Abstract
Pyroligneous acid (PA) was evaluated as a potential alternative to therapeutic antibiotics in poultry. Antimicrobial activity of PA was studied at acidic pH (2.0) and neutral pH (7.0) of the liquid against Salmonella enterica and Lactobacillus acidophilus. Acidic PA gave a MIC value of 0.8% (v/v) and 1.6% (v/v), and neutralized PA gave a MIC value of 1.6% (v/v) and 3.2% (v/v) against S. enterica and L. acidophilus respectively. Acidic PA was evaluated at different concentrations in a simulated poultry digestive tract and cecal fermentation to study its effect on the cecal microflora and fermentation profile. PA at a concentration of 1.6% (v/v) completely inhibited S. enterica and was also found to have a similar effect on lactobacilli count as compared with the control (p = 0.17). Additionally, PA at this concentration was found not to have a significant effect on acetic acid production after 24 h of cecal fermentation (p = 0.20).
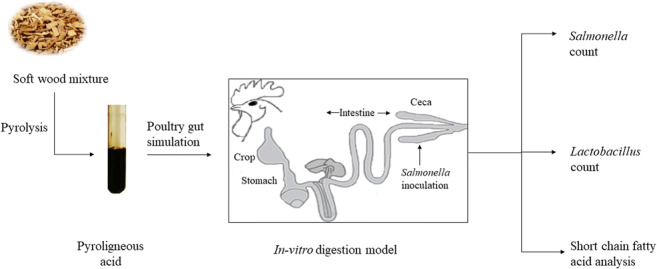
Graphical abstract
Keywords: Pyroligneous acid, Salmonella, Lactobacillus, Poultry gut simulation, SCFA production
Introduction
Bacterial gastroenteritis is a significant issue in the animal as well as human health. Salmonella enterica is a prevalent pathogen in poultry. The normal gut flora in poultry develops between 3 and 6 weeks of age, hence chicken is highly susceptible to Salmonella infections [1]. Salmonella is the most common cause of bacterial gastroenteritis in humans globally, and is transmitted to humans via the consumption of contaminated poultry products [2]. Hence, it is essential to implement effective control measures during the growing phase of broilers to decrease or eliminate the S. enterica load in the poultry gastrointestinal tract (GIT). In poultry, the ceca are the main site of colonization for Salmonella. The ceca are the most densely and diversely populated section of the poultry GIT and dysbiosis, i.e., an imbalance in the gut flora can decrease the host resistance to Salmonella infection.
Klasing et al. had suggested that host susceptibility to pathogens can be reduced by modification of the poultry diet [3]. Therapeutic antibiotics are one such feed additive that has been used to control bacterial enteritis caused by Salmonella. Trimethoprim, polymyxin B, and salinomycin sodium are some antibiotics that were shown to reduce or eliminate S. enterica counts in poultry [4]. However, the use of antibiotics comes at a price of selection and emergence of drug resistance in the environment [5].
Due to increased public health concerns from consumers, it has become essential for the food industry to look at alternatives for antibiotics in food production. Several prebiotics, such as oligofructose and inulin, have been extensively studied as an alternative to therapeutic antibiotics in broilers. Prebiotics favor the growth of beneficial bacteria in the gut, which can eliminate gut pathogens by competitive exclusion, i.e., the competition for nutrients and attachment sites [6]. Additionally, the selective proliferation of beneficial bacteria can produce antimicrobials such as short-chain fatty acids, which can potentially inhibit Salmonella. However, the results with these have not been consistent, and therefore, other alternatives need to be explored [7].
Pyroligneous acid (PA) is produced by the pyrolysis of lignocellulosic biomass. While the supplementation of PA in poultry feed has been shown to have a beneficial effect on broiler health, its use as a therapeutic agent has not been well explored [8, 9]. PA has a complex composition, consisting of several organic compounds [10, 11], each of which has a different mechanism of action for antimicrobial activity, thus rendering it difficult for pathogens to develop resistance. Furthermore, Watarai et al. (2005) reported that in an in vitro study, PA promoted the growth of beneficial bacteria such as Bifidobacterium [12]. Hence, both the direct and indirect antimicrobial actions of PA can be exploited synergistically to develop a suitable, effective, and environmentally friendly alternative to therapeutic antibiotics in poultry production, with a possible growth promotion action.
In the current study, a PA obtained from Pyrovac Inc. by the pyrolysis of a softwood mixture was evaluated in vitro for its anti-Salmonella efficiency as an alternative to therapeutic antibiotics in poultry production. Additionally, the effect of PA on Lactobacillus, which functions as a probiotic in poultry gut, was also studied. Before proceeding to poultry feeding trials, which are time consuming as well as expensive, it would be helpful to carry out an in vitro screening of the PA in simulated poultry digestion and cecal fermentation to evaluate its effect on pathogenic as well as beneficial gut bacteria. Therefore, the effect of the supplementation of PA on short-chain fatty acid (SCFA) production by cecal fermentation as well as Lactobacilli counts was also studied.
