS nanoparticles showed no significant difference with P value which was greater than P > 0.05 (Table 5). An IC50 value of 33.42 mM was obtained which indicated that the CdS nanoparticles were more effective against the A431 cells compare with B16F10 cells. Earlier studies have indicated that the CdS nanoparticles produce reactive oxygen species (ROS) by either forming electron hole pairs to transfer electron to oxygen or by destroying the intracellular antioxidant system by direct interaction of CdS nanoparticles or by elevating the ROS molecules by the release of Cd2+ ions [36–38]. It is important to note that CdS nanoparticles produce ROS molecules both in presence or absence of light. Green and Howman reported that while the microsized CdS did not elevate ROS molecules, the nanoparticles promoted the intracellular ROS levels by 20–30%. Besides producing ROS molecules, the depletion of intracellular glutathione induces cellular oxidative stress which is regarded as the main reason for cytotoxicity. The depletion of glutathione may be due to chelation of cadmium ions reported in the study [39–43].
Fig. 5.
a Percentage inhibition of B16F10 cells from CdS nanoparticles. b Cytotoxicity of positive control (5-ALA) against B16F10 cells. c Percentage inhibition of A431 cells from CdS nanoparticles. d Cytotoxicity of positive control (5-ALA) against A431 cells
Table 5.
ANOVA results of CdS nanoparticles and 5-ALA treatment against B16F10 and A431 cells
| Cell type | F | P value | Statistical significant (P < 0.005) | Geisser-Greenhouse’s epsilon | R square |
|---|---|---|---|---|---|
| B16F10 Cell | |||||
| CdS nanoparticles | 6.452 | 0.0479 | Yes | 0.5329 | 0.5634 |
| Positive control (5-ALA) | 1.411 | 0.3568 | No | 0.5007 | 0.4137 |
| A431 cell | |||||
| CdS nanoparticles | 1.067 | 0.3580 | No | 0.5952 | 0.1758 |
| Positive control (5-ALA) | 1.321 | 0.3169 | No | 0.4806 | 0.4001 |
Conclusion
In the present work, CdS nanoparticles were synthesized using biological route with Escherichia coli. The morphological and structural studies showed that the nanoparticles were nearly 40–80 nm in size and possessed various functional groups like O-H, N-H, C=C, C-N and C-H which showed that proteins play an important role as capping agents in stabilizing the nanoparticles. The XRD studies also revealed that the nanoparticles were face-centered crystals with lattice plane spacing (d) of 3.2 nm. The nanoparticles were tested for antimicrobial and antifungal activity against a set of foodborne pathogens which showed better activity against Pseudomonas aeruginosa, Bacillus licheniformis, Escherichia coli and Aspergillus flavus than standard antibiotics. The RBC lysis assay of cadmium sulfide nanoparticles at a concentration of 0.2 mM showed nearly 33.55% haemolytic activity which was lower than sodium dodecyl sulphate. The cytotoxic effect of CdS nanoparticles was tested on Mus musculus skin melanoma (B16F10) and human epidermoid carcinoma (A431) cell lines which showed a high inhibition of 75.71% at a concentration of 0.2 mM against B16F10 and 81.53% inhibition against A431 cells lines at 0.1-mM concentration. The inhibition was compared against a standard anticancerous drug 5-Aminolevulinic Acid (5-ALA) which showed an inhibition of 31.95% against B16F10 cells and 33.45% against A431 cells at a concentration of 1 mM. The results showed that the cadmium sulfide nanoparticles had a better cytotoxic activity against cells than standard anticancer drug. The cadmium sulfide nanoparticles can thus be conjugated or capped with biological component and used for drug targeting, fluorophores and bio-labelling agents in minimum quantities.
Electronic supplementary material
(PDF 224 kb)
Acknowledgments
The authors acknowledge the Management of JSS Science and Technology University, Mysuru for providing all facilities to carry out this work.