Materials and methods
Production and analysis of PA
The PA was produced by the pyrolysis of a softwood mixture (white pine, spruce, and fir) that was obtained from Belle-Ripe (www.belle-ripe.com), a biomass supplier company. Pyrolysis of the biomass was carried out in Pyrovac Inc., Lambert-de-Lauzon, QC, Canada, at 475 °C as described previously [13]. The composition of the PA was analyzed by gas chromatography-mass spectrometry (GC-MS) on an Agilent 5890 gas chromatograph. A 30-m long DB-5ms fused silica capillary with an inner diameter of 0.25 mm and coated with a 0.25-mm film thickness of cross-linked 5% phenyl methylpolysiloxane was used for separation of the PA components. The oven of the GC was maintained at 50 °C for 2 min, after which it was programmed to reach 290 °C at 5 °C min−1, and the final temperature was maintained for 10 min. The temperature of the injector was set to 280 °C with split mode (with a split ratio of 1/30). Helium, set with a flow rate of 1 mL min−1, was used as the carrier gas in the GC. The column end was introduced into the ion source of a mass detector (Agilent 5970) operated in the elector impact ionization mode. The mass spectrometer was operated at the following conditions—transfer line 270 °C, ion source 250 °C, and electron energy 70 eV. After every 0.8 s, the mass range (m/z) of 30–500 Da was scanned. Manual evaluation of the computerized match was done to maintain the quality of identification. The selected target compounds were identified by matching the retention time and mass spectra with known standard compounds. A series of standard mixture solutions (such as phenol, cresol, guaiacol, syringol, catechol, eugenol, and levoglucosan) at different concentrations were used as calibration solutions for quantification of the identified components of PA [14].
Antibacterial activity
Native PA (pH 2) and PA neutralized to pH 7 (with 10-M NaOH) were filter sterilized, and the antibacterial activity of these filtrates was tested against two bacterial strains—a pathogen (Salmonella enterica) and a probiotic (Lactobacillus acidophilus). A pathogenic poultry isolate of S. enterica was obtained from Dr. Diarra’s Lab (Agriculture and Agri-Food Canada, British Columbia) and grown in brain heart infusion (BHI) broth. L. acidophilus (NRRL B-23431), a probiotic strain, was grown in De Man, Rogosa, and Sharpe (MRS) broth. The MIC of the PA samples was determined against the two strains by the broth microdilution method as described by Fernandez et al. [15]. Briefly, 125 μL of sterile broth was added into the wells of a sterile 96-well plate. A total of 125 μL of PA was added to the first well, following which two-fold serial dilutions were carried out. To each well, 50 μL of a 1:1000 dilution of the overnight bacterial culture was added to give a final cell concentration of ~ 106 colony-forming units (CFU) mL−1. The experiments were carried out in triplicates, and controls with bacterial inoculum without PA as well as sterile broth were was also maintained. Plates were incubated at 37 °C for 24 h, and optical densities were measured using a spectrophotometer at 600 nm.
Cecal inoculum preparation
Cecal samples from chicken raised without antibiotics were obtained from Abattoir Agri-Bio Inc., QC, Canada. The contents of ceca from five birds were squeezed and pooled into sterile tubes under aseptic conditions. From this pooled sample, 0.1 g of cecal content was diluted with 300 mL of sterile 0.1-M anaerobic phosphate-buffered saline (pH 6.8) and mixed to obtain a homogenous solution, which was used as inoculum in the in vitro experiment [16, 17].
In vitro poultry digestion and cecal fermentation
Poultry feed used in this study was obtained from Agri-marché, QC, Canada, and its composition is given in (Table 1). In vitro digestion was carried out with 0.25 g of feed with five concentrations of PA—0.08% (v/w), 0.16% (v/w), 0.8% (v/w), 1.6% (v/w), and 4% (v/w). Basal feed without any supplementation was used as control. In vitro digestion was carried out by subsequent incubations with 0.1-M HCl, 1.5-M HCl, and 1-M NaHCO3 as described previously [18]. At the end of digestion, 5 mL of cecal slurry was added to each tube, and incubation was carried out at 37 °C for 24 h under anaerobic conditions [17]. Samples were withdrawn at 0 h and after 24 h of inoculation for analysis.
Table 1.
Composition of the poultry feed*
| Component | Composition |
|---|---|
| Crude protein | 18.4% |
| Raw fiber | 4.00% |
| Calcium | 0.8% |
| Phosphorus | 0.6% |
| Sodium | 0.19% |
| Vitamin A | 101,000 IU/kg |
| Vitamin D | 4984 IU/kg |
| Vitamin E | 50 IU/kg |
All values in %w/w, until specified
*As given by Agri-Marché
Bacterial enumeration
Samples were diluted sequentially in sterile saline by a dilution factor of 10, following which they were incubated on BPLS (brilliant green phenol red lactose sucrose) agar supplemented with sulfa mandelate at 37 °C for 24 h for enumeration of Salmonella and on MRS (De Man, Rogosa, and Sharpe) agar at 37 °C for 48 h in anaerobic conditions for enumeration of lactic acid bacteria [19].
Short-chain fatty acid analysis
For the quantification of SCFAs, the samples were centrifuged at 13,000×g for 10 min at 4 °C, following which the supernatant was filtered through a 0.22-μm syringe filter. To 500 μL of the filtered supernatant, 500 μL of 100-mM H2SO4 was added and extraction with diethyl ether was carried out. A gas chromatograph (6820GE; Agilent, Santa Clara, CA) equipped with a glass column (HP-innovax, 30 m by 0.320 mm) was used for the quantification of acetic acid, butyric acid, propionic acid, valeric acid, and iso-valeric acid. The temperatures of the detector, injection port, and column were 225 °C, 200 °C, and 200 °C, respectively, and SCFA concentrations were determined by comparison of peak heights of samples with those of standards [20].
Statistical analyses
Statistical analyses and SCFA production were carried out using STATISTICA (StatSoft, Tulsa, Oklahoma, USA). All results were expressed as the mean value of duplicates with standard deviation. An analysis of variance (ANOVA) was used to test the significance of the difference, and the difference was considered significant at a p value < 0.05.
Results and discussion
Characterization of PA
The chemical composition of the PA used in this study is given in Table 2. The PA was found to be consisted of known microbial inhibitors such as organic acids, phenolics, aldehydes, and ketones, as compared with previously reported literature.
Table 2.
Chemical composition of PA used in the current study
| Compound | Wt% |
|---|---|
| Water | 45 |
| Sugars** | 16 |
| Pyrolytic lignin | 9 |
| Acids* | 7.5 |
| Catechol | 5.6 |
| Methylguaiacol | 1.9 |
| Guaiacol | 1.5 |
| Methylisopropylcyclohexanone | 1.5 |
| Methylcatechol | 1.2 |
| Cresol | 0.9 |
| Furanone | 0.8 |
| Acetyloxycatechol | 0.8 |
| Ethylguaiacol | 0.6 |
| Hydroquinone | 0.6 |
| Propenylcatechol | 0.6 |
| Phenol | 0.5 |
| Triacetin | 0.5 |
| Acetyloxymethylfuraldehyde | 0.5 |
| Acetyldihydrofuranone | 0.5 |
| Maltol | 0.5 |
| Trimethylbenzenediol | 0.5 |
| Dimethoxybenzenebutyric acid | 0.5 |
| Dioxolane | 0.4 |
| Hexenone | 0.4 |
| Vanillin | 0.4 |
| Dimethylbenzenediol | 0.4 |
| Hydroxymethoxyphenylpropanone | 0.4 |
| Dimethylphenol | 0.3 |
| Eugenol | 0.3 |
| Dihydroxybenzenepropanone | 0.2 |
| Dihydroxyoctadienedione | 0.1 |
| Hydroxymethylcinnamaldehyde | 0.1 |
| Total | 100 |
*Mainly formic, acetic, and propionic acids
**Anhydro sugars, mainly levoglucosan
Organic acids, which account for the low pH of the sample, are produced by the thermal pyrolysis of xylans [21]. The present PA sample consisted of 7.5% of organic acids (mainly acetic, propionic, and formic acid), as compared with those previously reported by Rattanawut et al. (2.8%) [22], Hou et al. (3.10%) [23], and Oramahi et al. (between 3.6 and 4.3%) [24]. Phenolics, such as catechol (5.6%) and methylcatechol (1.2%), were also observed in higher concentrations as compared with previous studies by Xu et al. (4.62%) [25] and Fagernäs et al. (0.16%) [26]. Other antimicrobial compounds such as ketones (e.g., methylisopropylcyclohexanone (1.5%)), lactones (e.g., furanone (0.8%)), and aldehydes (such vanillin (0.4%) and hydroxymethylcinnamaldehyde (0.1%)) were also observed.