Funding information
This work is financially assisted by the Technical Education Quality Improvement Program (TEQIP), Government of India, in the form of PhD research assistantship to Dr. Aishwarya Shivashankarappa.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mohanapuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future application. J Nanoparticles Res. 2008;10:507–517. [Google Scholar]
- 2.Debaditya B, Rajinder K. Nanotechnology and potential for microorganisms. Crit Rev Biotechnol. 2005;25(4):199–204. doi: 10.1080/07388550500361994. [DOI] [PubMed] [Google Scholar]
- 3.Kalishwaralal K, Babu RS, Venkataraman D, Mohd B, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B: Biointerfaces. 2008;65(1):150–153. doi: 10.1016/j.colsurfb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Bruna N, Collao B, Tello A, Caraventes P, Silva ND, Monras JP, Aenishanslins NO, Flores M, Gonzalez RE, Bravo D, Donoso JMP. Synthesis of salt stable fluorescent nanoparticles (quantum dots) by polyextremophile halophilic bacteria. Sci Rep. 2019;9(1):1953. doi: 10.1038/s41598-018-38330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh BR, Dwivedi S, Al-Khedhairy AA, Musarrat J. Colloids and surfaces. B Biointerfaces. 2011;85(2):207–213. doi: 10.1016/j.colsurfb.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Ramrakhiani M. Luminescence of cadmium sulfide nanoparticles and nanocomposites. International Journal of Luminescence and Applications. 2013;3(1):15–22. [Google Scholar]
- 7.Rzigalinski BA, Strobl JS. Cadmium containing nanoparticles: perspective on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol. 2009;238:280–288. doi: 10.1016/j.taap.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakkar TN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6(2):257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Abd E-R, LE Shanshoury-El E, Ebeid ME. Rapid biosynthesis of cadmium sulfide nanoparticles using culture supernatants of Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633 and Lactobacillus acidophilus DSMZ 20079T. Afr J Biotechnol. 2012;11(31):7957–7965. [Google Scholar]
- 10.Kowshik M, Deshmukh N, Vogel W, Urban J, Kulkarni SK, Paknikar KM. Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol Bioeng. 2002;78(5):583–588. doi: 10.1002/bit.10233. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad A, Mukherjee P, Mandal D, Senapati S, Khan MI, Kumar R, Sastry MJ (2002) Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus Fusarium oxysporum. J Am Chem Soc 16;124 (41):12108-12109 [DOI] [PubMed]
- 12.Mousavi RA, Sephay AA, Fazeli MR (2012) Biosynthesis, purification and characterization of cadmium sulfide nanoparticles using Enteriobacteriaceae and their applications. Scientific Research Publications 1(1)
- 13.CLSI (2018) Performance standards for antimicrobial disk susceptibility tests; approved standard, 13th Edition. CLSI document MO2 [ISBN 1–56238–834-7]”
- 14.Aishwarya S, Sanjay KR. Conjugation study of 5-aminolevulinic acid with microbial synthesized gold nanoparticles to evaluate its effect on skin melanoma and epidermoid arcinoma cell lines using photodynamic cancer therapy. Gold Bull. 2018;51:11–19. [Google Scholar]
- 15.Vives MA, Infante MR, Garcia E, Selve C, Maugras M, Vinadrell MP. Erythrocyte hemolysis and shape changes induced by new lysine-derivative surfactant. Chem Biol Interact. 1999;118(1):1–18. doi: 10.1016/s0009-2797(98)00111-2. [DOI] [PubMed] [Google Scholar]
- 16.Bai H, Zhang Z, Guo Y, Jia W. Biological synthesis of size-controlled cadmium sulfide nanoparticles using immobilized Rhodobacter sphaeroides. Nanoscale Res Lett. 2009;4:717–723. doi: 10.1007/s11671-009-9303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweeney RY, Mao C, Gao X, Burt JL, Belcher AM, Georgiou G, Iverson BL. Bacterial biosynthesis of cadmium sulfide nanoparticles. Chem Biol. 2004;11(11):1553–1559. doi: 10.1016/j.chembiol.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Mercy A, Salvaraj RS, Boaz BM, Anandhi A, Kanagadurai R. Indian J Pure Appl Phys. 2013;51:448–452. [Google Scholar]
- 19.Malarkodi C, Rajeshkumar S, Paulkumar K, Jobitha GG, Vanaja M, Annadurai G. Biosynthesis of semiconductor nanoparticles by using sulfur reducing bacteria Serratia nematodiphila. Adv Nano Res. 2013;1(2):83–91. [Google Scholar]
- 20.Rajeshkumar S, Ponnanikajamideen M, Malarkodi C, Malini M, Annadurai G. Microbe mediated synthesis of antimicrobial semiconductor nanoparticles by marine bacteria. J Nanostruct Chem. 2014;4:96. [Google Scholar]
- 21.Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbial Biotechnol. 2006;69(5):485–492. doi: 10.1007/s00253-005-0179-3. [DOI] [PubMed] [Google Scholar]
- 22.Sanghi R, Verma P. A facile green extracellular biosynthesis of CdS nanoparticles by immobilized fungus. Chem Eng J. 2009;155:886–891. [Google Scholar]