The feedstock used for pyrolysis determines the composition of the PA produced by the thermal degradation of plant lignocellulose. Softwoods (e.g., pine) are rich in 2-methoxyphenol, which is a major component of lignin and undergoes demethylation during pyrolysis, leading to the formation of phenols, cresols, and catechol [27]. The higher abundance of phenolics can also be attributed to the high temperatures (425–575 °C) at which pyrolysis is carried out [28]. The pyrolysis of xylans present in softwoods leads to the production of ketones and aldehydes, while the thermal degradation of hemicellulose produces lactones [11, 21].
Antibacterial activity
The antibacterial activity of acidic and neutralized PA was evaluated against Lactobacillus acidophilus and Salmonella enterica. Acidic PA gave an MIC of 0.8% (v/v) and 1.6% (v/v) against S. enterica and L. acidophilus respectively, while neutralized PA gave an MIC value of 1.6% (v/v) and 3.2% (v/v) against S. enterica and L. acidophilus respectively (Fig. 1). This indicates that acidic pH would be more efficient as a possible antimicrobial agent because it needed half the concentration of neutralized PA to inhibit Salmonella growth.
Fig. 1.
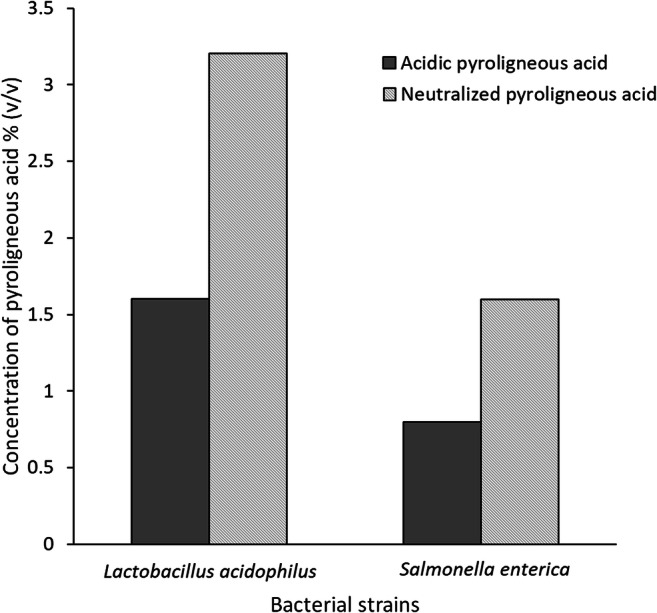
Comparison of MIC values against S. enterica and L. acidophilus for acidic and neutralized PA
It has been previously postulated that organic compounds such as organic acids and phenolics are primarily responsible for the antimicrobial activity of PA [29]. The mechanism of antibacterial activity of phenolics is via inactivation of bacterial enzymes and disruption of cell membranes [30]. Hydroxylated phenolics such as catechol have been reported to have a strong antimicrobial activity, due to the presence of multiple hydroxyl groups [31]. Undissociated organic acids can diffuse into bacterial cells and lower the pH of the cytoplasm, which leads to inhibition of enzyme activity and cell leakage [6]. PA is composed of several organic compounds (phenolics, organic acids, aldehydes, ketones), each of which has a weak antimicrobial activity. While the antibacterial activity of PA could be explained by a possible synergistic effect of its components, in the present study, it was observed that acidic PA was found to be more efficient as compared with neutralized PA. These results, in addition to the higher content of organic acids in the PA, could indicate that the antibacterial activity could primarily be associated with the pH of the PA. Additionally, it was also observed that the minimum concentration of PA needed for inhibition of Lactobacillus was double of that required for Salmonella inhibition. Therefore, it could be inferred that at a concentration needed to inhibit Salmonella, acidic PA would have no detrimental effect on the beneficial lactic acid bacteria. Watarai et al. (2005) previously reported that wood vinegar liquid from evergreen oak could have two simultaneous effects, i.e., inhibition of pathogenic bacteria, such as S. enteritidis, and the proliferation of beneficial bacteria such as Bifidobacterium thermiphilum, both of which would promote broiler health and productivity [12]. Based on the above, since the MIC value of acidic PA against Salmonella showed no detrimental effect on Lactobacillus, it would be interesting to study the effect of PA in cecal fermentation in a simulated poultry digestive tract.
Effect of PA on bacterial counts in cecal fermentation
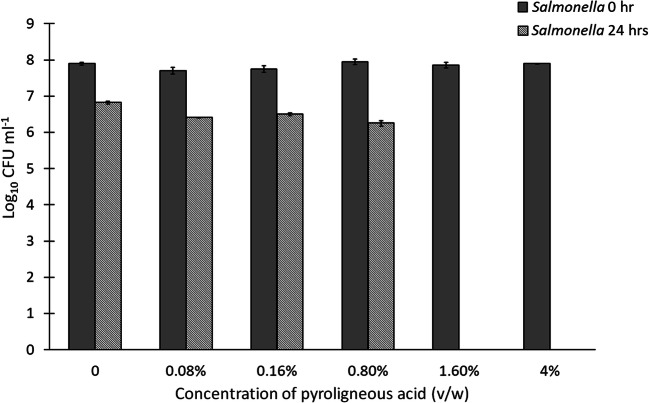
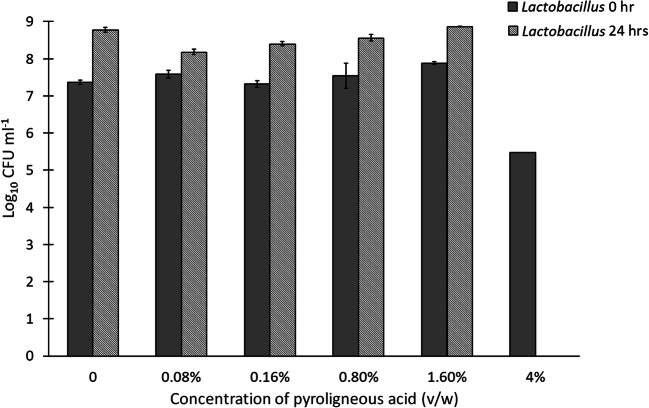
At the end of 24 h of cecal fermentation, there was a significant difference between the treatment groups for Salmonella counts (p = 0.00004). While any supplementation of PA was shown to decrease Salmonella counts, it was observed that PA at a concentration of 1.6% (v/w) inhibited Salmonella completely, and at a concentration of 4% (v/w), there was a total inhibition of all bacteria (Fig. 2). For the count of total lactobacilli also, a significant difference was observed among the treatment groups (p = 0.0005). Increasing concentrations of PA from 0.08 (v/w) to 1.6% (v/w) were found to increase Lactobacilli counts (Fig. 3). At 4% v/w concentration of PA, no bacterial growth was observed on the plate. Post hoc analysis by using the Neuman Keuls test showed that the mean Lactobacilli count obtained with 1.6% (v/w) of PA was statistically comparable to that obtained with the control (p = 0.74), while the counts with 0.08% (v/w), 0.16% (v/w), and 0.8% (v/w) were lesser than that of the control (p = 0.01, 0.37, and 0.01, respectively). The MIC against S. enterica was found to be two-fold higher in the cecal fermentation study as compared with the broth microdilution. When the PA was added to the feed, there could have been a partial adsorption of the PA by the feed particles, resulting in the reduced availability PA to “interact” with the S. enterica in the simulated hindgut. This may potentially explain the increased MIC value in the cecal fermentation study.
Fig. 2.
CFU counts for Salmonella with different concentrations of PA
Fig. 3.
CFU counts for Lactobacilli with different concentrations of PA
For the use of PA as feed additives, several studies have been carried out in swine rearing. Choi et al. (2009) reported that pigs fed with PA (produced from pyrolysis of oak chips at 500–700 °C) had higher counts of ileal Lactobacilli, which could be attributed to the presence of acidifiers, mainly organic acids in the PA, which consequently could reduce counts of harmful coliforms [32]. Similar results were also obtained by Wang et al. (2013), with piglets fed with bamboo PA with an acidifier, and they attributed this to the selective antibacterial activity of the feed supplements against acid-intolerant pathogenic bacteria and maintenance of the acid-tolerant Lactobacilli [33]. Kupittayanant and Kupittayanant observed that PA had a protective effect against diarrhea in weaning piglets by decreasing counts of Escherichia coli and increasing counts of Lactobacillus spp. [34]. Rattanawut (2013) reported that in Betong chicken, bamboo PA (which has acetic acid as its main component) was shown to reduce fecal E. coli and Salmonella counts [35].
In the current study, 1.6% (v/w) PA was shown to completely inhibit Salmonella and has no direct negative effect on cecal Lactobacilli when compared with the in vitro control. Since the proliferation of beneficial bacteria is also dependent on the host immunomodulatory responses, it would be interesting to study the effect of PA supplementation at this concentration in the field.