- 23.Chen G, Yi B, Zeng G, Niu Q, Yan M, Chen A, Du J, Huang J, Zhang Q. Facile green extracellular biosynthesis of CdS quantum dots by white rot fungus Phanerochaete chrysosprium. Colloids Surfaces B: Biointerfaces. 2014;117:199–205. doi: 10.1016/j.colsurfb.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Zhu H, Jiang R, Xiao L, Chang Y, Guan Y, Li X, Zeng G. Photocatalytic decolorization and degradation of Congo Red on innovative crosslinked chitosan/nano-CdS composite catalyst under visible light irradiation. J Hazard Mater. 2009;169(1–3):933–940. doi: 10.1016/j.jhazmat.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 25.Malarkodi C, Rajeshkumar S, Paulkumar K, Vanaja M, Gnanajobitha G, Annadurai G (2014) Biosynthesis and antimicrobial activity of semiconductor nanoparticles against oral pathogens, Bioinorganic Chemistry and Application [DOI] [PMC free article] [PubMed]
- 26.Pandian SRK, Deepak V, Kalishwarala K, Gurunathan S. Biologically synthesized fluorescent CdS NPs encapsulated by PHB. Enzym Microb Technol. 2011;48(4–5):319–325. doi: 10.1016/j.enzmictec.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Fragoso P, Reyes-Esparza J, Leon-Buitimea A, Rodriguez-Fragoso L. Synthesis, characterization and toxicological evaluation of maltodextrin capped cadmium sulfide nanoparticles in human cell lines and chicken embryos. J Nanobiotechnol. 2012;10(47):1–11(47). doi: 10.1186/1477-3155-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravikumar S, Gokulakrishnan R, Boomi P (2012) In vitro antimicrobial activity of the metal oxide nanoparticles against urinary tract infectious bacterial pathogens. Asian Pacific Journal of Tropical Disease:85–89
- 29.Vanaja M, Annadurai G. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl Nanosci. 2013;3(3):217–223. [Google Scholar]
- 30.Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications and toxicity effects. International Nano Letters. 2012;2:32. [Google Scholar]
- 31.Rezaei-Zarchi S, Javed A, Ghani MJ, Soufian S, Firouzabadi FB, Moghaddam AB, Mirjilili SH. Comparative study of antimicrobial activities of TiO2 and CdO nanoparticles against the pathogenic strain of Escherichia coli. Iran J Pathol. 2010;5(2):83–89. [Google Scholar]
- 32.Zhang H, Chen G. Potent antimicrobial activities of Ag/TiO2 nanocomposite powders synthesized by a one–pot sol–gel method. Environ Sci Technol. 2009;43(8):2905–2910. doi: 10.1021/es803450f. [DOI] [PubMed] [Google Scholar]
- 33.He L, Liu Y, Mustapha A, Lin M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res. 2011;166(3):207–215. doi: 10.1016/j.micres.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Kim KJ, Sung WS, Suh BK, Moon SK, Choi JS, Kim JG, Lee DG. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals. 2009;22(2):235–242. doi: 10.1007/s10534-008-9159-2. [DOI] [PubMed] [Google Scholar]
- 35.Kim KJ, Sung WS, Moon SK, Choi JS, Kim JG, Lee DG. Antifungal activity of silver nanoparticles on dermatophytes. J Microbiol Biotechnol. 2008;18(8):1482–1484. [PubMed] [Google Scholar]
- 36.Cho SJ, Maysinge D, Jain M, Roder B, Hackbarth S, Winnik FM. Longterm exposure to CdTe quantum dots causes functional impairments in live cells. Langmuir. 2007;23:1974–1980. doi: 10.1021/la060093j. [DOI] [PubMed] [Google Scholar]
- 37.Dailianis S, Piperakis SM, Kaloyianni M. Cadmium effects on ROS production and DNA damage via adrenergic receptors stimulation: role of Na+/H+ exchanger and PKC. Free Radic Res. 2005;39:1059–1070. doi: 10.1080/10715760500243765. [DOI] [PubMed] [Google Scholar]
- 38.Yang PM, Chen HC, Tsai JS, Lin LY. Cadmium induces Ca2+-dependent necrotic cell death through calpain-triggered mitochondrial depolarization and reactive oxygen species-mediated inhibition of nuclear factor-kappaB activity. Chem Res Toxicol. 2007;20:406–415. doi: 10.1021/tx060144c. [DOI] [PubMed] [Google Scholar]
- 39.Green M, Howman E. Semiconductor quantum dots and free radical induced DNA nicking. Chem Commun. 2005;121:123. doi: 10.1039/b413175d. [DOI] [PubMed] [Google Scholar]
- 40.Lovric J, Cho SJ, Winnik FM, Maysinger D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem Biol. 2005;12:1227–1234. doi: 10.1016/j.chembiol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Osseni RA, Debbasch C, Christen MO, Rat P, Warnet JM. Tacrine-induced reactive oxygen species in a human liver cell line: the role of anethole dithiolethione as a scavenger. Toxicol in Vitro. 1999;13:683–688. doi: 10.1016/s0887-2333(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 42.Gomer CJ, Ryter SW, Ferrario A, Rucker N, Wong S, Fisher AM. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56(10):2355–2360. [PubMed] [Google Scholar]
- 43.Schoursoeder A, Goldberg MS, Kstrup C, Wang Y, Jiang S, Joseph BJ, Levins CG, Kannan ST, Langer R, Andersen DG. Remotely activated protein producing nanoparticles. Nano Lett. 2012;12(6):2685–2689. doi: 10.1021/nl2036047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 224 kb)