Effect of PA on SCFA production in cecal fermentation
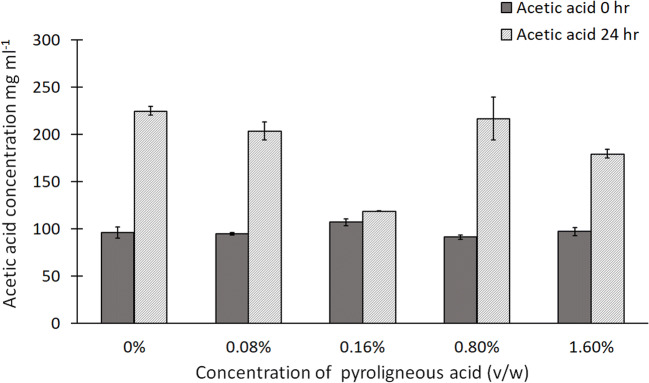
Since no bacterial growth was observed at the PA concentration of 4% (v/w), SCFA analysis was not done for this treatment. For the other treatments, only acetic acid was detected. It was observed that after 24 h of fermentation, there was no significant difference in the production of acetic acid among the remaining treatment groups (p = 0.2) (Fig. 4). Therefore, it can be inferred that supplementation of PA at 1.6% (v/w) did not modify the cecal fermentation profile as compared with the control in vitro. Previous studies have reported that wood vinegar can modify cecal microflora as well as nutrient absorption. This along with its capacity to inhibit intestinal pathogens such as Salmonella can be exploited to reduce the load of therapeutic antibiotics in poultry.
Fig. 4.
Production of acetic acid by cecal bacteria with different concentrations of PA
Conclusion
The PA used in the current study was produced from a softwood mixture comprising of white pine, spruce, and fir, and was found to consist of antimicrobial compounds such as organic acids, phenolics, and vanillin, the synergistic action of which could account for the high antibacterial activity in vitro even at low concentrations. The supplementation of PA at 1.6% (v/w) was shown to inhibit Salmonella completely and has no negative effect for Lactobacilli counts or the production of acetic acid in vitro. Therefore, it would be of interest to study the effect of PA supplementation in the field against an artificial Salmonella challenge.
Abbreviations
- GIT
Gastrointestinal tract
- PA
Pyroligneous acid
- SCFA
Short-chain fatty acids
- GC-MS
Gas chromatography-mass spectrometry
- MIC
Minimum inhibitory concentration
- BHI
Brain heart infusion
- MRS
De Man, Rogosa, and Sharpe
- BPLS
Brilliant green phenol red lactose sucrose
- ANOVA
Analysis of variance
Authors’ contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Gayatri Suresh and Hooshang Pakdel. The first draft of the manuscript was written by Gayatri Suresh, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding information
This work was supported by the Natural Sciences and Engineering Research Council of Canada (Engage Grant 122842) and Fonds de recherche du Québec - Nature et technologies (FRQNT Equipe).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Highlights
• Pyroligneous acid (PA) produced by softwood pyrolysis and analyzed by GC-MS.
• MIC determined for acidic and neutralized PA against S. enterica and Lactobacillus.
• In vitro cecal fermentation with feed with different PA concentrations for 24 h.
• 1.6% (v/v) PA inhibited Salmonella completely and had no effect on Lactobacilli.
• 1.6% (v/v) PA had no significant effect on SCFA production.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang Y, Tellez G, Latorre JD, Ray PM, Hernandez X, Hargis BM, Ricke SC, Kwon YM. Salmonella excludes Salmonella in poultry: confirming an old paradigm using conventional and barcode-tagging approaches. Front Vet Sci. 2018;5:101. doi: 10.3389/fvets.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant AQ, Hashem F, Parveen S. Salmonella and campylobacter: antimicrobial resistance and bacteriophage control in poultry. Food Microbiol. 2016;53(Part B):104–109. doi: 10.1016/j.fm.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Klasing K. Nutritional modulation of resistance to infectious diseases. Poult Sci. 1998;77(8):1119–1125. doi: 10.1093/ps/77.8.1119. [DOI] [PubMed] [Google Scholar]
- 4.Vandeplas S, Dauphin RD, Beckers Y, Thonart P, Thewis A. Salmonella in chicken: current and developing strategies to reduce contamination at farm level. J Food Prot. 2010;73(4):774–785. doi: 10.4315/0362-028X-73.4.774. [DOI] [PubMed] [Google Scholar]
- 5.Card RM, Cawthraw SA, Nunez-Garcia J, Ellis RJ, Kay G, Pallen MJ, Woodward MJ, Anjum MF. An in-vitro chicken gut model demonstrates transfer of a multidrug resistance plasmid from Salmonella to commensal Escherichia coli. MBio. 2017;8(4):e00777–e00717. doi: 10.1128/mBio.00777-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suresh G, Das RK, Kaur Brar S, Rouissi T, Avalos Ramirez A, Chorfi Y, Godbout S. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit Rev Microbiol. 2018;44(3):318–335. doi: 10.1080/1040841X.2017.1373062. [DOI] [PubMed] [Google Scholar]
- 7.Eeckhaut V, Wang J, Van Parys A, Haesebrouck F, Joossens M, Falony G, Raes J, Ducatelle R, Van Immerseel F. The probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front Microbiol. 2016;7:1416. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samanya M, K-e Y. Morphological changes of the intestinal villi in chickens fed the dietary charcoal powder including wood vinegar compounds. J Poult Sci. 2001;38(4):289–301. doi: 10.2141/jpsa.38.289. [DOI] [Google Scholar]
- 9.Youn B, Nam K, Chang K, Hwang S, Choe I. Effects of wood vinegar addition for meat quality improvement of old layer. Korean J Poult Sci. 2005;32(2):101–106. [Google Scholar]
- 10.Grewal A, Abbey L, Gunupuru LR. Production, prospects and potential application of pyroligneous acid in agriculture. J Anal Appl Pyrolysis. 2018;135:152–159. doi: 10.1016/j.jaap.2018.09.008. [DOI] [Google Scholar]
- 11.Mathew S, Zakaria ZA. Pyroligneous acid—the smoky acidic liquid from plant biomass. Appl Microbiol Biotechnol. 2015;99(2):611–622. doi: 10.1007/s00253-014-6242-1. [DOI] [PubMed] [Google Scholar]
- 12.Watarai S. Eliminating the carriage of Salmonella enterica serovar Enteritidis in domestic fowls by feeding activated charcoal from bark containing wood vinegar liquid (Nekka-Rich) Poult Sci. 2005;84(4):515–521. doi: 10.1093/ps/84.4.515. [DOI] [PubMed] [Google Scholar]
- 13.Roy C, Blanchette D, de Caumia B (2000) Industrial scale demonstration of the Pyrocycling (TM) process for the conversion of biomass to biofuels and chemicals. Paper presented at the First World Conference and Exhibition on Biomass for Energy and Industry, Sevilla, Spain, June 5-9, 2000
- 14.Suresh G, Pakdel H, Rouissi T, Brar SK, Fliss I, Roy C. In vitro evaluation of antimicrobial efficacy of pyroligneous acid from softwood mixture. Biotechnol Res Innov. 2019;3(1):47–53. doi: 10.1016/j.biori.2019.02.004. [DOI] [Google Scholar]
- 15.Fernandez B, Le Lay C, Jean J, Fliss I. Growth, acid production and bacteriocin production by probiotic candidates under simulated colonic conditions. J Appl Microbiol. 2013;114(3):877–885. doi: 10.1111/jam.12081. [DOI] [PubMed] [Google Scholar]
- 16.Meimandipour A, Shuhaimi M, Hair-Bejo M, Azhar K, Kabeir BM, Rasti B, Yazid AM. In vitro fermentation of broiler cecal content: the role of lactobacilli and pH value on the composition of microbiota and end products fermentation. Lett Appl Microbiol. 2009;49(4):415–420. doi: 10.1111/j.1472-765X.2009.02674.x. [DOI] [PubMed] [Google Scholar]
- 17.Donalson LM, Kim WK, Chalova VI, Herrera P, McReynolds JL, Gotcheva VG, Vidanović D, Woodward CL, Kubena LF, Nisbet DJ, Ricke SC. In vitro fermentation response of laying hen cecal bacteria to combinations of fructooligosaccharide prebiotics with alfalfa or a layer ration. Poult Sci. 2008;87(7):1263–1275. doi: 10.3382/ps.2007-00179. [DOI] [PubMed] [Google Scholar]
- 18.Suresh G, Santos DU, Rouissi T, Brar SK, Mehdi Y, Godbout S, Chorfi Y, Ramirez AA. Production and in-vitro evaluation of an enzyme formulation as a potential alternative to feed antibiotics in poultry. Process Biochem. 2019;80:9–16. doi: 10.1016/j.procbio.2019.01.023. [DOI] [Google Scholar]
- 19.Giannenas I, Papaneophytou C, Tsalie E, Triantafillou E, Tontis D, Kontopidis G. The effects of benzoic acid and essential oil compounds in combination with protease on the performance of chickens. J Anim Feed Sci. 2014;23(1):73–81. doi: 10.22358/jafs/65719/2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei F, Yin Y, Wang Y, Deng B, Yu HD, Li L, Xiang C, Wang S, Zhu B, Wang X. Higher-level production of volatile fatty acids in vitro by chicken gut microbiotas than by human gut microbiotas as determined by functional analyses. Appl Environ Microbiol. 2012;78(16):5763–5772. doi: 10.1128/AEM.00327-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefanidis SD, Kalogiannis KG, Iliopoulou EF, Michailof CM, Pilavachi PA, Lappas AA. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J Anal Appl Pyrolysis. 2014;105:143–150. doi: 10.1016/j.jaap.2013.10.013. [DOI] [Google Scholar]
- 22.Rattanawut J, Todsadee A, K-e Y. Effects of bamboo charcoal powder including vinegar supplementation on performance, eggshell quality, alterations of intestinal villi and intestinal pathogenic bacteria populations of aged laying hens. Ital J Anim Sci. 2017;16(2):259–265. doi: 10.1080/1828051X.2017.1283544. [DOI] [Google Scholar]
- 23.Hou X, Qiu L, Luo S, Kang K, Zhu M, Yao Y. Chemical constituents and antimicrobial activity of wood vinegars at different pyrolysis temperature ranges obtained from Eucommia ulmoides Olivers branches. RSC Adv. 2018;8(71):40941–40949. doi: 10.1039/C8RA07491G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oramahi HA, Yoshimura T, Diba F, Setyawati D. Antifungal and antitermitic activities of wood vinegar from oil palm trunk. J Wood Sci. 2018;64(3):311–317. doi: 10.1007/s10086-018-1703-2. [DOI] [Google Scholar]
- 25.Xu H, Zhao J, Yang J, Xie J, Zhang N, Jiang J. Effects of apple and pear wood vinegar components on Pleurotus ostreatus mycelium growth. BioResources. 2020;15(2):2961–2970. doi: 10.15376/biores.15.2.2961-2970. [DOI] [Google Scholar]
- 26.Fagernäs L, Kuoppala E, Tiilikkala K, Oasmaa A. Chemical composition of birch wood slow pyrolysis products. Energy Fuel. 2012;26(2):1275–1283. doi: 10.1021/ef2018836. [DOI] [Google Scholar]
- 27.Li R, Narita R, Nishimura H, Marumoto S, Yamamoto SP, Ouda R, Yatagai M, Fujita T, Watanabe T. Antiviral activity of phenolic derivatives in pyroligneous acid from hardwood, softwood, and bamboo. ACS Sustain Chem Eng. 2017;6(1):119–126. doi: 10.1021/acssuschemeng.7b01265. [DOI] [Google Scholar]
- 28.Butt D. Formation of phenols from the low-temperature fast pyrolysis of radiata pine (Pinus radiata): part II. Interaction of molecular oxygen and substrate water. J Anal Appl Pyrolysis. 2006;76(1):48–54. doi: 10.1016/j.jaap.2005.01.009. [DOI] [Google Scholar]
- 29.Wei Q, Ma X, Dong J. Preparation, chemical constituents and antimicrobial activity of pyroligneous acids from walnut tree branches. J Anal Appl Pyrolysis. 2010;87(1):24–28. doi: 10.1016/j.jaap.2009.09.006. [DOI] [Google Scholar]
- 30.Harada K, Iguchi A, Yamada M, Hasegawa K, Nakata T, Hikasa Y. Determination of maximum inhibitory dilutions of bamboo pyroligneous acid against pathogenic bacteria from companion animals: an in vitro study. J Vet Adv. 2013;3(11):300–305. [Google Scholar]
- 31.Abas FZ, Zakaria ZA, Ani FN. Antimicrobial properties of optimized microwave-assisted pyroligneous acid from oil palm fiber. J Appl Pharm Sci. 2018;8(07):65–71. doi: 10.7324/JAPS.2018.8711. [DOI] [Google Scholar]
- 32.Choi JY, Shinde PL, Kwon IK, Song YH, Chae BJ. Effect of wood vinegar on the performance, nutrient digestibility and intestinal microflora in weanling pigs. Asian Australas J Anim Sci. 2009;22(2):267–274. doi: 10.5713/ajas.2009.80355. [DOI] [Google Scholar]
- 33.Wang HF, Gao K, Wang C, Zhang WM, Liu JX. Effects of feeding bamboo vinegar and acidifier as an antibiotic substitute on the growth performance and intestinal bacterial communities of weaned piglets. Acta Agric Scand A Anim Sci. 2013;63(3):143–150. doi: 10.1080/09064702.2013.845244. [DOI] [Google Scholar]
- 34.Kupittayanant P, Kupittayanant S. Effects of wood vinegar on the protection of diarrhea in weaning pigs. Planta Med Int Open. 2017;4(S 01):Mo-PO-242. doi: 10.1055/s-0037-1608288. [DOI] [Google Scholar]
- 35.Rattanawut J (2013) Effects of dietary bamboo charcoal powder including bamboo vinegar liquid supplementation on growth performance, fecal microflora population and intestinal morphology in Betong chickens. J Poult Sci:0130109. 10.2141/jpsa.0130109